Abstract
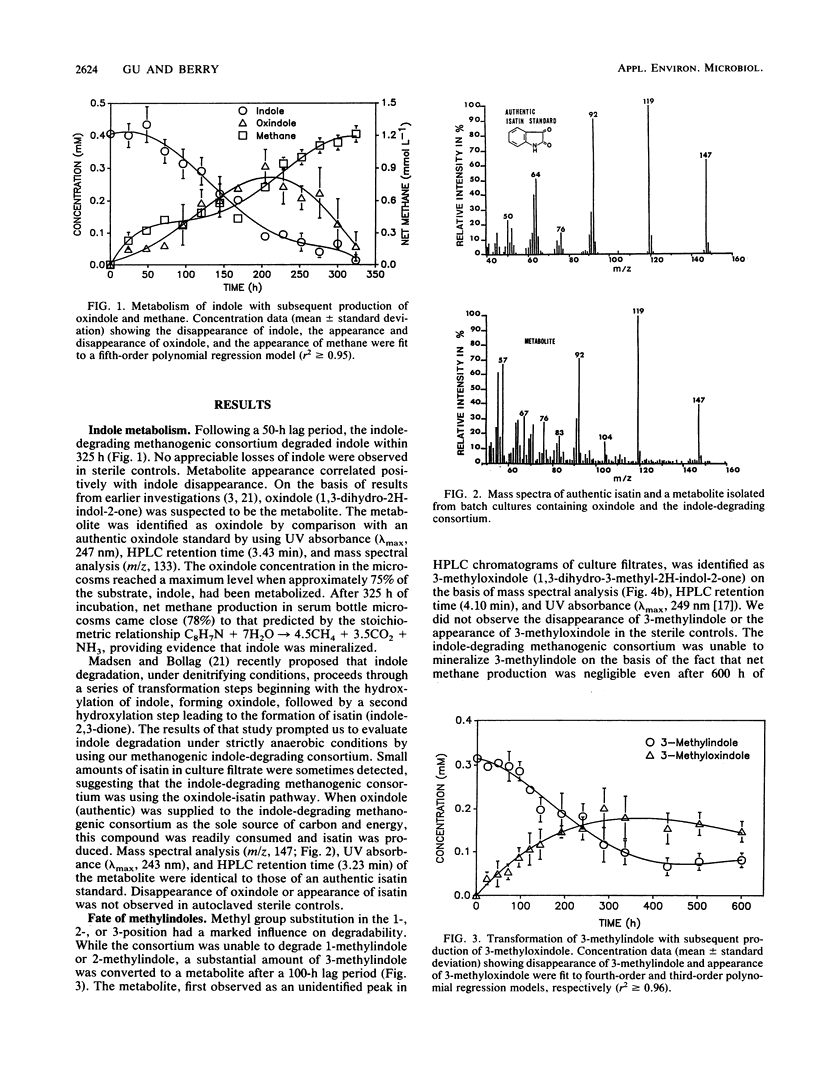
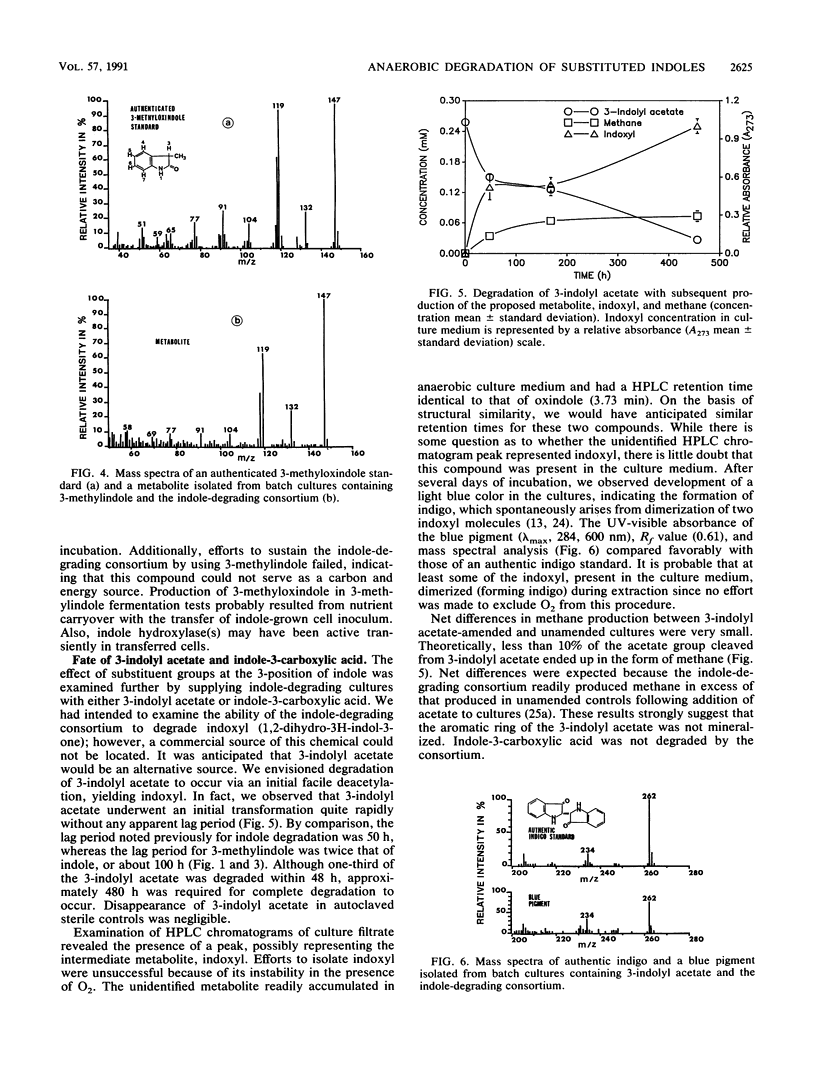
Degradation of indole by an indole-degrading methanogenic consortium enriched from sewage sludge proceeded through a two-step hydroxylation pathway yielding oxindole and isatin. The ability of this consortium to hydroxylate and subsequently degrade substituted indoles was investigated. Of the substituted indoles tested, the consortium was able to transform or degrade 3-methylindole and 3-indolyl acetate. Oxindole, 3-methyloxindole, and indoxyl were identified as metabolites of indole, 3-methylindole, and 3-indolyl acetate degradation, respectively. Isatin (indole-2,3-dione) was produced as an intermediate when the consortium was amended with oxindole, providing evidence that degradation of indole proceeded through successive hydroxylation of the 2- and 3-positions prior to ring cleavage between the C-2 and C-3 atoms on the pyrrole ring of indole. The presence of a methyl group (-CH3) at either the 1- or 2-position of indole inhibited the initial hydroxylation reaction. The substituted indole, 3-methylindole, was hydroxylated in the 2-position but not in the 3-position and could not be further metabolized through the oxindole-isatin pathway. Indoxyl (indole-3-one), the deacetylated product of 3-indolyl acetate, was not hydroxylated in the 2-position and thus was not further metabolized by the consortium. When an H atom or electron-donating group (i.e., -CH3) was present at the 3-position, hydroxylation proceeded at the 2-position, but the presence of electron-withdrawing substituent groups (i.e., -OH or -COOH) at the 3-position inhibited hydroxylation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. Methanogenic fermentation of the naturally occurring aromatic amino acids by a microbial consortium. Biochem Soc Trans. 1980 Oct;8(5):625–627. doi: 10.1042/bst0080625. [DOI] [PubMed] [Google Scholar]
- Berry D. F., Francis A. J., Bollag J. M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol Rev. 1987 Mar;51(1):43–59. doi: 10.1128/mr.51.1.43-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. F., Madsen E. L., Bollag J. M. Conversion of indole to oxindole under methanogenic conditions. Appl Environ Microbiol. 1987 Jan;53(1):180–182. doi: 10.1128/aem.53.1.180-182.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R., Berry D., Tiedje J. M. Anaerobic biodegradation of phenolic compounds in digested sludge. Appl Environ Microbiol. 1983 Jul;46(1):50–54. doi: 10.1128/aem.46.1.50-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr Reductive degradation of pyrimidines. I. The isolation and characterization of a uracil fermenting bacterium, Clostridium uracilicum nov. spec. J Bacteriol. 1957 Feb;73(2):220–224. doi: 10.1128/jb.73.2.220-224.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. R., Hammond A. C., Breeze R. G., Potchoiba M. J., Heinemann W. W. Effect of monensin on bovine ruminal 3-methylindole production after abrupt change to lush pasture. Am J Vet Res. 1983 Jan;44(1):118–122. [PubMed] [Google Scholar]
- Dürre P., Andreesen J. R. Purine and glycine metabolism by purinolytic clostridia. J Bacteriol. 1983 Apr;154(1):192–199. doi: 10.1128/jb.154.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M., Wada H. The bacterial oxidation of indole. Biochim Biophys Acta. 1968 Apr 16;158(1):70–78. doi: 10.1016/0304-4165(68)90073-1. [DOI] [PubMed] [Google Scholar]
- Holcenberg J. S., Stadtman E. R. Nicotinic acid metabolism. 3. Purification and properties of a nicotinic acid hydroxylase. J Biol Chem. 1969 Mar 10;244(5):1194–1203. [PubMed] [Google Scholar]
- Kamath A. V., Vaidyanathan C. S. New pathway for the biodegradation of indole in Aspergillus niger. Appl Environ Microbiol. 1990 Jan;56(1):275–280. doi: 10.1128/aem.56.1.275-280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen E. L., Francis A. J., Bollag J. M. Environmental factors affecting indole metabolism under anaerobic conditions. Appl Environ Microbiol. 1988 Jan;54(1):74–78. doi: 10.1128/aem.54.1.74-78.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEBEK O. K., JAGER H. Divergent pathways of indole metabolism in Chromobacterium violaceum. Nature. 1962 Nov 24;196:793–795. doi: 10.1038/196793a0. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Stadtman T. C., Pastan I., Smith L. D. Clostridium barkeri sp. n. J Bacteriol. 1972 May;110(2):758–760. doi: 10.1128/jb.110.2.758-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. T., Carlson J. R. Dissimilation of tryptophan and related indolic compounds by ruminal microorganisms in vitro. Appl Microbiol. 1974 Mar;27(3):540–548. doi: 10.1128/am.27.3.540-548.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


