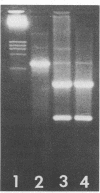
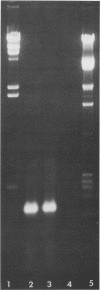
Abstract
Vibrio vulnificus is a human pathogen associated with consumption of raw oysters. During the colder months the organism apparently enters a viable but nonculturable state and thus cannot be cultured by ordinary bacteriological methods. For this reason, another means of detecting this bacterium is necessary. In the present study we utilized the polymerase chain reaction (PCR) to detect V. vulnificus DNA, thus eliminating the problem of nonculturability. DNA from both culturable and nonculturable cells of V. vulnificus was amplified by PCR with primers flanking a 340-bp fragment of the cytotoxin-hemolysin gene. As little as 72 pg of DNA from culturable cells and 31 ng of DNA from nonculturable cells could be detected. Fifty cycles of a two-step reaction (30 s [each] at 94 and 65 degrees C) were found to be optimal as well as more time efficient than the three-step PCR. The total procedure from the point of DNA extraction to observation on a gel required less than 8 h. Possible reasons for the difficulties encountered in amplifying DNA from nonculturable cells, e.g., gene rearrangement or loss of the hemolysin gene, are discussed.
Full text
PDF

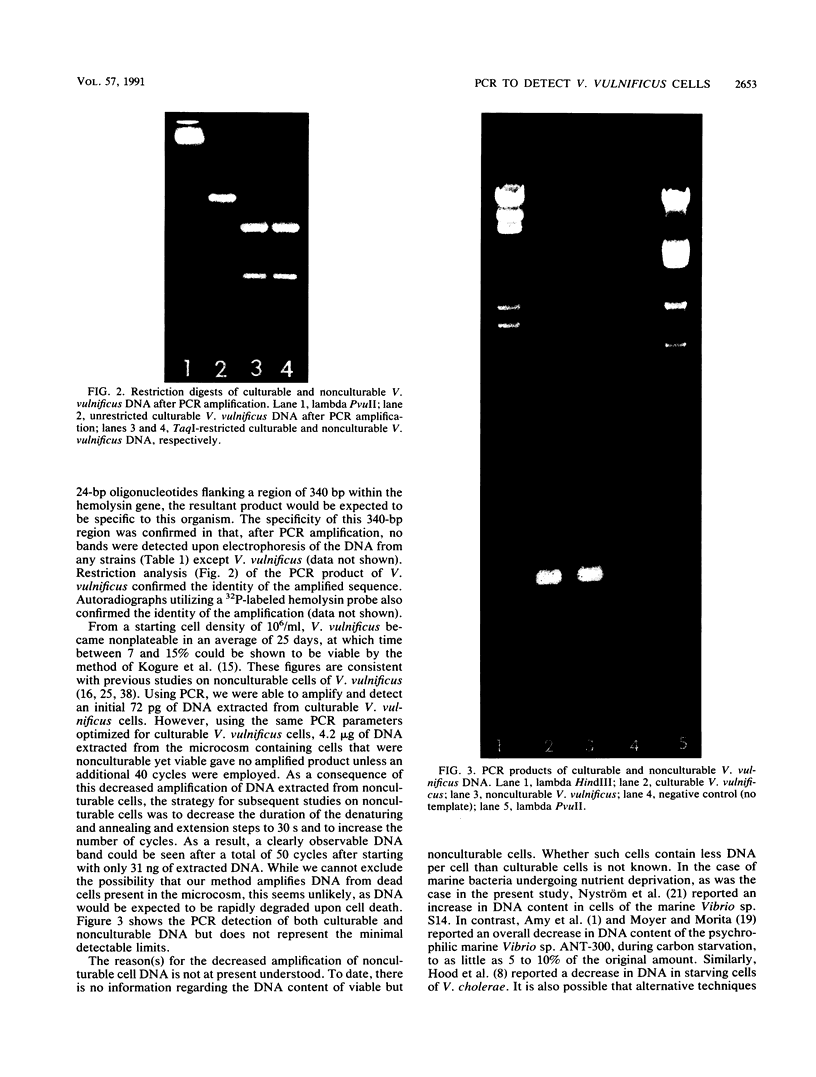


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A. K., Mahbubani M. H., Atlas R. M. Detection of viable Legionella pneumophila in water by polymerase chain reaction and gene probe methods. Appl Environ Microbiol. 1991 Feb;57(2):597–600. doi: 10.1128/aem.57.2.597-600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton P. R., Tamplin M. L., Huq A., Colwell R. R. Enumeration of Vibrio cholerae O1 in Bangladesh waters by fluorescent-antibody direct viable count. Appl Environ Microbiol. 1987 Dec;53(12):2862–2865. doi: 10.1128/aem.53.12.2862-2865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M. A., Yamanaka H., Miyoshi S., Aziz K. M., Shinoda S. Ecology of Vibrio mimicus in aquatic environments. Appl Environ Microbiol. 1989 Aug;55(8):2073–2078. doi: 10.1128/aem.55.8.2073-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Keasler S. P., Trucksess M. W., Feng P., Kaysner C. A., Lampel K. A. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Appl Environ Microbiol. 1991 Mar;57(3):707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Colwell R. R. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J Bacteriol. 1973 Jan;113(1):24–32. doi: 10.1128/jb.113.1.24-32.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysner C. A., Abeyta C., Jr, Wekell M. M., DePaola A., Jr, Stott R. F., Leitch J. M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987 Jun;53(6):1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl Environ Microbiol. 1982 Oct;44(4):820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Stroh E. M. Temporal relationship of Vibrio parahaemolyticus in patients and the environment. J Clin Microbiol. 1988 Sep;26(9):1754–1756. doi: 10.1128/jcm.26.9.1754-1756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klontz K. C., Lieb S., Schreiber M., Janowski H. T., Baldy L. M., Gunn R. A. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981-1987. Ann Intern Med. 1988 Aug 15;109(4):318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- Knight I. T., Shults S., Kaspar C. W., Colwell R. R. Direct detection of Salmonella spp. in estuaries by using a DNA probe. Appl Environ Microbiol. 1990 Apr;56(4):1059–1066. doi: 10.1128/aem.56.4.1059-1066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Wright A. C., Roberts D. M., Wood P. K., Simpson L. M., Oliver J. D. Identification of environmental Vibrio vulnificus isolates with a DNA probe for the cytotoxin-hemolysin gene. Appl Environ Microbiol. 1987 Jan;53(1):193–195. doi: 10.1128/aem.53.1.193-195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer C. L., Morita R. Y. Effect of growth rate and starvation-survival on cellular DNA, RNA, and protein of a psychrophilic marine bacterium. Appl Environ Microbiol. 1989 Oct;55(10):2710–2716. doi: 10.1128/aem.55.10.2710-2716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Oliver J. D., Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991 Aug;173(16):5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Flärdh K., Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990 Dec;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill K. R., Jones S. H., Grimes D. J. Incidence of Vibrio vulnificus in northern New England water and shellfish. FEMS Microbiol Lett. 1990 Oct;60(1-2):163–167. doi: 10.1016/0378-1097(90)90365-w. [DOI] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl Environ Microbiol. 1982 Dec;44(6):1404–1414. doi: 10.1128/aem.44.6.1404-1414.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983 Mar;45(3):985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole M. D., Oliver J. D. Experimental pathogenicity and mortality in ligated ileal loop studies of the newly reported halophilic lactose-positive Vibrio sp. Infect Immun. 1978 Apr;20(1):126–129. doi: 10.1128/iai.20.1.126-129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. C., Morris J. G., Jr, Maneval D. R., Jr, Richardson K., Kaper J. B. Cloning of the cytotoxin-hemolysin gene of Vibrio vulnificus. Infect Immun. 1985 Dec;50(3):922–924. doi: 10.1128/iai.50.3.922-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Wright A. C., Kaper J. B., Morris J. G., Jr The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae E1 Tor hemolysin gene. Infect Immun. 1990 Aug;58(8):2706–2709. doi: 10.1128/iai.58.8.2706-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]