Abstract
In Rhizobium-legume symbiosis, the plant host controls and optimizes the nodulation process by autoregulation. Tn5 mutants of Rhizobium leguminosarum bv. phaseoli TAL 182 which are impaired at various stages of symbiotic development, were used to examine autoregulation in the common bean (Phaseolus vulgaris L.). Class I mutants were nonnodulating, class II mutants induced small, distinct swellings on the roots, and a class III mutant formed pink, bacterium-containing, but ineffective nodules. A purine mutant (Ade-) was nonnodulating, while a pyrimidine mutant (Ura-) formed small swellings on the roots. Amino acid mutants (Leu-, Phe-, and Cys-) formed mostly empty white nodules. Each of the mutants was used as a primary inoculant on one side of a split-root system to assess its ability to suppress secondary nodulation by the wild type on the other side. All mutants with defects in nodulation ability, regardless of the particular stage of blockage, failed to induce a suppression response from the host. Only the nodulation-competent, bacterium-containing, but ineffective class III mutant induced a suppression response similar to that induced by the wild type. Suppression was correlated with the ability of the microsymbiont to proliferate inside the nodules but not with the ability to initiate nodule formation or the ability to fix nitrogen. Thus, the presence of bacteria inside the nodules may be required for the induction of nodulation suppression in the common bean.
Full text
PDF

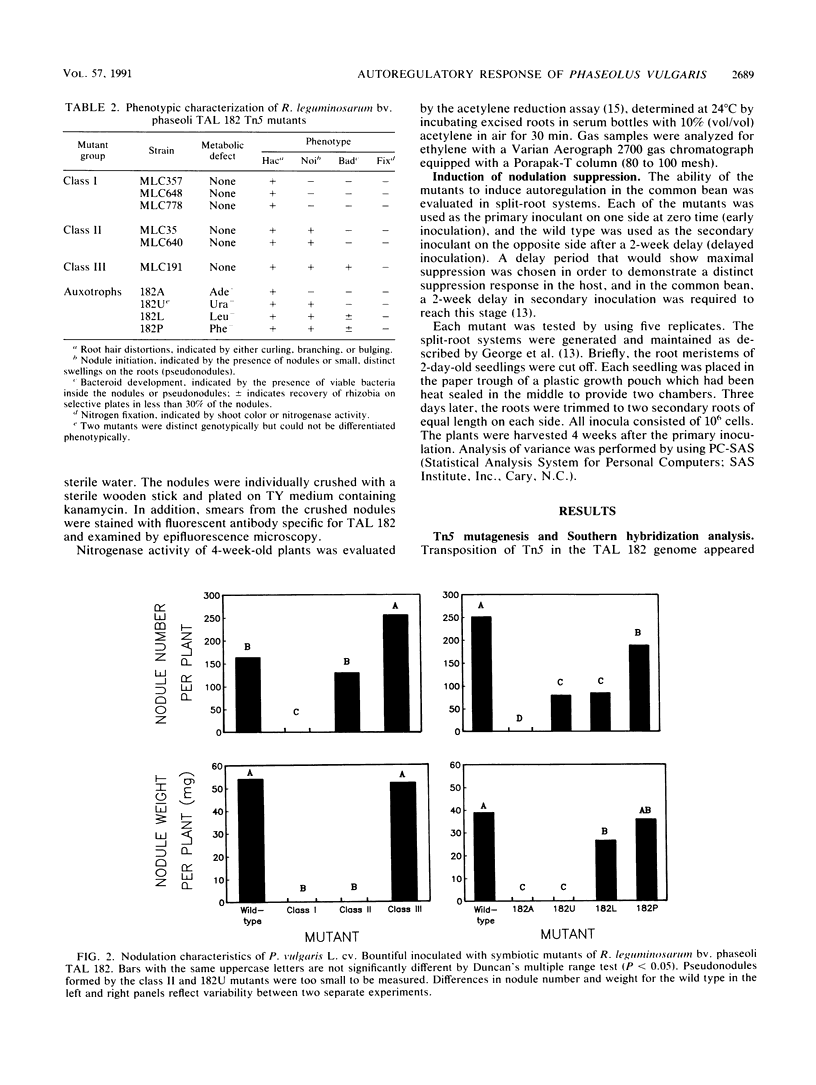

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Gresshoff P. M. Alfalfa Controls Nodulation during the Onset of Rhizobium-induced Cortical Cell Division. Plant Physiol. 1991 Feb;95(2):366–373. doi: 10.1104/pp.95.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Lagares A., Bauer W. D. Rhizobium meliloti exopolysaccharide Mutants Elicit Feedback Regulation of Nodule Formation in Alfalfa. Plant Physiol. 1990 Feb;92(2):368–374. doi: 10.1104/pp.92.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves A. C., Mathews A., Day D. A., Carter A. S., Carroll B. J., Gresshoff P. M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986 Oct;82(2):588–590. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic S. P., Ridge R. W., Chen H. C., Redmond J. W., Batley M., Rolfe B. G. Induction of pathogenic-like responses in the legume Macroptilium atropurpureum by a transposon-induced mutant of the fast-growing, broad-host-range Rhizobium strain NGR234. J Bacteriol. 1988 Apr;170(4):1848–1857. doi: 10.1128/jb.170.4.1848-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984 May;75(1):125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J. E., Nakao P., Bohlool B. B., Gresshoff P. M. Lack of Systemic Suppression of Nodulation in Split Root Systems of Supernodulating Soybean (Glycine max [L.] Merr.) Mutants. Plant Physiol. 1989 Aug;90(4):1347–1352. doi: 10.1104/pp.90.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Somasegaran P., Bohlool B. B. Single-Strain versus Multistrain Inoculation: Effect of Soil Mineral N Availability on Rhizobial Strain Effectiveness and Competition for Nodulation on Chick-Pea, Soybean, and Dry Bean. Appl Environ Microbiol. 1990 Nov;56(11):3298–3303. doi: 10.1128/aem.56.11.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]