Abstract
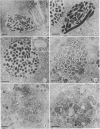
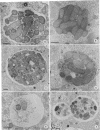
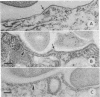
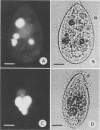
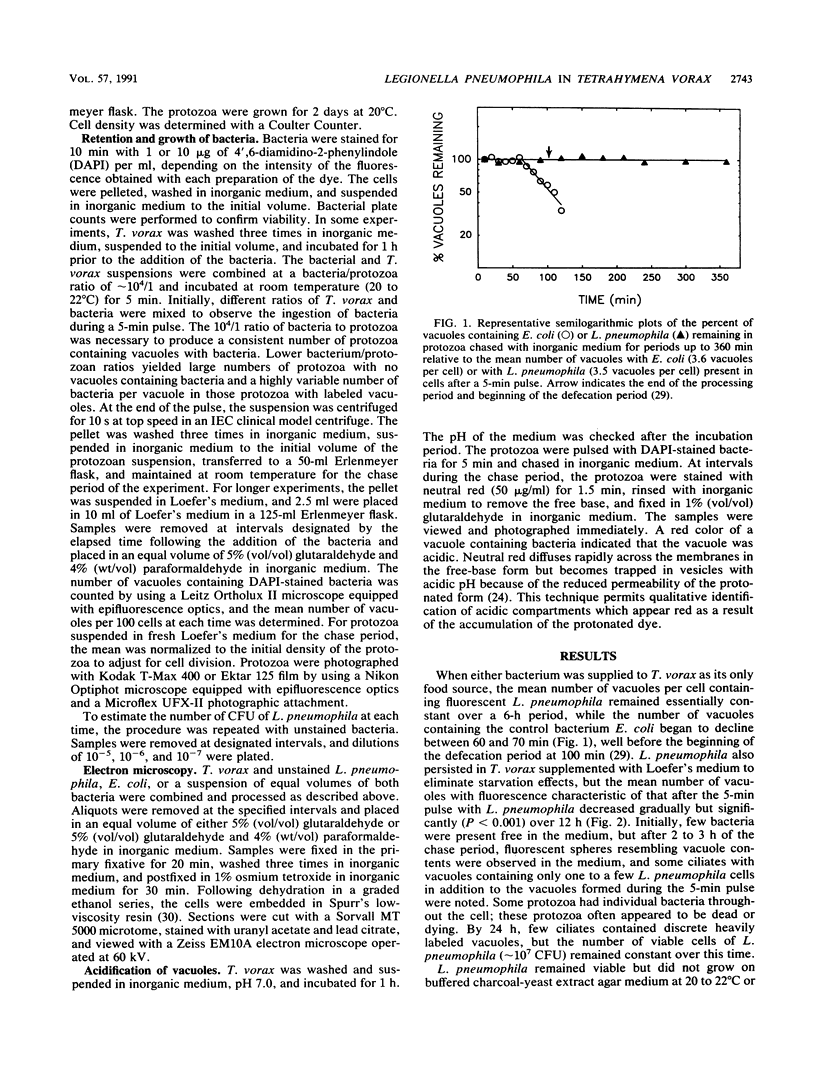
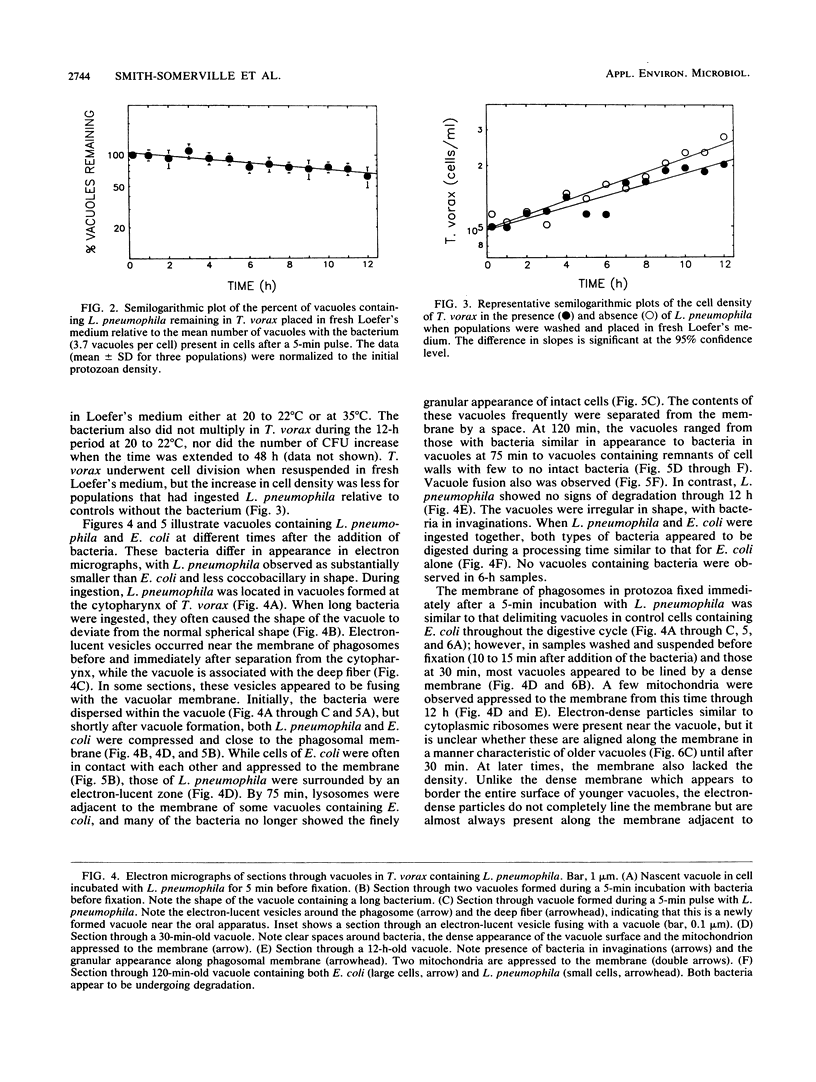
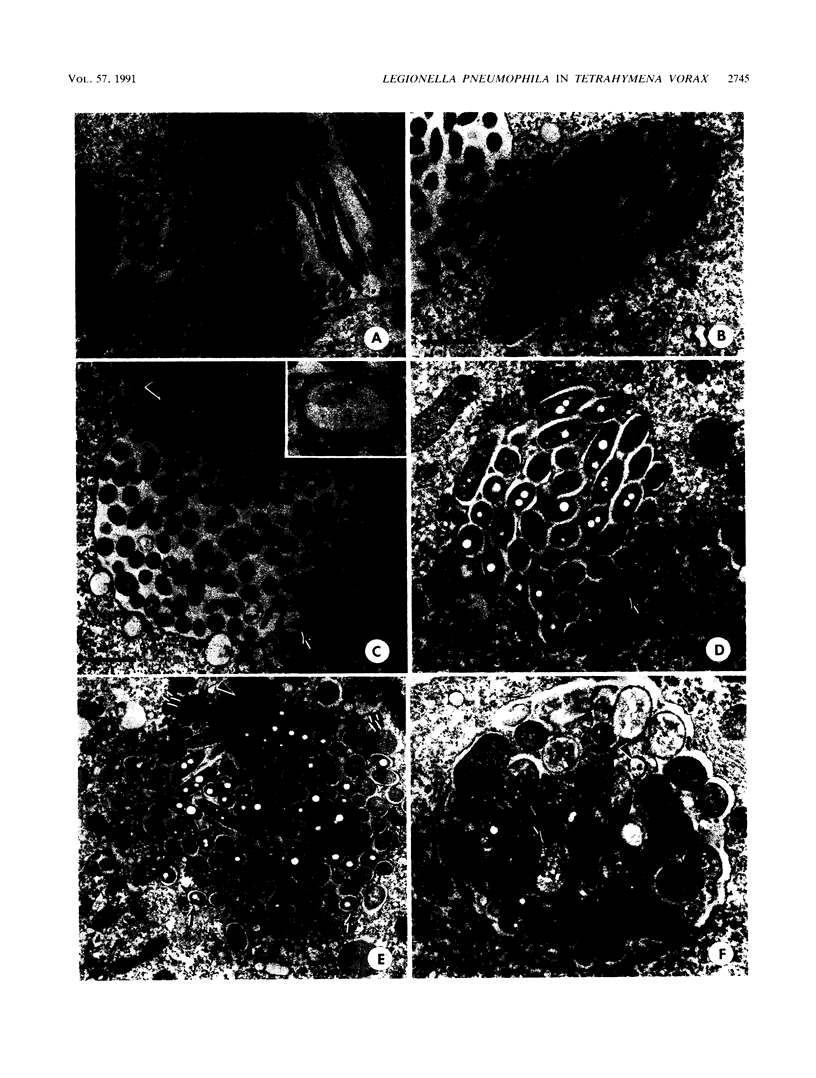
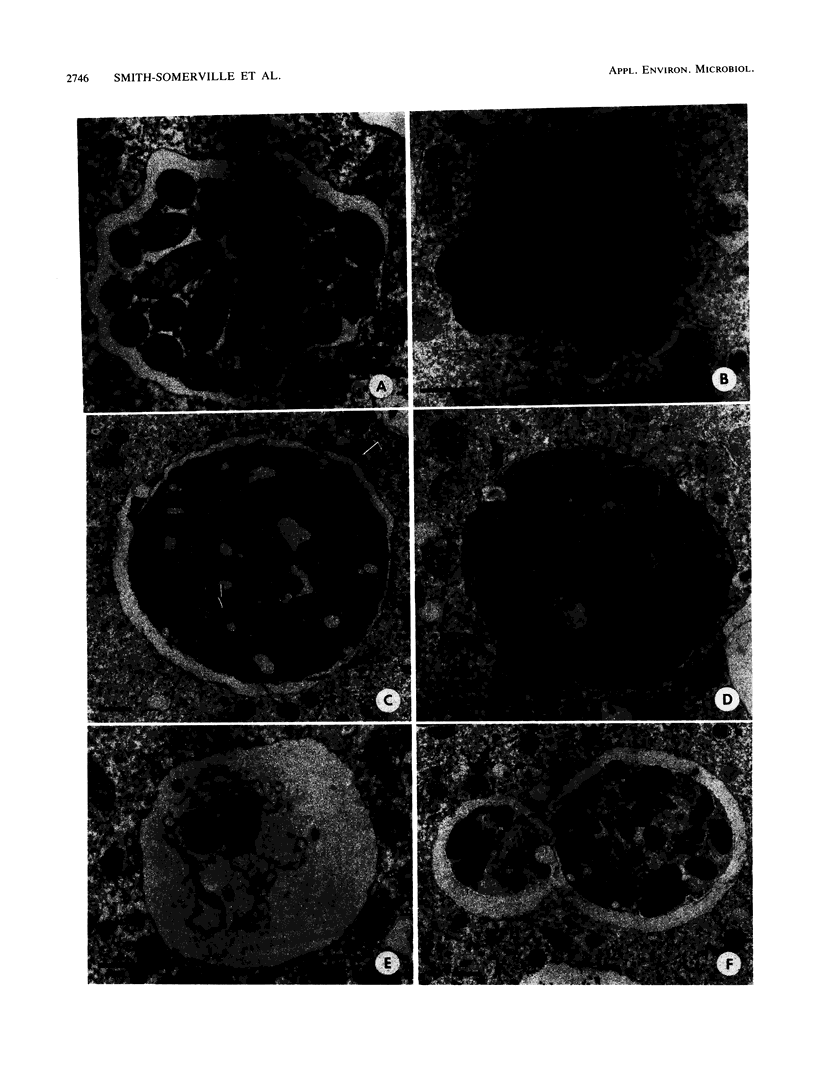
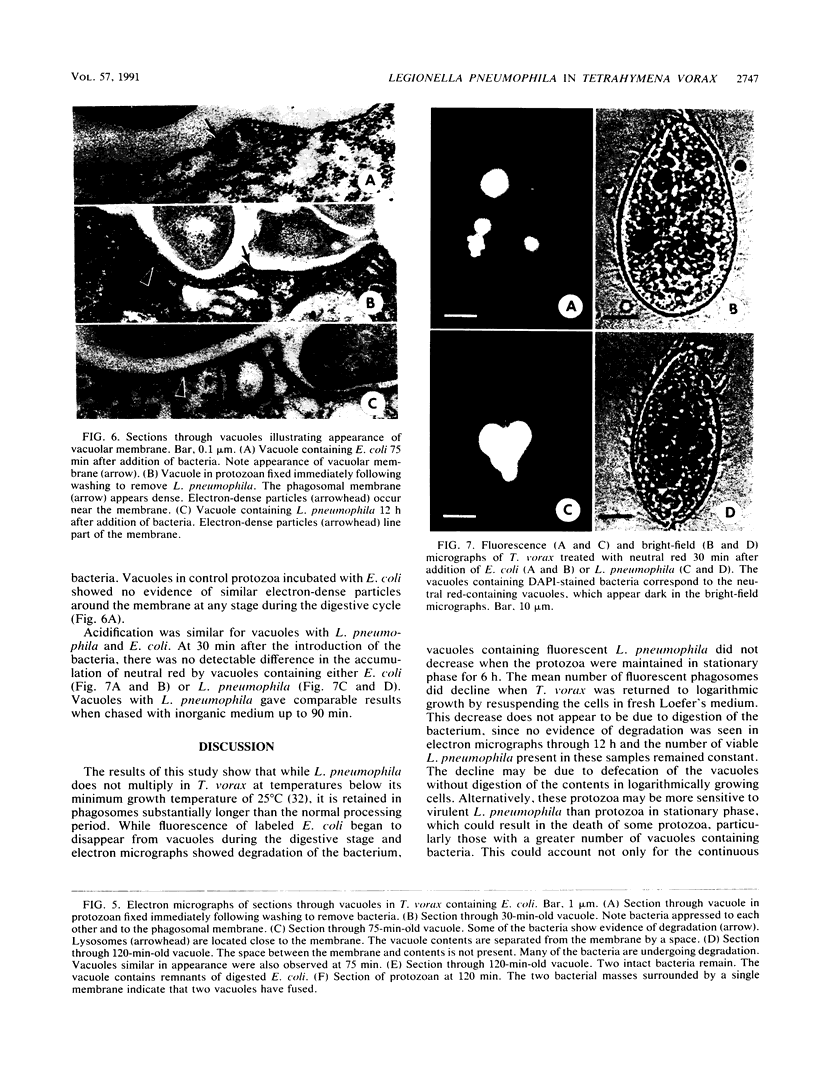
The processing of phagosomes containing Legionella pneumophila and Escherichia coli were compared in Tetrahymena vorax, a hymenostome ciliated protozoan that prefers lower temperatures. L. pneumophila did not multiply in the ciliate when incubated at 20 to 22 degrees C, but vacuoles containing L. pneumophila were retained in the cells for a substantially longer time than vacuoles with E. coli. Electron micrographs showed no evidence of degradation of L. pneumophila cells through 12 h, while E. coli cells in the process of being digested were observed in vacuoles 75 min after the addition of the bacterium. T. vorax ingested L. pneumophila normally, but by 10 to 15 min, the vacuolar membrane appeared denser than that surrounding nascent or newly formed phagosomes. In older vacuoles, electron-dense particles lined portions of the membrane. Acidification of the phagosomes indicated by the accumulation of neutral red was similar in T. vorax containing L. pneumophila or E. coli. This ciliate could provide a model for the analysis of virulence-associated intracellular events independent of the replication of L. pneumophila.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Fok A. K. Nonlysosomal vesicles (acidosomes) are involved in phagosome acidification in Paramecium. J Cell Biol. 1983 Aug;97(2):566–570. doi: 10.1083/jcb.97.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand C. M., Skinner A. R., Malic A., Kurtz J. B. Interaction of L. pneumophilia and a free living amoeba (Acanthamoeba palestinensis). J Hyg (Lond) 1983 Oct;91(2):167–178. doi: 10.1017/s0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaree J. M., Fields B. S., Feeley J. C., Gorman G. W., Martin W. T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986 Feb;51(2):422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhse H. E., Jr Microstome-macrostome transformation in Tetrahymena vorax strain V2 type S induced by a transforming principle, stomatin. J Protozool. 1967 Nov;14(4):608–613. doi: 10.1111/j.1550-7408.1967.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Dondero T. J., Jr, Rendtorff R. C., Mallison G. F., Weeks R. M., Levy J. S., Wong E. W., Schaffner W. An outbreak of Legionnaires' disease associated with a contaminated air-conditioning cooling tower. N Engl J Med. 1980 Feb 14;302(7):365–370. doi: 10.1056/NEJM198002143020703. [DOI] [PubMed] [Google Scholar]
- England A. C., 3rd, Fraser D. W., Mallison G. F., Mackel D. C., Skaliy P., Gorman G. W. Failure of Legionella pneumophila sensitivities to predict culture results from disinfectant-treated air-conditioning cooling towers. Appl Environ Microbiol. 1982 Jan;43(1):240–244. doi: 10.1128/aem.43.1.240-244.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Barbaree J. M., Shotts E. B., Jr, Feeley J. C., Morrill W. E., Sanden G. N., Dykstra M. J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986 Sep;53(3):553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Shotts E. B., Jr, Feeley J. C., Gorman G. W., Martin W. T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984 Mar;47(3):467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Cherry W. B., Orrison L. H., Thacker L. Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl Environ Microbiol. 1979 Jun;37(6):1239–1242. doi: 10.1128/aem.37.6.1239-1242.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J. B., Bartlett C. L., Newton U. A., White R. A., Jones N. L. Legionella pneumophila in cooling water systems. Report of a survey of cooling towers in London and a pilot trial of selected biocides. J Hyg (Lond) 1982 Jun;88(3):369–381. doi: 10.1017/s0022172400070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz C. S., Chen J. X., Shuman H. A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988 Jun;56(6):1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. K., Patton C. M., Feeley J. C., Johnson S. E., Gorman G., Martin W. T., Skaliy P., Mallison G. F., Politi B. D., Mackel D. C. Isolation of the Legionnaires' disease bacterium from environmental samples. Ann Intern Med. 1979 Apr;90(4):664–666. doi: 10.7326/0003-4819-90-4-664. [DOI] [PubMed] [Google Scholar]
- Newsome A. L., Baker R. L., Miller R. D., Arnold R. R. Interactions between Naegleria fowleri and Legionella pneumophila. Infect Immun. 1985 Nov;50(2):449–452. doi: 10.1128/iai.50.2.449-452.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrison L. H., Cherry W. B., Milan D. Isolation of Legionella pneumophilia from cooling tower water by filtration. Appl Environ Microbiol. 1981 May;41(5):1202–1205. doi: 10.1128/aem.41.5.1202-1205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T. J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980 Dec;33(12):1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler C. A., Staehelin L. A. Oral cavity of Tetrahymena pyriformis. A freeze-fracture and high-voltage electron microscopy study of the oral ribs, cytostome, and forming food vacuole. J Ultrastruct Res. 1979 Feb;66(2):132–150. doi: 10.1016/s0022-5320(79)90130-8. [DOI] [PubMed] [Google Scholar]
- Tyndall R. L., Domingue E. L. Cocultivation of Legionella pneumophila and free-living amoebae. Appl Environ Microbiol. 1982 Oct;44(4):954–959. doi: 10.1128/aem.44.4.954-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky R. M., Wolford R., McNamara A. M., Yee R. B. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl Environ Microbiol. 1985 May;49(5):1197–1205. doi: 10.1128/aem.49.5.1197-1205.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenbach A. L., Thompson G. A., Jr Studies of membrane formation in Tetrahymena pyriformis. VIII. On the origin of membranes surrounding food vacuoles. J Protozool. 1974 Nov;21(5):745–751. doi: 10.1111/j.1550-7408.1974.tb03744.x. [DOI] [PubMed] [Google Scholar]