Abstract
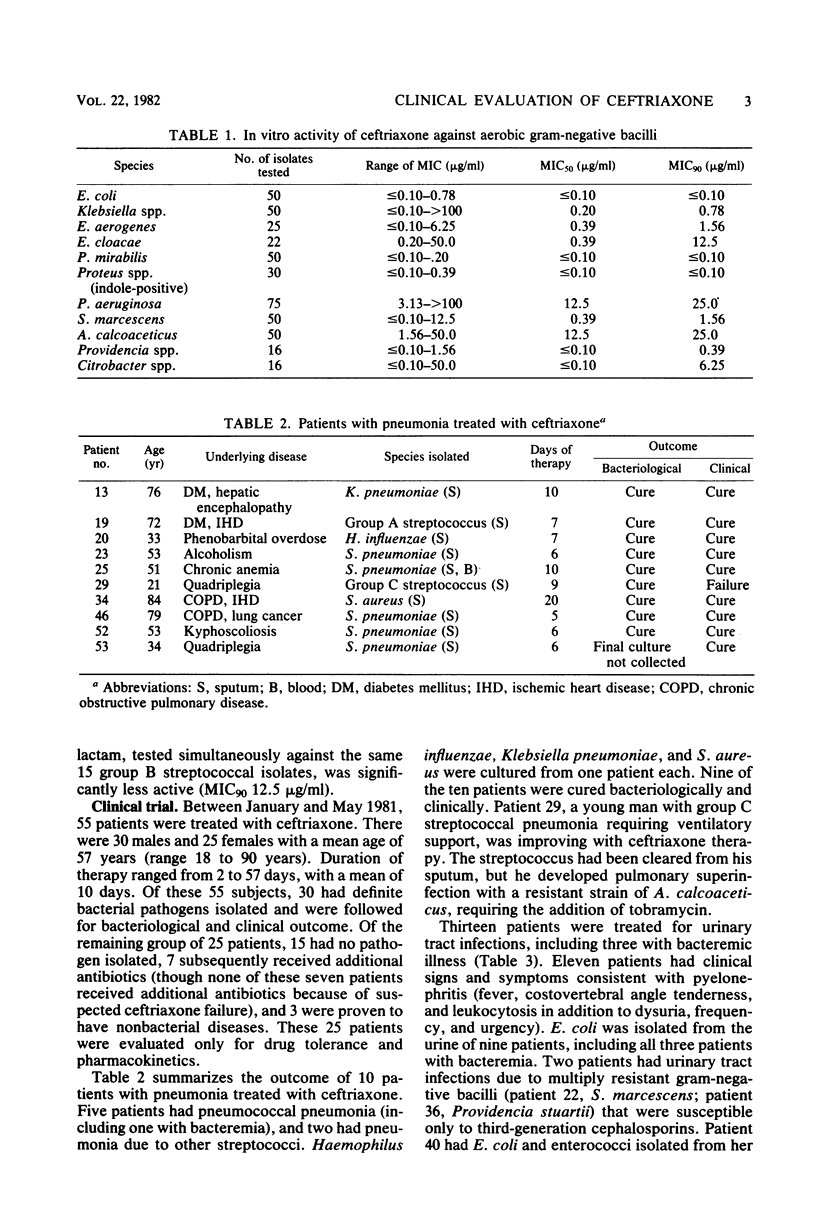
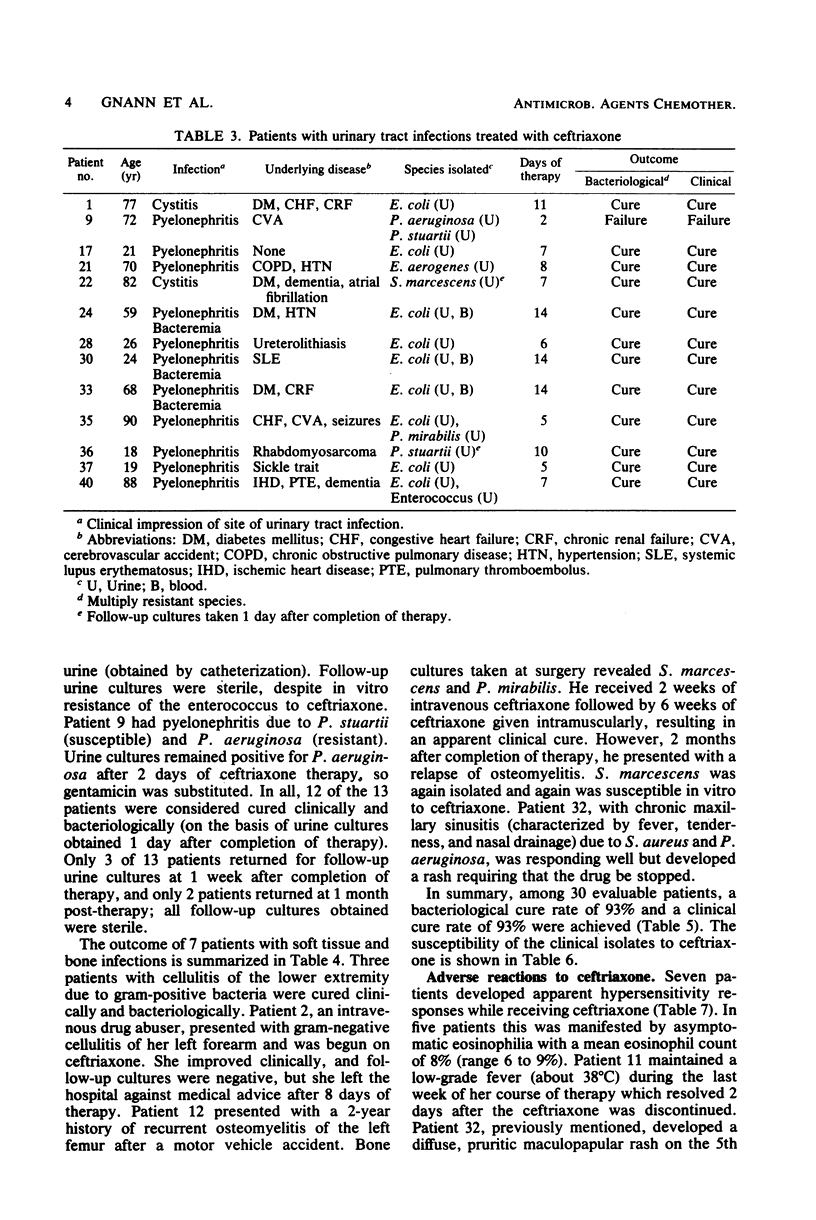
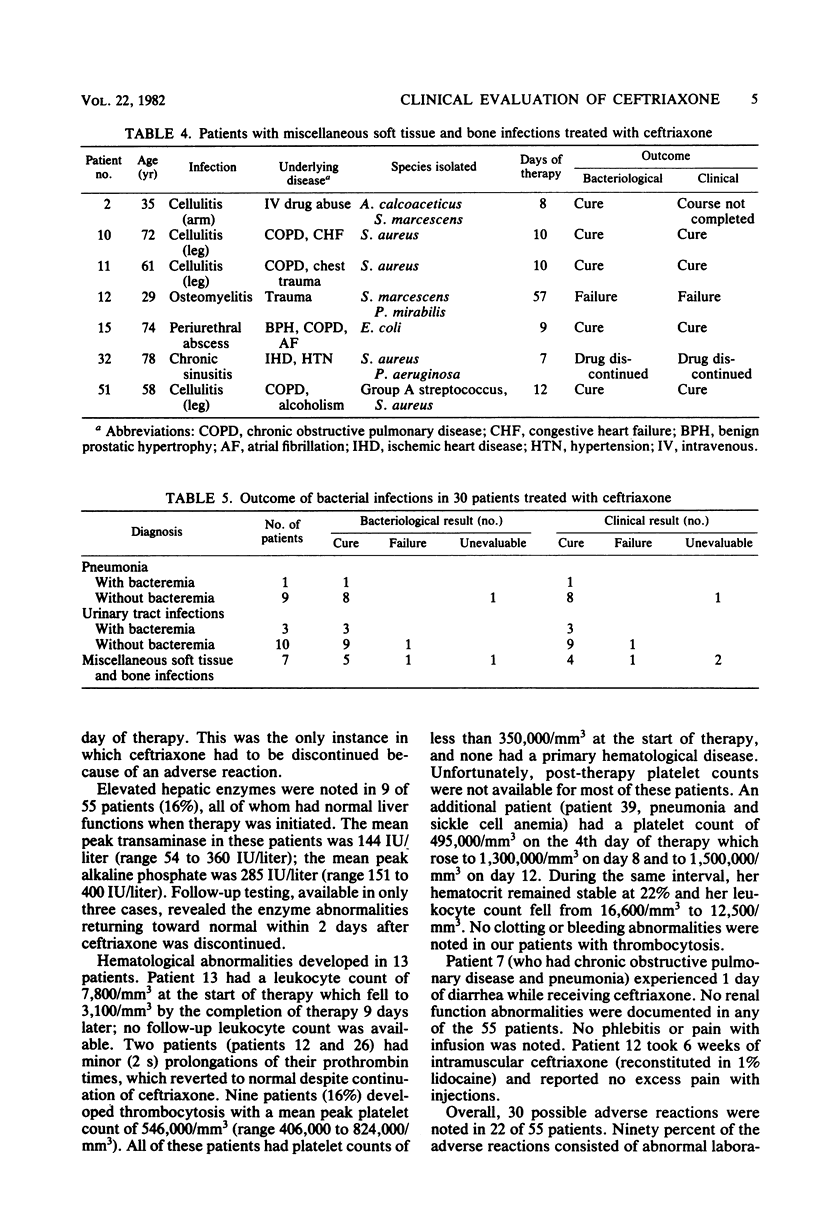
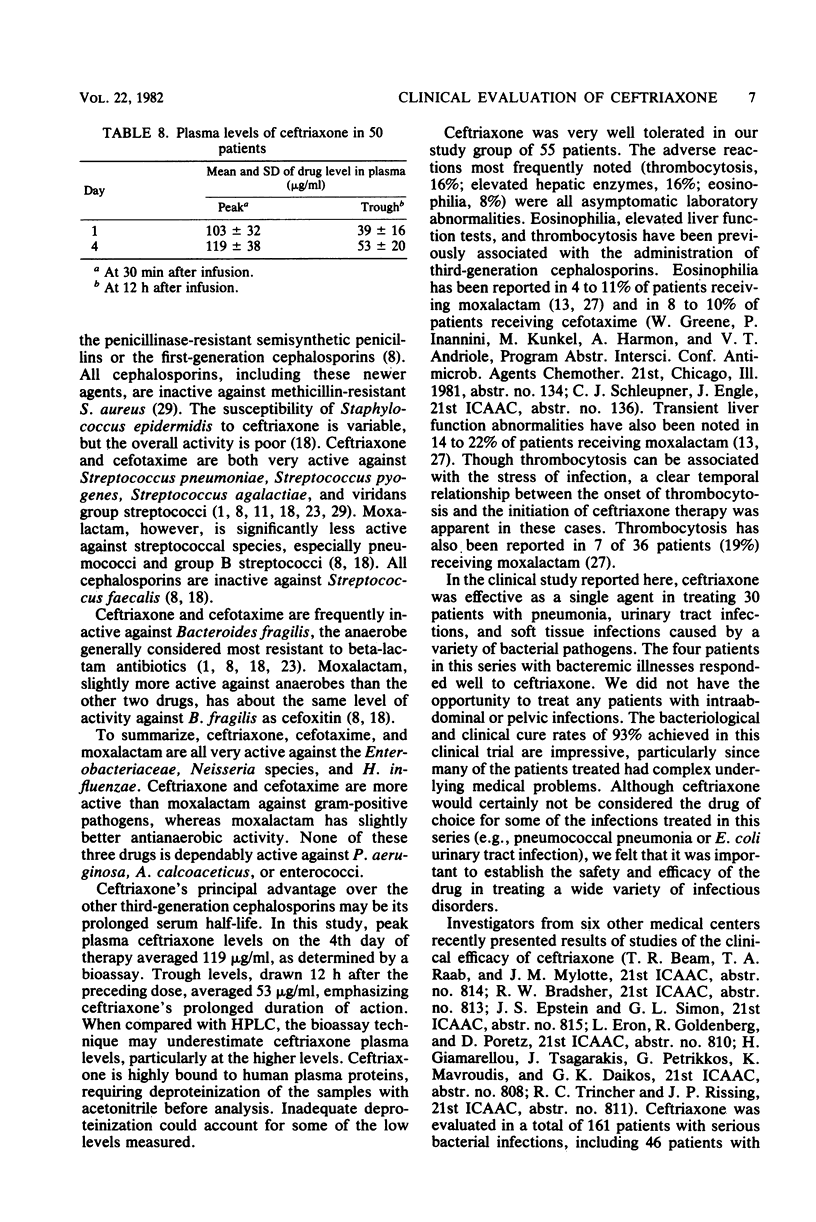
The in vitro activity of ceftriaxone against 437 clinical isolates of gram-negative bacilli was determined. Ceftriaxone was found to have high in vitro activity against Enterobacteriaceae, with the exception of Enterobacter cloacae. Ceftriaxone was only minimally active against Pseudomonas aeruginosa and Acinetobacter calcoaceticus. We evaluated the clinical efficacy and toxicity of ceftriaxone in 55 adult patients. Bacterial infection was confirmed by the isolation of etiological bacteria in 30 patients. Infectious disorders treated included 10 pneumonias, 13 urinary tract infections, and 7 soft tissue or bone infections. Pathogens identified were 25 isolates of gram-negative bacilli, 5 isolates of Staphylococcus aureus, 5 isolates of pneumococci, and 4 isolates of other streptococci. The overall efficacy of ceftriaxone was excellent. The clinical cure rate was 93%, and the bacteriological cure rate was 93%. A total of 30 adverse reactions were noted in 22 of 55 patients receiving ceftriaxone, but only one necessitated discontinuation of treatment. Adverse effects frequently noted were elevated hepatic enzymes (16%), thrombocytosis (16%), and eosinophilia (8%). Ceftriaxone is an effective and well-tolerated antimicrobial agent that appears promising for the treatment of serious gram-negative bacillary infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M. G., Brusch J. L., Barza M., Weinstein L. Bactericidal activity and pharmacology of cefazolin. Antimicrob Agents Chemother. 1973 Oct;4(4):396–401. doi: 10.1128/aac.4.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskid G., Christenson J. G., Cleeland R., DeLorenzo W., Trown P. W. In vivo activity of ceftriaxone (Ro 13-9904), a new broad-spectrum semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981 Aug;20(2):159–167. doi: 10.1128/aac.20.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoz M., Denis F., Félix H., Diop Mar I. Treatment of purulent meningitis with a new cephalosporin-Rocephin (Ro 13-9904). Clinical, bacteriological and pharmacological observations in 24 cases. Chemotherapy. 1981;27 (Suppl 1):57–61. doi: 10.1159/000238030. [DOI] [PubMed] [Google Scholar]
- Chau P. Y., Ng W. S., Ling J., Arnold K. In vitro susceptibility of Salmonella to various antimicrobial agents, including a new cephalosporin, Ro 13-9904. Antimicrob Agents Chemother. 1981 Jan;19(1):8–11. doi: 10.1128/aac.19.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado D. G., Brau C. J., Cobbs C. G., Dismukes W. E. In vitro activity of LY127935, a new 1-oxa cephalosporin, against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1979 Dec;16(6):864–868. doi: 10.1128/aac.16.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T. C., Ehret J. Comparative in vitro studies of Ro 13-9904, a new cephalosporin derivative. Antimicrob Agents Chemother. 1981 Mar;19(3):435–442. doi: 10.1128/aac.19.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., Eley A. Activity of a new cephalosporin antibiotic, Ro 13-9904 against dense populations of selected enterobacteria. Antimicrob Agents Chemother. 1981 Jan;19(1):66–71. doi: 10.1128/aac.19.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymès R., Lutz A., Schrinner E. Experimental evaluation of HR756, a new cephalosporin derivative: pre-clinical study. Infection. 1977;5(4):259–260. doi: 10.1007/BF01640793. [DOI] [PubMed] [Google Scholar]
- Hinkle A. M., Bodey G. P. In vitro evaluation of Ro 13-9904. Antimicrob Agents Chemother. 1980 Oct;18(4):574–578. doi: 10.1128/aac.18.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura T., Matsumoto Y., Okada N., Mine Y., Nishida M., Goto S., Kuwahara S. Ceftizoxime (FK 749), a new parenteral cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1979 Nov;16(5):540–548. doi: 10.1128/aac.16.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston W. K., Elliott A. M., Dismukes W. E., Avent C. K., Cobbs C. G. Clinical evaluation of moxalactam. Antimicrob Agents Chemother. 1981 Jul;20(1):88–97. doi: 10.1128/aac.20.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy R., Münch R., Blaser J., Bhend H., Siegenthaler W. Human pharmacology of cefotaxime (HR 756), a new cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):127–133. doi: 10.1128/aac.16.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchou B., Tran Van Tho, Armengaud M. Diffusion of ceftriaxone (Ro 13-9004/001) in the cerebrospinal fluid. Comparison with other beta-lactam antibiotics in dogs with healthy meninges and in dogs with experimental meningitis. Chemotherapy. 1981;27 (Suppl 1):37–41. doi: 10.1159/000238027. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Minami S., Muraoka T., Saikawa I., Mitsuhashi S. In vitro antibacterial activity of cefoperazone (T-1551), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1979 Dec;16(6):731–735. doi: 10.1128/aac.16.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Fu K. P., Aswapokee P. Antibacterial activity of a new 1-oxa cephalosporin compared with that of other beta-lactam compounds. Antimicrob Agents Chemother. 1979 Aug;16(2):141–149. doi: 10.1128/aac.16.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. S., Chau P. Y., Arnold K. In vitro susceptibility of Haemophilus influenzae and neisseria gonorrhoeae to Ro 13-9904 in comparison with other beta-lactam antibiotics. Antimicrob Agents Chemother. 1981 May;19(5):925–926. doi: 10.1128/aac.19.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner R., Weiss U., Brombacher U., Lanz P., Montavon M., Furlenmeier A., Angehrn P., Probst P. J. Ro 13-9904/001, a novel potent and long-acting parenteral cephalosporin. J Antibiot (Tokyo) 1980 Jul;33(7):783–786. doi: 10.7164/antibiotics.33.783. [DOI] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Warren C., Phillips I. In vitro antibacterial activity and susceptibility of the cephalosporin Ro 13-9904 to beta-lactamases. Antimicrob Agents Chemother. 1980 Aug;18(2):292–298. doi: 10.1128/aac.18.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton S., Nelson J. D., McCracken G. H., Jr In vitro susceptibility of gram-negative bacilli from pediatric patients to moxalactam, cefotaxime, Ro 13-9904, and other cephalosporins. Antimicrob Agents Chemother. 1980 Sep;18(3):476–479. doi: 10.1128/aac.18.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack M. P., Wheldon D. B., Swann R. A., Perks E. Cefsulodin, a cephalosporin with specific antipseudomonal activity; in vitro studies of the drug alone and in combination. J Antimicrob Chemother. 1979 Nov;5(6):687–691. doi: 10.1093/jac/5.6.687. [DOI] [PubMed] [Google Scholar]
- Stoeckel K. Pharmacokinetics of Rocephin, a highly active new cephalosporin with an exceptionally long biological half-life. Chemotherapy. 1981;27 (Suppl 1):42–46. doi: 10.1159/000238028. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Rotschafer J., Solliday J., Crossley K. B. Moxalactam therapy for a wide spectrum of bacterial infections in adults. Antimicrob Agents Chemother. 1981 May;19(5):740–744. doi: 10.1128/aac.19.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. In vitro activity of Ro 13-9904, a new beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Feb;19(2):222–225. doi: 10.1128/aac.19.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Baker S., Wright N., Livingston R. The pharmacokinetics of LY127935, a broad spectrum oxa-beta-lactam. J Antimicrob Chemother. 1980 May;6(3):319–322. doi: 10.1093/jac/6.3.319. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T. T., Shibata S. A., Herbert P., Oill P. A. In vitro activity of Ro 13-9904, cefuroxime, cefoxitin, and ampicillin against Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Aug;18(2):355–356. doi: 10.1128/aac.18.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]


