Abstract
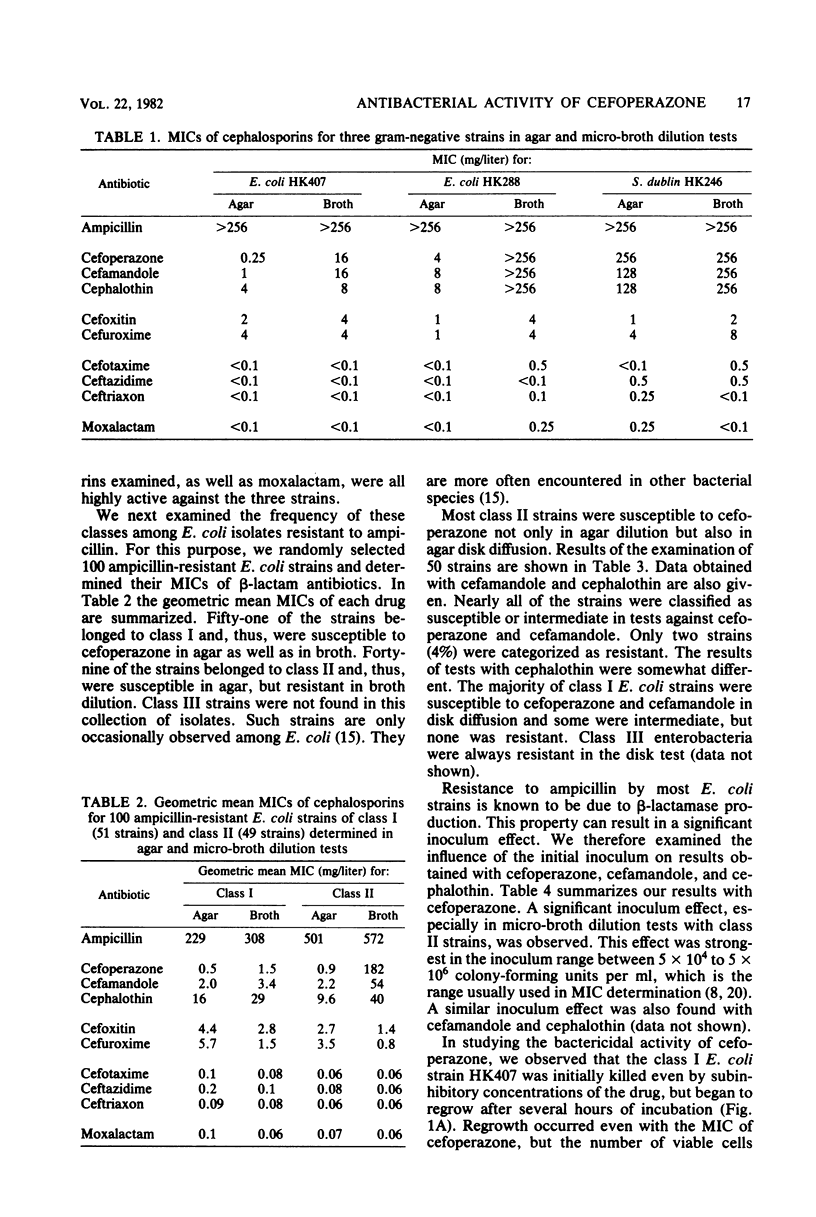
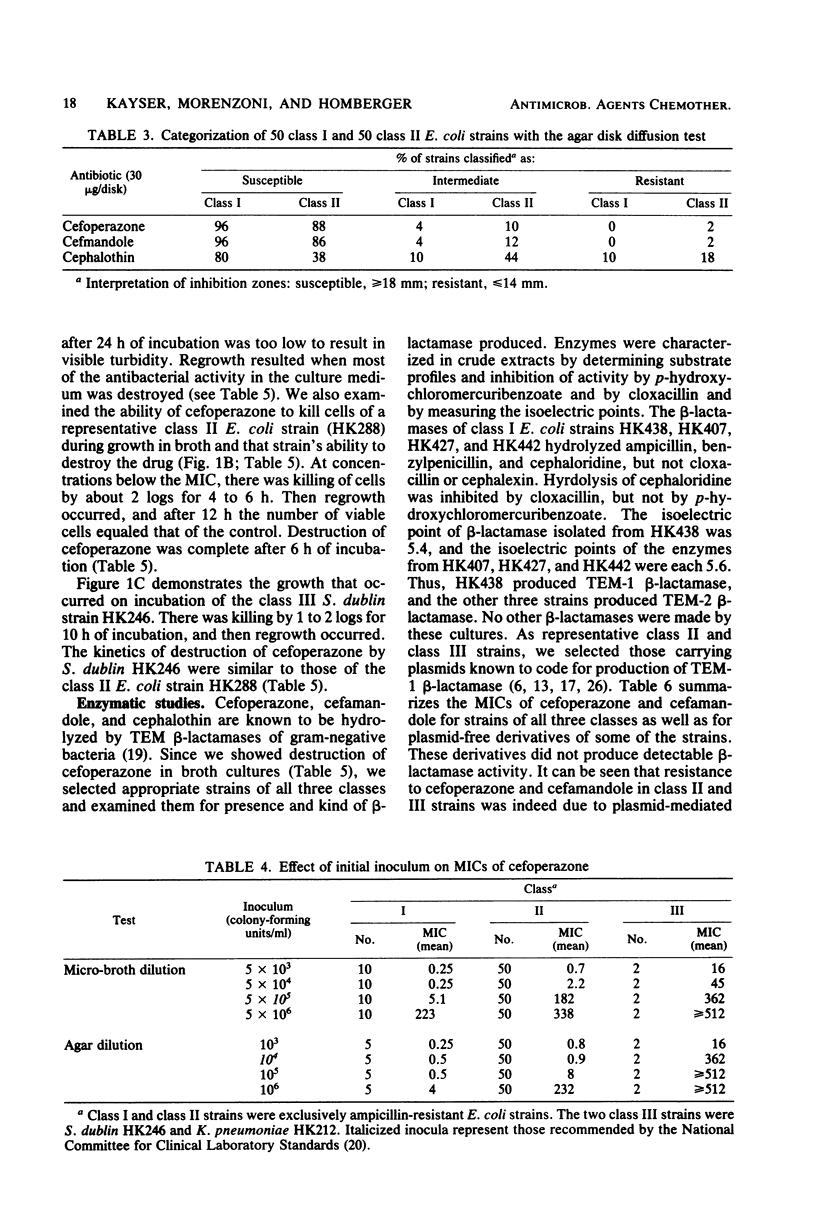
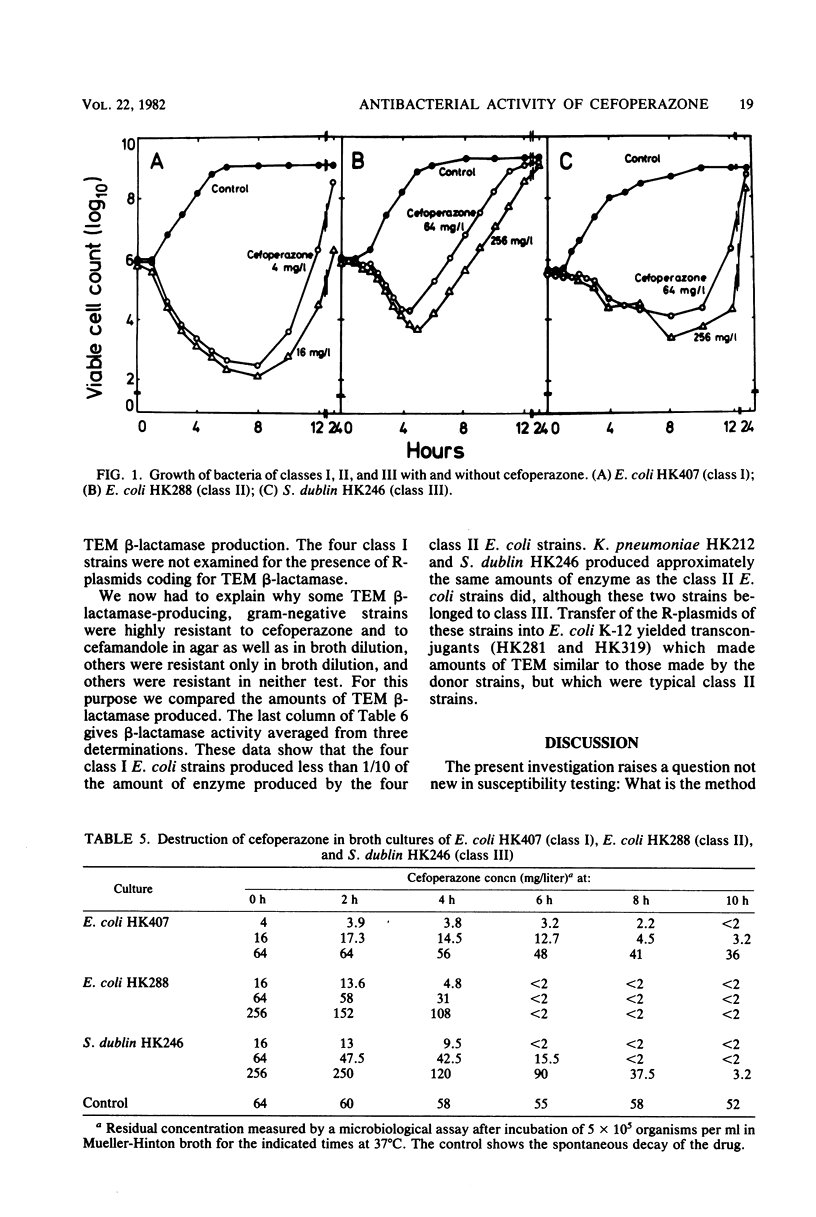
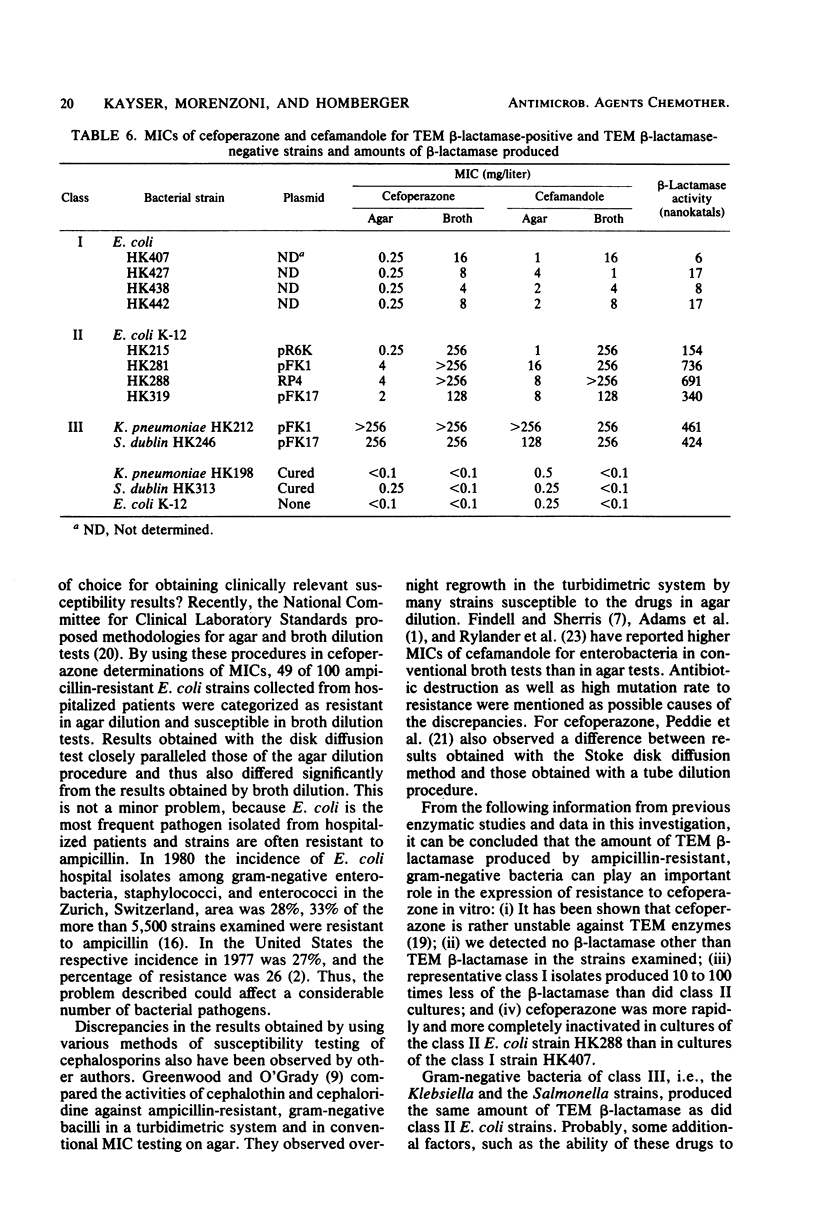
Examination of the activity of cefoperazone against ampicillin-resistant, gramnegative bacteria in agar dilution and simultaneously in broth dilution revealed that strains could be divided into three classes: class I strains were susceptible in agar (mean minimal inhibitory concentration [MIC], 0.5 mg/liter) as well as in broth dilution (mean MIC, 1.5 mg/liter), class II strains were susceptible in agar (MIC, 0.9 mg/liter), but resistant in broth dilution (MIC, 182 mg/liter); and class III strains were highly resistant in both test systems. Among 100 randomly selected ampicillin-resistant Escherichia coli cultures, 51 belonged to class I and 49 belonged to class II. Class III E. coli strains were much rarer. Similar results were obtained with cefamandole and cephalothin, but not with six other second-and third-generation cephalosporins. MICs of cefoperazone against cultures of all three classes were influenced by initial inoculum size. The inoculum effect was greatest with class II strains. Examination of bactericidal activity by cefoperazone showed killing of class I and class II E. coli strains and of class III strains of other genera during the first hours of incubation and regrowth after the drug was destroyed by the action of TEM β-lactamase (penicillinase). Representative class I bacteria produced 10 to 100 times less TEM β-lactamase than did class II strains. It appeared that the quantitative difference in TEM production was the reason for the different resistance phenotypes in class I and class II strains. Salmonella and Klebsiella strains of class III produced the same amounts of TEM β-lactamase as did class II E. coli strains. Probably, some factors other than β-lactamase contributed to the class III phenotype in these species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams H. G., Stilwell G. A., Turck M. In vitro evaluation of cefoxitin and cefamandole. Antimicrob Agents Chemother. 1976 Jun;9(6):1019–1024. doi: 10.1128/aac.9.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNER E. J., MICKLEWAIT J. S., BRODIE J. L., KIRBY W. M. NATURAL AND ACQUIRED RESISTANCE OF KLEBSIELLA-AEROBACTER TO CEPHALOTHIN AND CEPHALORIDINE. Proc Soc Exp Biol Med. 1965 Jun;119:536–541. doi: 10.3181/00379727-119-30231. [DOI] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findell C. M., Sherris J. C. Susceptibility of Enterobacter to cefamandole: evidence for a high mutation rate to resistance. Antimicrob Agents Chemother. 1976 Jun;9(6):970–974. doi: 10.1128/aac.9.6.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Resistance categories of enterobacteria to beta-lactam antibiotics. J Infect Dis. 1975 Sep;132(3):233–240. doi: 10.1093/infdis/132.3.233. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T. INHIBITION OF PENICILLINASES FROM GRAM-POSITIVE AND GRAM-NEGATIVE BACTERIA BY SUBSTRATE ANALOGUES. Nature. 1964 Mar 7;201:999–1001. doi: 10.1038/201999a0. [DOI] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Devaud M., Largiadér F., Binswanger V. Acquisition of multiple antibiotic resistance by Salmonella dublin from the gramnegative hospital flora, in a kidney allograft recipient. Zentralbl Bakteriol Orig A. 1978 Sep;241(3):308–318. [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Munzinger J. Empfindlichkeit von Bakterien gegenüber Chemotherapeutika (Zürich 1980). Teil I. Von Spitalpatienten isolierte Bakterien. Schweiz Med Wochenschr. 1982 Mar 20;112(12):411–416. [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre R., Schulz E., Freiesleben H. Influence of R-plasmid mediated beta-lactamase production on the therapeutic efficacy of cefoxitin and cefamandole in experimental chemotherapy. J Antimicrob Chemother. 1980 Sep;6(5):633–638. doi: 10.1093/jac/6.5.633. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S., Minami S., Matsubara N., Yotsuji A., Kurashige S., Saikawa I. In vitro and in vivo antibacterial activity of cefoperazone. Clin Ther. 1980;3(Spec Issue):1–13. [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Peddie B. A., Bishop V., Bailey R. R. Sensitivity of bacterial isolates to cefoperazone. Drugs. 1981;22 (Suppl 1):27–28. doi: 10.2165/00003495-198100221-00007. [DOI] [PubMed] [Google Scholar]
- Rylander M., Brorson J. E., Johnsson J., Norrby R. Comparison between agar and broth minimum inhibitory concentrations of cefamandole, Cefoxitin, and cefuroxime. Antimicrob Agents Chemother. 1979 Apr;15(4):572–579. doi: 10.1128/aac.15.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. T. Sulphydryl groups essential for the penicillinase activity of Aerobacter cloacae. Nature. 1963 Mar 2;197:900–901. doi: 10.1038/197900a0. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Casey J. I., Ruch P. A., Stumpf L. L., Finland M. Rapid microassay of gentamicin, kanamycin, neomycin, streptomycin, and vancomycin in serum or plasma. J Lab Clin Med. 1971 Sep;78(3):457–463. [PubMed] [Google Scholar]
- Schmid B., Kayser F. H. Resistenze gegen beta-Lactam-Antibiotika und Aminoglykoside bei gramnegativen Bakterien. 1. Molekulare und gentische Charakterisierung von R-Faktoren. Zentralbl Bakteriol Orig A. 1976 Apr;234(3):371–383. [PubMed] [Google Scholar]


