Abstract
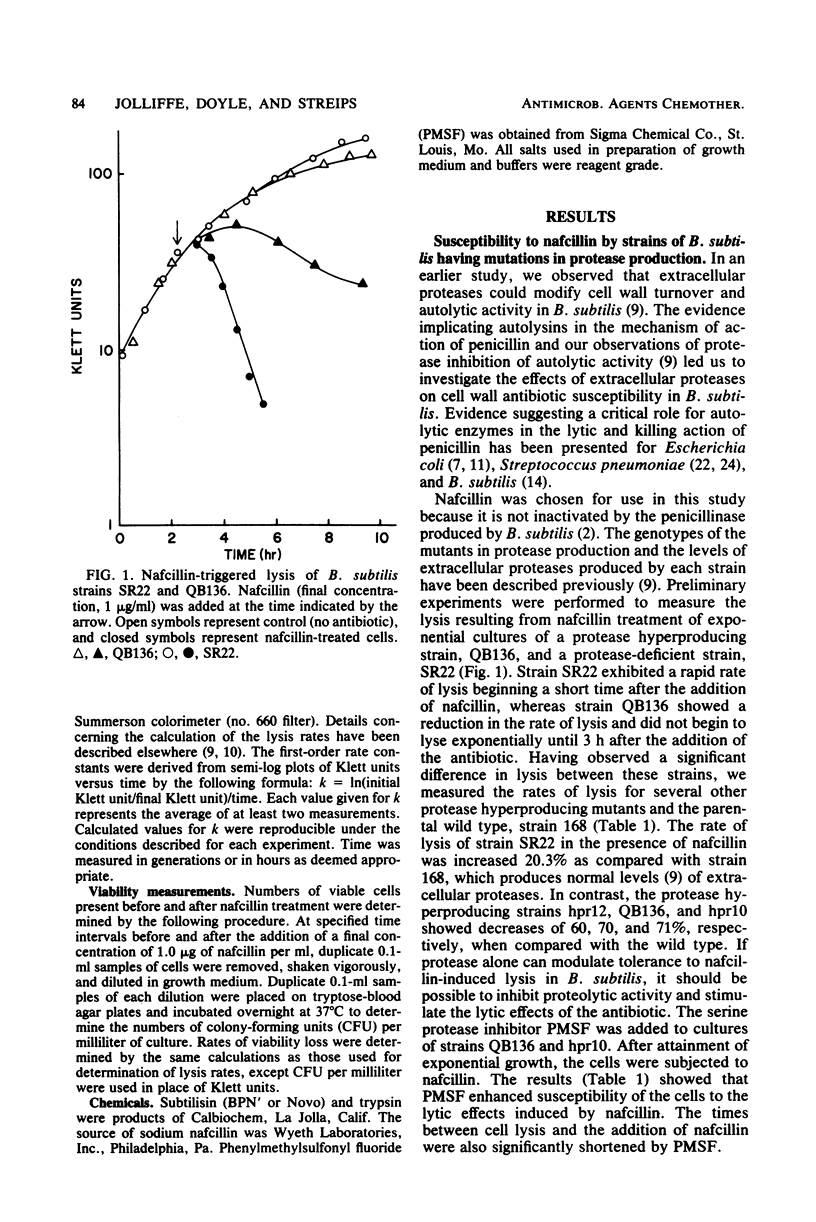
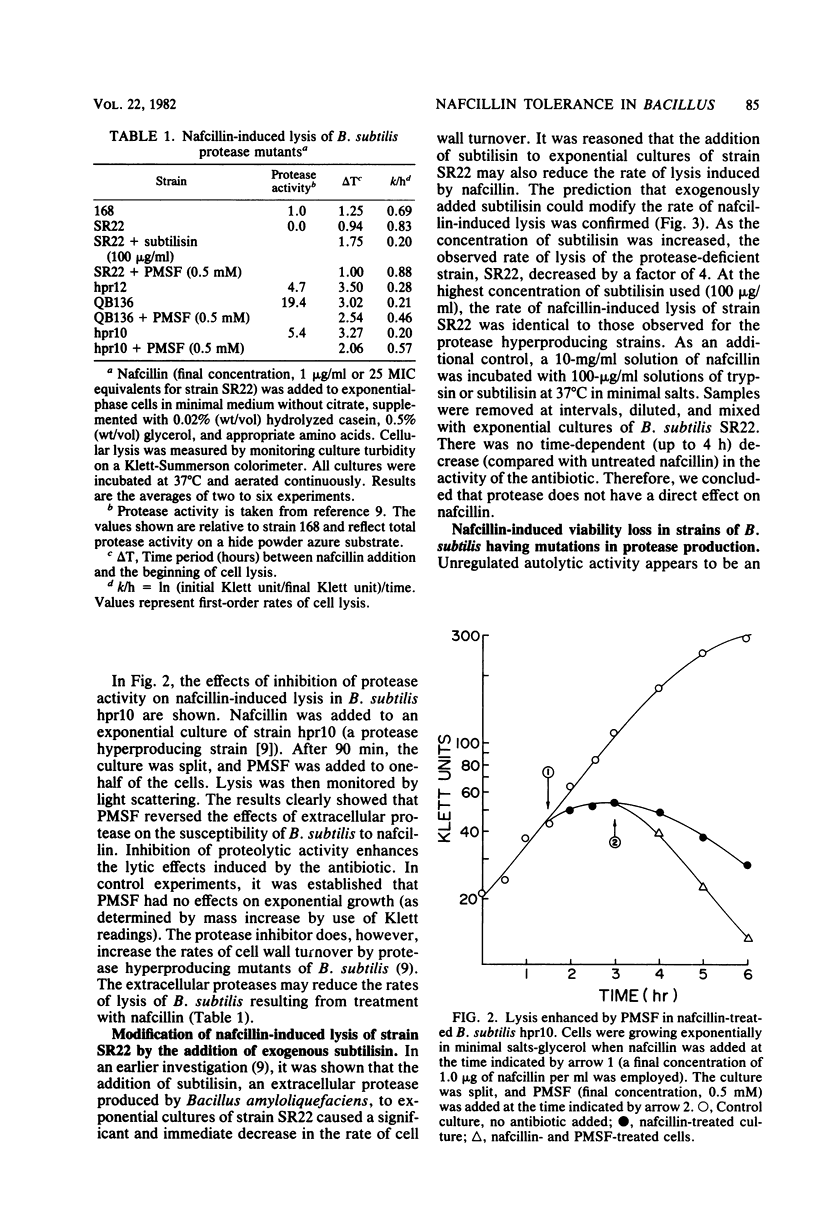
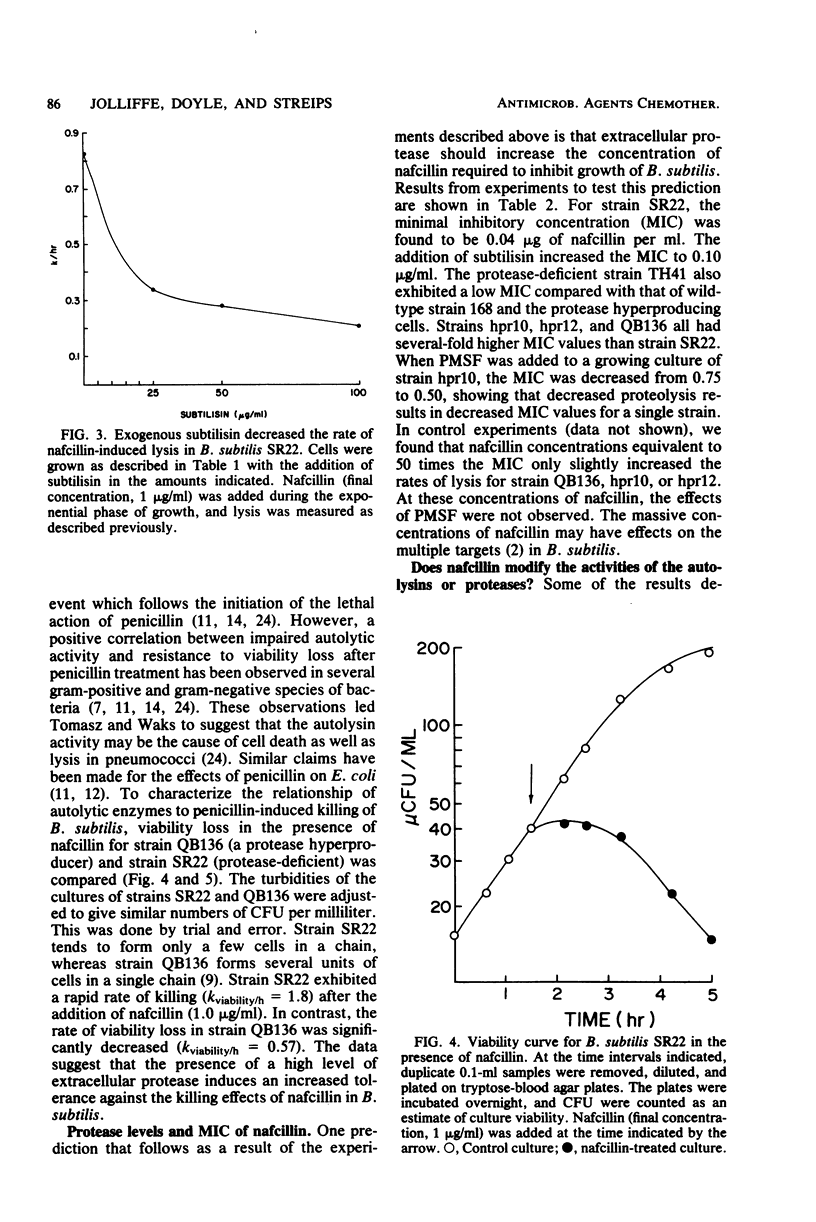
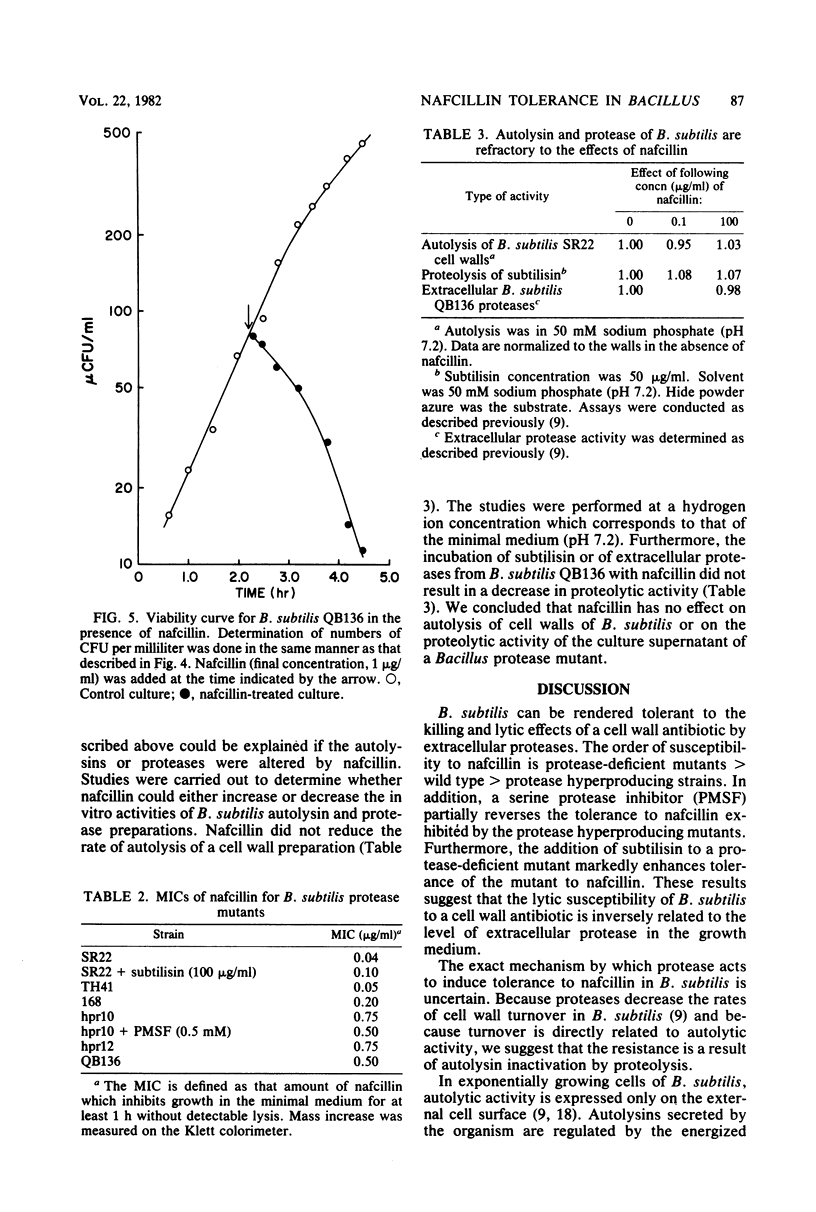
Mutants of Bacillus subtilis capable of secreting high amounts of protease were highly tolerant to the lethal and lytic effects of nafcillin. Protease-deficient mutants were more susceptible. However, when subtilisin was added to exogenously to a protease-deficient strain, the organism assumed the characteristics of nafcillin tolerance. Similarly, when phenylmethylsulfonyl fluoride, a serine protease inhibitor, was added to the tolerant strains, they became susceptible to nafcillin-induced lysis. The effects of nafcillin on B. subtilis were studied with both viability determinations and assay of cellular lysis. The minimum inhibitory concentrations of nafcillin tended to be higher for the protease hyperproducing strains, but these values could be reduced by the protease inhibitor. No loss of antibiotic activity was observed when nafcillin was incubated with either subtilisin or trypsin. Furthermore, protease and autolysin from B. subtilis were not modified by nafcillin. The results showed that extracellular proteases could render B. subtilis relatively tolerant to the killing and lytic effects of a cell wall antibiotic. The proteases were probably acting on the autolysins of the organism, thereby increasing tolerance to nafcillin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best G. K., Best N. H., Koval A. V. Evidence for participation of autolysins in bactericidal action of oxacillin on Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Dec;6(6):825–830. doi: 10.1128/aac.6.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. Extracellular proteases modify cell wall turnover in Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1199–1208. doi: 10.1128/jb.141.3.1199-1208.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Escherichia coli mutants tolerant to beta-lactam antibiotics. J Bacteriol. 1979 Dec;140(3):955–963. doi: 10.1128/jb.140.3.955-963.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Dec;16(6):838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koischwitz H., Kleinkauf H. Gramicidin S-synthetase. Preparation of the multienzymic complex with a high specific activity. Biochim Biophys Acta. 1976 May 13;429(3):1041–1051. doi: 10.1016/0005-2744(76)90349-1. [DOI] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Doyle R. J., Streips U. N., Langemeier S. O. Transport and incorporation of N-acetyl-D-glucosamine in Bacillus subtilis. J Bacteriol. 1982 Apr;150(1):8–15. doi: 10.1128/jb.150.1.8-15.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M. Autolytic activity and its association with the development of competence in group H streptococci. J Bacteriol. 1973 Aug;115(2):607–614. doi: 10.1128/jb.115.2.607-614.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor R. H., Scott D. F., Best G. K. Oxacillin-induced lysis of Staphylococcus aureus. Antimicrob Agents Chemother. 1979 Aug;16(2):134–140. doi: 10.1128/aac.16.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Pooley H. M., Thurman P. F., Taylor C. Wall and membrane growth in bacilli and their mutants. Ann Microbiol (Paris) 1974 Sep;125 B(2):135–147. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Biochemical and genetical approaches to the mechanism of action of penicillin. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):273–283. doi: 10.1098/rstb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Tomasz A. On the mechanism of the irreversible antimicrobial effects of beta-lactams. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):303–308. doi: 10.1098/rstb.1980.0047. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]


