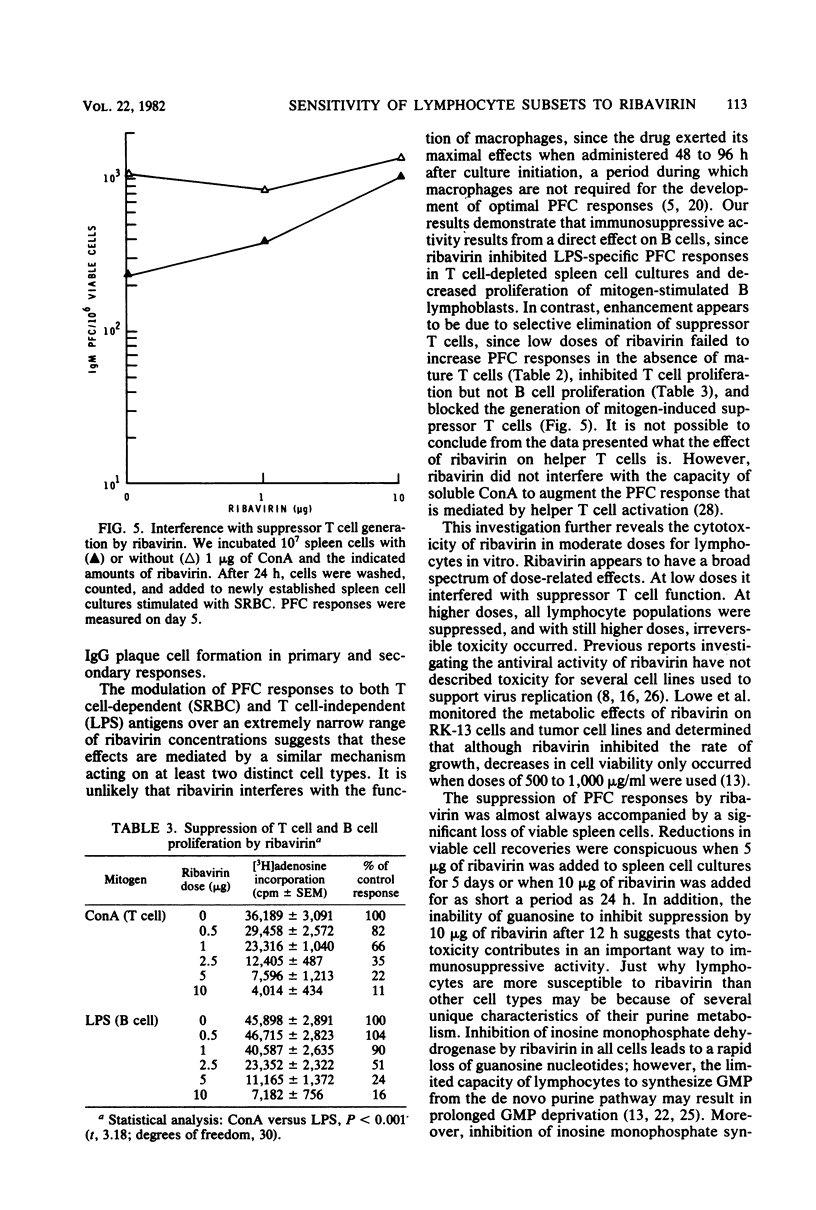
Abstract
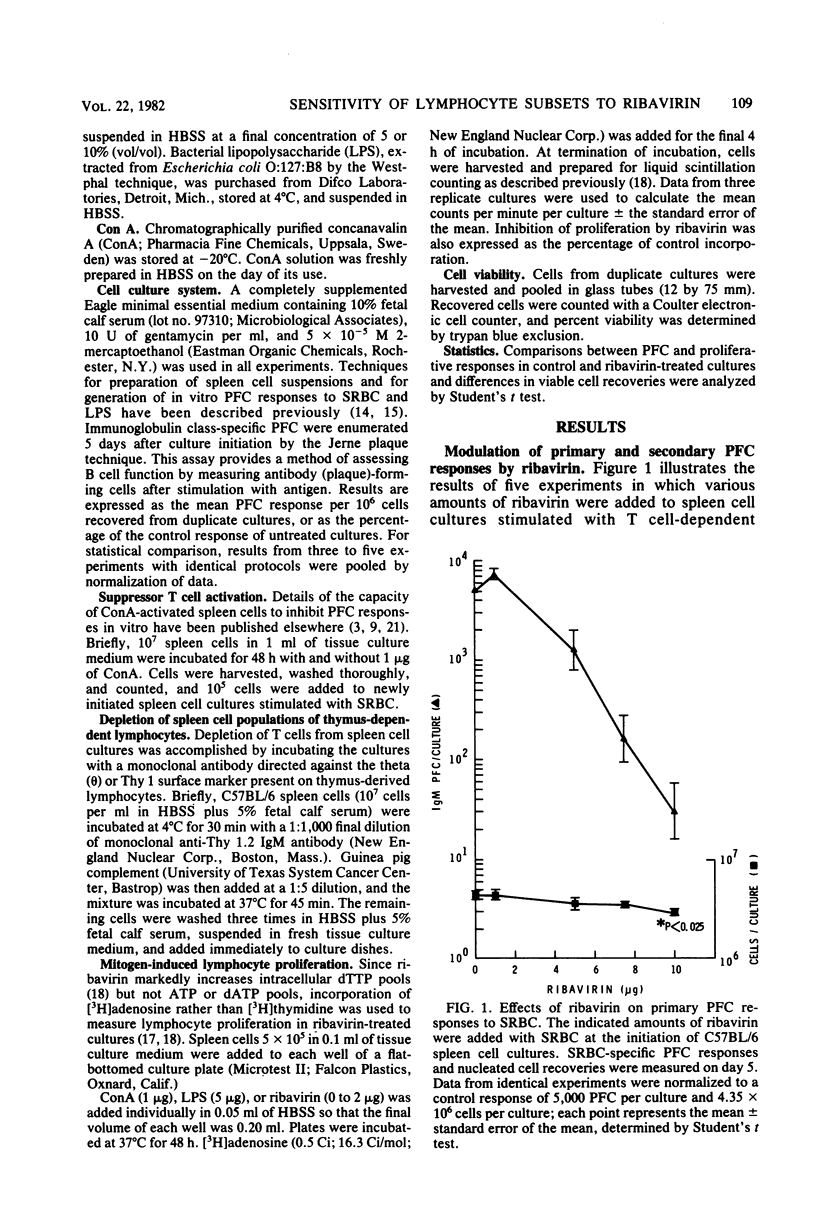
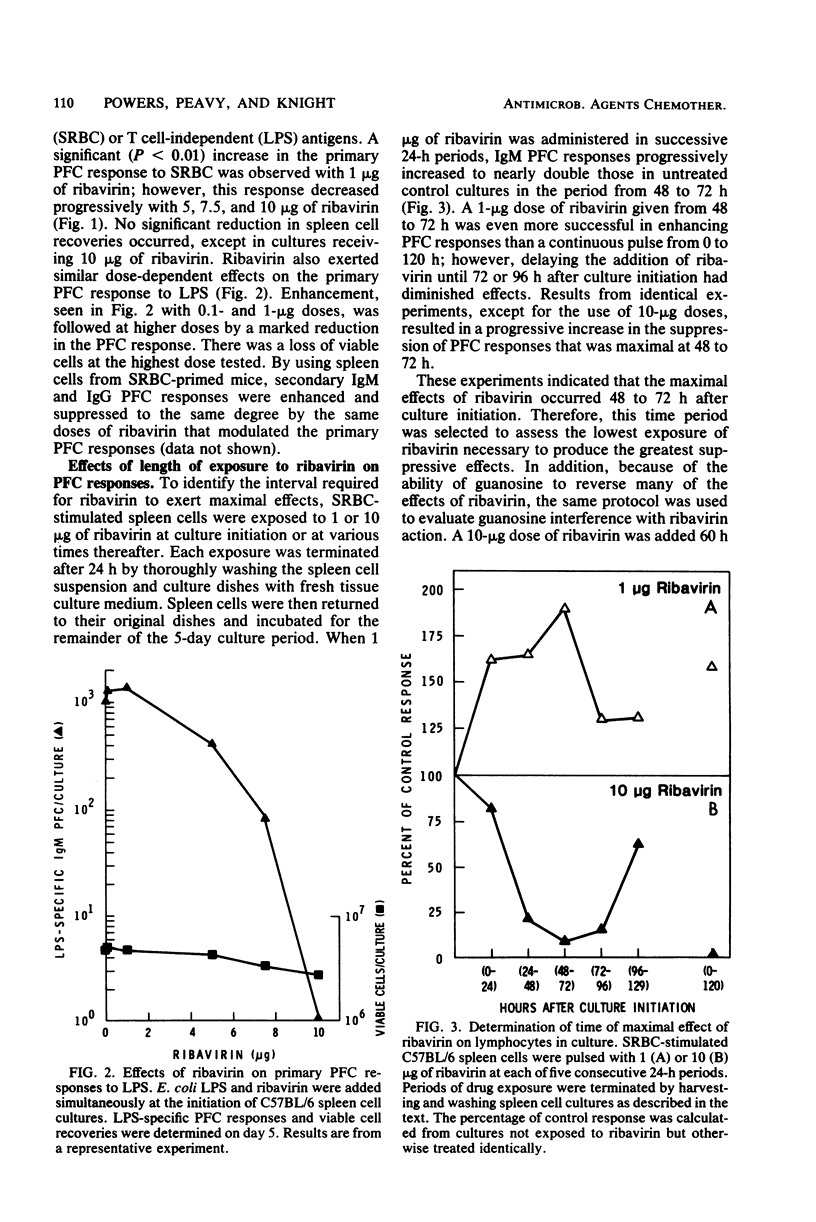
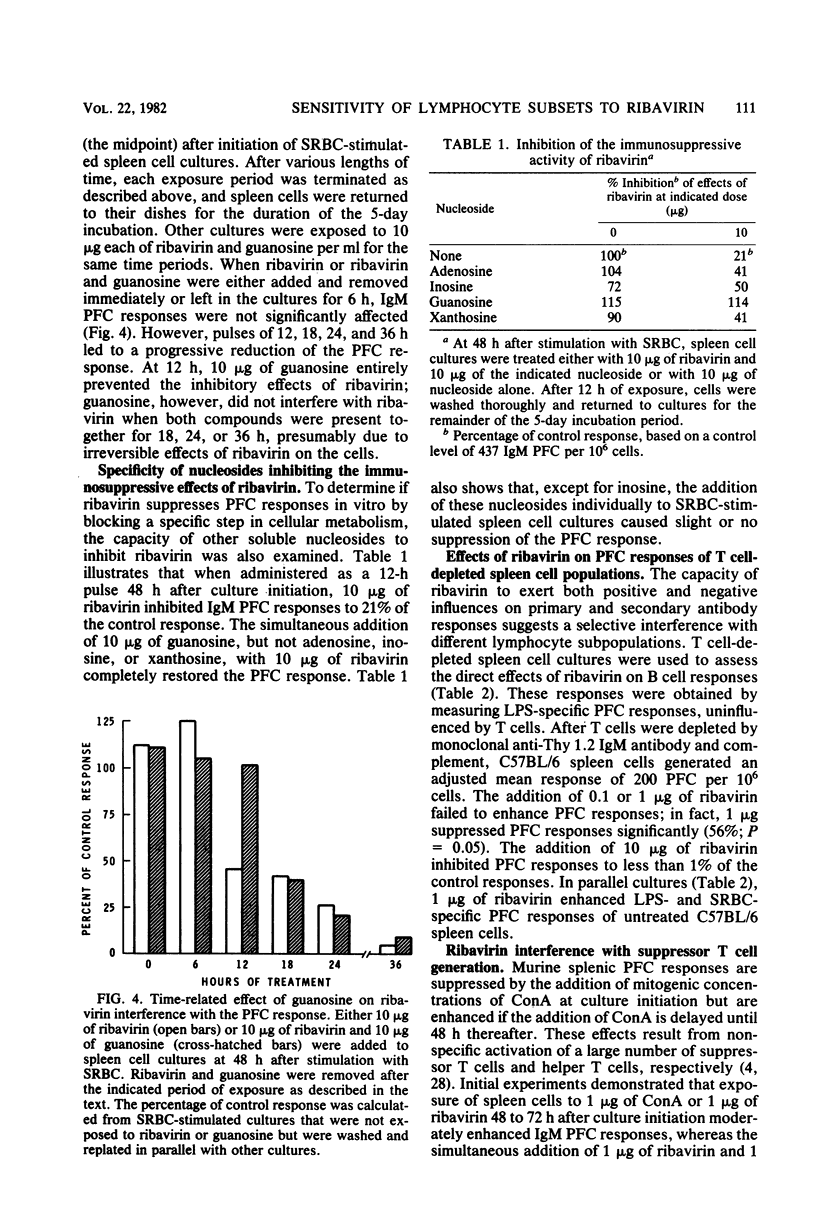
The present studies were designed to examine the effects of ribavirin (1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide), a broad-spectrum antiviral agent, on the generation of murine antibody responses in vitro. Whereas primary and secondary sheep erythrocyte-specific, plaque-forming cell responses by normal murine spleen cells were enhanced by low concentrations of ribavirin (1 microgram per culture), they were strongly inhibited by higher concentrations of ribavirin (5 to 10 micrograms per culture). Both phenomena occurred with the greatest magnitude when spleen cells were exposed to ribavirin 48 to 72 h after culture initiation. Enhancement appeared to result from selective interference with suppressor T cells, since ribavirin failed to augment lipopolysaccharide-specific plaque-forming cell responses in T cell-depleted spleen cell cultures but inhibited concanavalin A-induced lymphocyte proliferation and suppressor T cell generation in cultures of normal spleen cells. The immunosuppressive properties of ribavirin were mediated by a direct antiproliferative effect and, at higher concentrations, a cytotoxic effect for B lymphocytes, since the drug inhibited plaque-forming cell responses in T cell-depleted spleen cell cultures, suppressed lipopolysaccharide-induced lymphocyte proliferation and reduced viable spleen cell recoveries.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D. A., Kaye J., Matsumoto S., Seegmiller J. E., Thompson L. Biochemical basis for the enhanced toxicity of deoxyribonucleosides toward malignant human T cell lines. Proc Natl Acad Sci U S A. 1979 May;76(5):2430–2433. doi: 10.1073/pnas.76.5.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. II. Evidence for separate stimulatory and inhibitory cells. J Exp Med. 1973 Dec 1;138(6):1496–1505. doi: 10.1084/jem.138.6.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. II. The requirement for macrophages in lymphoid cell collaboration. J Exp Med. 1972 May 1;135(5):1049–1058. doi: 10.1084/jem.135.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Lee J. J., Dosch H. M. Selective toxicity of purine deoxynucleosides for human lymphocyte growth and function. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1998–2002. doi: 10.1073/pnas.76.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Ullman B., Cohen A., Martin D. W., Jr Deoxyguanosine toxicity in a mouse T lymphoma: relationship to purine nucleoside phosphorylase-associated immune dysfunction. Cell. 1978 Jul;14(3):531–538. doi: 10.1016/0092-8674(78)90239-8. [DOI] [PubMed] [Google Scholar]
- Huffman J. H., Sidwell R. W., Khare G. P., Witkowski J. T., Allen L. B., Robins R. K. In vitro effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1973 Feb;3(2):235–241. doi: 10.1128/aac.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen L. W., Budman D. R., Williams G. W., Steinberg A. D., Gerber N. L. Ribavirin: efficacy in the treatment of murine autoimmune disease. Science. 1977 Feb 25;195(4280):787–789. doi: 10.1126/science.299957. [DOI] [PubMed] [Google Scholar]
- Klassen L. W., Williams G. W., Reinertsen J. L., Gerber N. L., Steinberg A. D. Ribavirin treatment in murine autoimmune disease. I. Therapeutic efficacy and effect on the immune response. Arthritis Rheum. 1979 Feb;22(2):145–154. doi: 10.1002/art.1780220207. [DOI] [PubMed] [Google Scholar]
- Knight V., McClung H. W., Wilson S. Z., Waters B. K., Quarles J. M., Cameron R. W., Greggs S. E., Zerwas J. M., Couch R. B. Ribavirin small-particle aerosol treatment of influenza. Lancet. 1981 Oct 31;2(8253):945–949. doi: 10.1016/s0140-6736(81)91152-1. [DOI] [PubMed] [Google Scholar]
- Lowe J. K., Brox L., Henderson J. F. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and virazole. Cancer Res. 1977 Mar;37(3):736–743. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Maidhof A., Taschner H., Zahn R. K. Virazole (1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide; a cytostatic agent. Biochem Pharmacol. 1977 Jun 1;26(11):1071–1075. doi: 10.1016/0006-2952(77)90246-5. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Shands J. W., Smith R. T. Selective effects of mitogens on subpopulations of mouse lymphoid cells. Cell Immunol. 1974 Mar 30;11(1-3):86–98. doi: 10.1016/0008-8749(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Koff W. C., Hyman D. S., Knight V. Inhibition of lymphocyte proliferative responses by ribavirin. Infect Immun. 1980 Aug;29(2):583–589. doi: 10.1128/iai.29.2.583-589.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Powers C. N., Knight V. Inhibition of murine plaque-forming cell responses in vivo by ribavirin. J Immunol. 1981 Mar;126(3):861–864. [PubMed] [Google Scholar]
- Pierce C. W. Immune responses in vitro. I. Cellular requirements for the immune response by nonprimed and primed spleen cells in vitro. J Exp Med. 1969 Aug 1;130(2):345–364. doi: 10.1084/jem.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholar E. M., Calabresi P. Identification of the enzymatic pathways of nucleotide metabolism in human lymphocytes and leukemia cells. Cancer Res. 1973 Jan;33(1):94–103. [PubMed] [Google Scholar]
- Scott G. H., Stephen E. L., Berendt R. F. Activity of amantadine, rimantadine, and ribavirin against swine influenza in mice and squirrel monkeys. Antimicrob Agents Chemother. 1978 Feb;13(2):284–288. doi: 10.1128/aac.13.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H., Khare G. P., Allen L. B., Witkowski J. T., Robins R. K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972 Aug 25;177(4050):705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Fontenelle L. J., Muzik H., Paterson A. R., Unger H., Brox L. W., Henderson J. F. Inhibitors of inosinate dehydrogenase activity in Ehrlich ascites tumor cells in vitro. Biochem Pharmacol. 1974 Oct 1;23(19):2727–2735. doi: 10.1016/0006-2952(74)90043-4. [DOI] [PubMed] [Google Scholar]
- Streeter D. G., Witkowski J. T., Khare G. P., Sidwell R. W., Bauer R. J., Robins R. K., Simon L. N. Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse H. Y., Dutton R. W. Separation of helper and suppressor T lymphocytes. II. Ly phenotypes and lack of DNA synthesis requirement for the generation of concanavalin A helper and suppressor cells. J Exp Med. 1977 Sep 1;146(3):747–758. doi: 10.1084/jem.146.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Carson D. A., Seegmiller J. E. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3042–3044. doi: 10.1073/pnas.75.7.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. Z., Knight V., Wyde P. R., Drake S., Couch R. B. Amantadine and ribavirin aerosol treatment of influenza A and B infection in mice. Antimicrob Agents Chemother. 1980 Apr;17(4):642–648. doi: 10.1128/aac.17.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]



