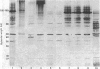
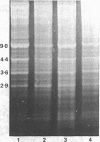
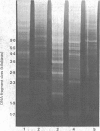
Abstract
OBJECTIVE--To define an outbreak of bacteraemia due to coagulase negative staphylococci highly resistant to ciprofloxacin in a leukaemia unit, investigate the source and mode of spread of the outbreak strain, and assess control measures. DESIGN--The outbreak strain was characterised by five different typing methods. Surveillance of patients, staff, and environment was carried out during the outbreak and five months after control measures were introduced. SETTING--A unit with 10 beds for adults with leukaemia and patients receiving bone marrow transplants. The outbreak occurred during a trial of ciprofloxacin for empirical treatment of neutropenic fevers. INTERVENTIONS--Ciprofloxacin was withdrawn from use in the unit and daily bathing with chlorhexidine gluconate solution started. Main outcome measure--The absence of bacteraemia due to the outbreak strain for five months after control measures. RESULTS--During the study 49 patients developed 21 episodes of bacteraemia due to the outbreak strain, which was ciprofloxacin resistant (minimum inhibitory concentration greater than or equal to 128 mg/l), susceptible to phage 155 A9C, and SII biotype and had characteristic immunoblot and DNA fingerprint features. There was a high amount of colonisation of patients but not staff with this strain, which was also wide spread in the environment. The control measures led to rapid resolution of the outbreak and disappearance of the strain from the unit. CONCLUSIONS--In areas where coagulase negative staphylococcal infections are common doctors must be aware of the possibility of cross infection with single strain, and the availability of more discriminatory methods of typing will facilitate the identification and control of such episodes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnie J. P., Lee W. A comparison of DNA and immunoblot fingerprinting of the SII biotype of coagulase negative staphylococci. Epidemiol Infect. 1988 Oct;101(2):203–212. doi: 10.1017/s095026880005411x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnie J. P., Lee W., Matthews R. C., Bayston R. Immunoblot fingerprinting of coagulase negative staphylococci. J Clin Pathol. 1988 Jan;41(1):103–107. doi: 10.1136/jcp.41.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell M., Phillips I. Hands as route of transmission for Klebsiella species. Br Med J. 1977 Nov 19;2(6098):1315–1317. doi: 10.1136/bmj.2.6098.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Parisi J. T., Bisno A. L., Simpson W. A., Beachey E. H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983 Aug;18(2):258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houang E. T., Marples R. R., Weir I., Mourant A. J., de Saxe M. J., Singleton B. Problems in the investigation of an apparent outbreak of coagulase-negative staphylococcal septicaemia following cardiac surgery. J Hosp Infect. 1986 Nov;8(3):224–232. doi: 10.1016/0195-6701(86)90117-9. [DOI] [PubMed] [Google Scholar]
- Humphreys H., Mulvihill E. Ciprofloxacin-resistant Staphylococcus aureus. Lancet. 1985 Aug 17;2(8451):383–383. doi: 10.1016/s0140-6736(85)92510-3. [DOI] [PubMed] [Google Scholar]
- Oppenheim B. A., Hartley J. W., Lee W., Burnie J. P. Outbreak of coagulase negative staphylococcus highly resistant to ciprofloxacin in a leukaemia unit. BMJ. 1989 Jul 29;299(6694):294–297. doi: 10.1136/bmj.299.6694.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Herwaldt L. A. Laboratory, clinical, and epidemiological aspects of coagulase-negative staphylococci. Clin Microbiol Rev. 1988 Jul;1(3):281–299. doi: 10.1128/cmr.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., Dekker A. W., Verhoef J. Ciprofloxacin for selective decontamination of the alimentary tract in patients with acute leukemia during remission induction treatment: the effect on fecal flora. J Infect Dis. 1985 Jul;152(1):104–107. doi: 10.1093/infdis/152.1.104. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Cashmore C., Leyland M. J. Ciprofloxacin-resistant staphylococci. Lancet. 1985 Oct 26;2(8461):949–949. doi: 10.1016/s0140-6736(85)90880-3. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Newman K. A., Wiernik P. H. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503–508. doi: 10.7326/0003-4819-97-4-503. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Dudnick D. V., Chapin M., Ho W. G., Gale R. P., Martin W. J. Coagulase-negative staphylococcal bacteremia in patients receiving immunosuppressive therapy. Arch Intern Med. 1983 Jan;143(1):32–36. [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek P. J., Lampe A. S., Berbée G. A., Thompson J., Mouton R. P. Epidemic of prosthetic valve endocarditis caused by Staphylococcus epidermidis. Br Med J (Clin Res Ed) 1985 Oct 5;291(6500):949–950. doi: 10.1136/bmj.291.6500.949. [DOI] [PMC free article] [PubMed] [Google Scholar]