Abstract
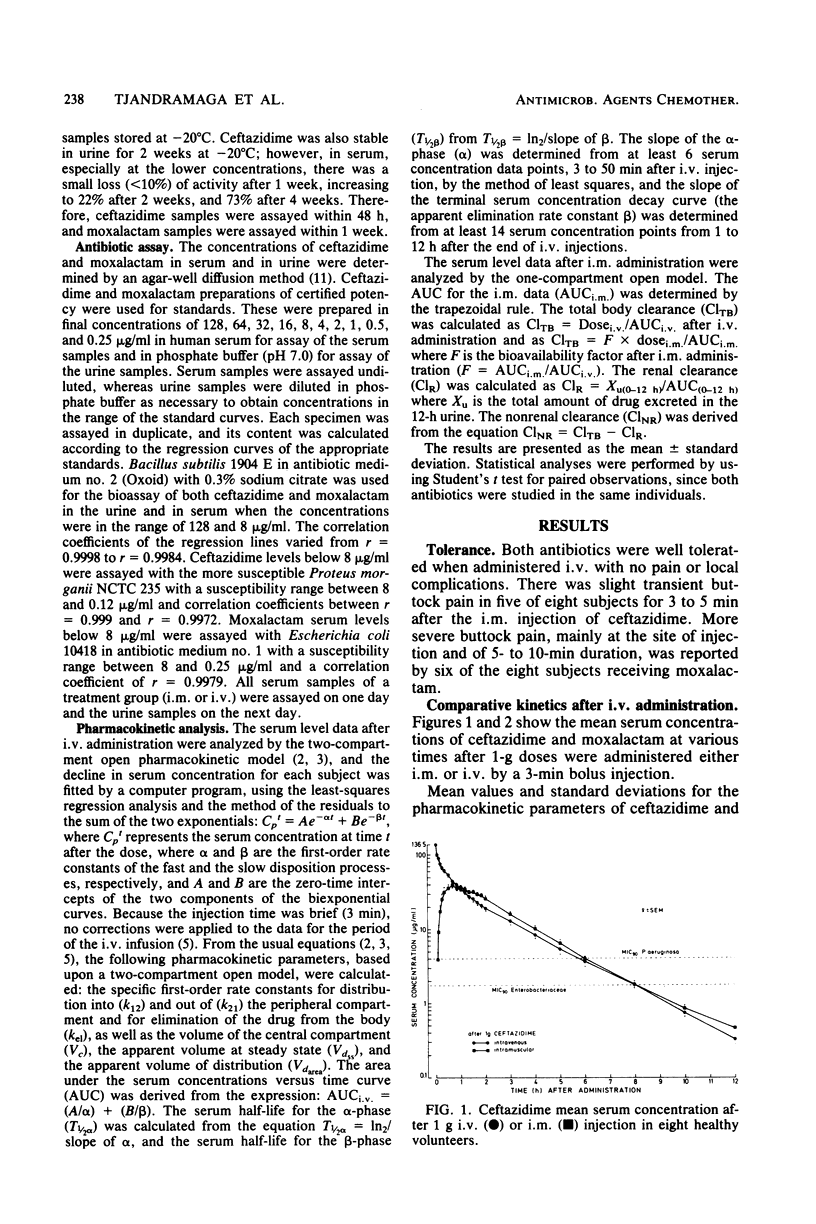
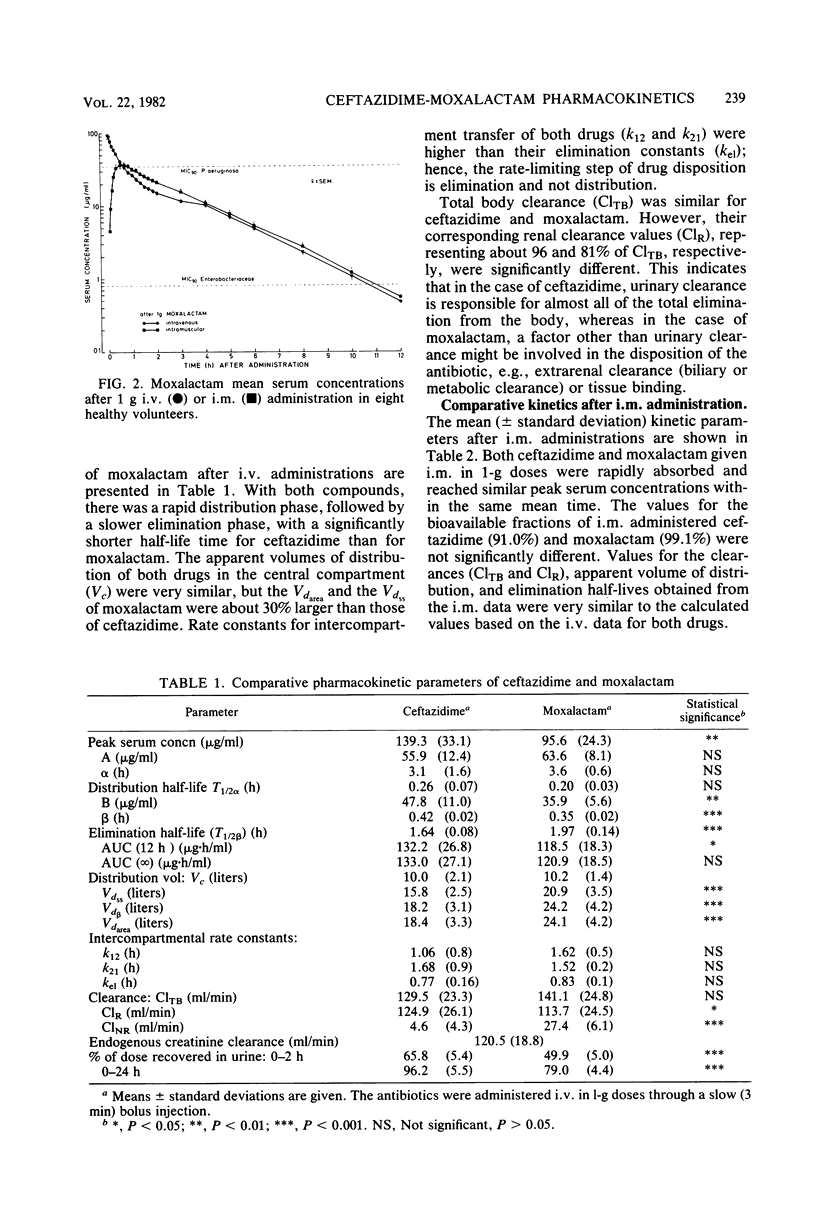
The pharmacokinetics of ceftazidime and moxalactam were compared after intravenous and intramuscular administration of single 1-g doses to eight healthy volunteers in a crossover study. The bioavailability of the antibiotics after administration by either route was almost complete. Both drugs had similar areas under the serum curves. Significant differences between ceftazidime and moxalactam were observed with respect to the apparent volume of distribution (18.4 and 24.1 liters, respectively), to the terminal half-life (1.6 versus 2.0 h), and to urinary recovery of the active compound (96 versus 79%). Ceftazidime was almost completely eliminated by renal excretion (greater than 96%), whereas about 20% of the moxalactam was eliminated by nonrenal mechanisms. The concentrations of ceftazidime and moxalactam in serum after a 1-g dose exceeded the concentrations required to inhibit 90% of the Enterobacteriaceae for about 8 and 10 h, respectively. The levels of ceftazidime and moxalactam in serum exceeded the 90% minimal inhibitory concentration of Pseudomonas aeruginosa for about 6 and 1 h, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barza M., Tally F. P., Jacobus N. V., Gorbach S. L. In vitro activity of LY127935. Antimicrob Agents Chemother. 1979 Sep;16(3):287–292. doi: 10.1128/aac.16.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Gorski S., Person A., Mangura C., Chmel H. Clindamycin elimination in patients with liver disease. J Antimicrob Chemother. 1981 Oct;8(4):277–281. doi: 10.1093/jac/8.4.277. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Fu K. P., Aswapokee P. Antibacterial activity of a new 1-oxa cephalosporin compared with that of other beta-lactam compounds. Antimicrob Agents Chemother. 1979 Aug;16(2):141–149. doi: 10.1128/aac.16.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Spyker D. A., Donowitz G. R., Bolton W. K., Sande M. A. Moxalactam and cefazolin: comparative pharmacokinetics in normal subjects. Antimicrob Agents Chemother. 1981 Apr;19(4):613–619. doi: 10.1128/aac.19.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Fu K. P., Neu H. C. Pharmacokinetics of moxalactam and cefazolin compared in normal volunteers. Antimicrob Agents Chemother. 1981 Feb;19(2):302–305. doi: 10.1128/aac.19.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandramaga T. B., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. Antimicrob Agents Chemother. 1978 Dec;14(6):829–837. doi: 10.1128/aac.14.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager G. M., White G. W., Zimelis V. M., Panwalker A. P. LY-127935: a novel beta-lactam antibiotic with unusual antibacterial activity. Antimicrob Agents Chemother. 1979 Sep;16(3):297–300. doi: 10.1128/aac.16.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. Comparison of in vitro activities of eight beta-lactamase-stable cephalosporins against beta-lactamase-producing gram-negative bacilli. Antimicrob Agents Chemother. 1981 Mar;19(3):407–413. doi: 10.1128/aac.19.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. GR-20263: a new aminothiazolyl cephalosporin with high activity against Pseudomonas and Enterobacteriaceae. Antimicrob Agents Chemother. 1980 May;17(5):807–812. doi: 10.1128/aac.17.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Bedford K. A. LY127935, a novel oxa-beta-lactam: an in vitro comparison with other beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Sep;16(3):341–345. doi: 10.1128/aac.16.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Baker S., Livingston R. Comparison of cefotaxime and moxalactam pharmacokinetics and tissue levels. Antimicrob Agents Chemother. 1980 Sep;18(3):369–371. doi: 10.1128/aac.18.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]


