Abstract
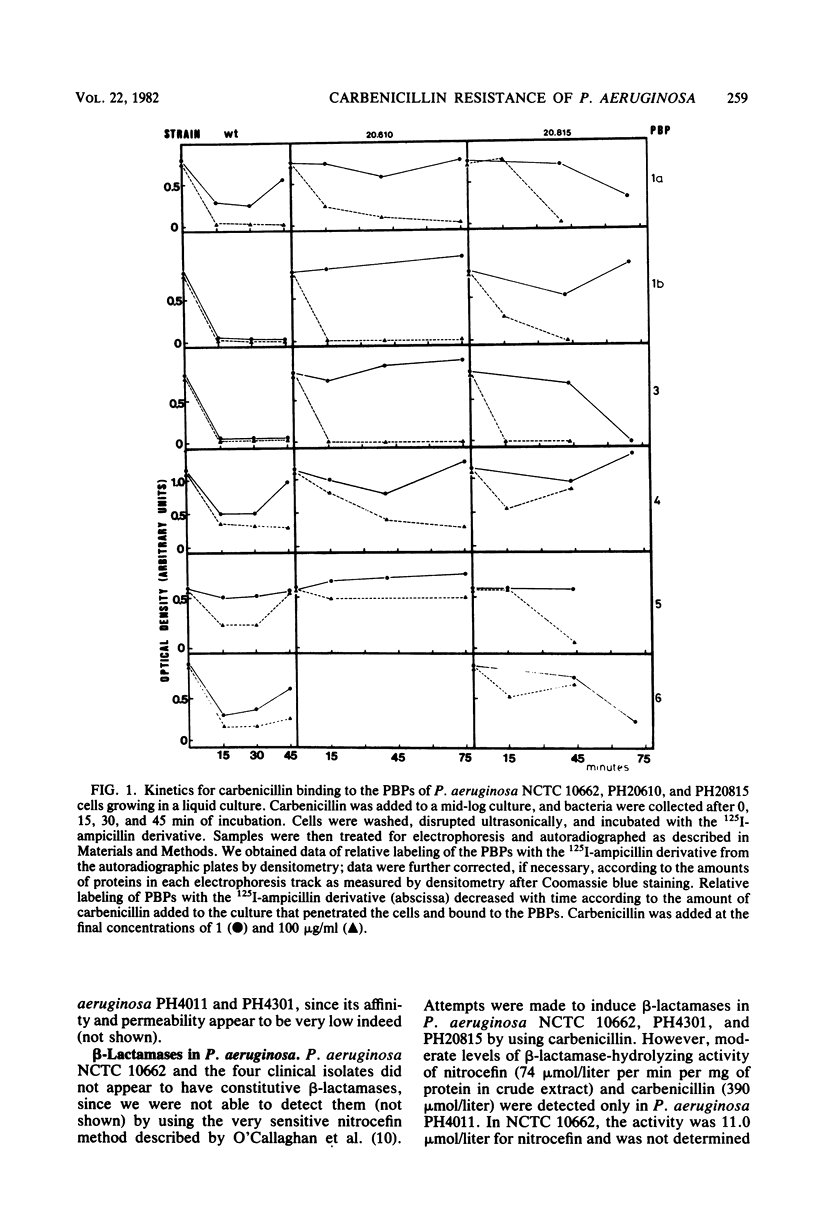
Four strains of Pseudomonas aeruginosa obtained from clinical isolates which are carbenicillin resistant were studied to find the cause(s) of resistance to this beta-lactam antibiotic. The electrophoresis patterns of the four strains (PH20610, PH20815, PH4011, and PH4301) were found to be different from those of a wild-type strain, P. aeruginosa NCTC 10662, and appeared to lack penicillin-binding protein 2. Affinity of other penicillin-binding proteins from strains PH20610 and PH20815 for carbenicillin seemed to be normal or slightly diminished. Electrophoretic patterns of penicillin-binding proteins from strains PH4011 and PH4301 had more profound differences, since the affinities of their penicillin-binding proteins 1a, 1b, and 4 for carbenicillin were decreased by nearly two orders of magnitude relative to the preparations from the wild-type strain. Kinetic studies on binding of carbenicillin to penicillin-binding proteins both in isolated membrane preparations and in intact cells revealed that carbenicillin penetration into resistant cells was a much slower process than in susceptible cells, suggesting that the outer envelope structures serve as an efficient barrier against carbenicillin entry into our P. aeruginosa strains from clinical isolates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Brown C., Boxall M., Boulton M. G. Modified peptidoglycan transpeptidase activity in a carbenicillin-resistant mutant of Pseudomonas aeruginosa 18s. Antimicrob Agents Chemother. 1978 Aug;14(2):246–251. doi: 10.1128/aac.14.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E. Mutation of Pseudomonas aeruginosa specifying reduced affinity for penicillin G. Antimicrob Agents Chemother. 1982 Feb;21(2):216–223. doi: 10.1128/aac.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Govan J. W., Fyfe J. A., Costerton J. W. Heterogeneity of antibiotic resistance in mucoid isolates of Pseudomonas aeruginosa obtained from cystic fibrosis patients: role of outer membrane proteins. Antimicrob Agents Chemother. 1981 Jun;19(6):1056–1063. doi: 10.1128/aac.19.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Nuchamowitz Y., Rubinstein E. Insensitivity of peptidoglycan biosynthetic reactions to beta-lactam antibiotics in a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):687–695. doi: 10.1128/aac.19.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton R. P., Bongaerts G. P., van Gestel M. Comparison of activity and beta-lactamase stability of cefotaxime with those of six other cephalosporins. Antimicrob Agents Chemother. 1979 Dec;16(6):757–760. doi: 10.1128/aac.16.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Tamura A., Yamagishi S. The bacterial outer-membrane permeability of beta-lactam antibiotics. J Antibiot (Tokyo) 1979 Jan;32(1):59–65. doi: 10.7164/antibiotics.32.59. [DOI] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Yamagishi S. A method for measuring the outer membrane-permeability of beta-lactam antibiotics in gram-negative bacteria. J Antibiot (Tokyo) 1977 Dec;30(12):1134–1136. doi: 10.7164/antibiotics.30.1134. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration through the gram-negative cell wall: a co-determinant of the efficacy of beta-lactam antibiotics. Int J Clin Pharmacol Biopharm. 1979 Mar;17(3):131–134. [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


