Abstract
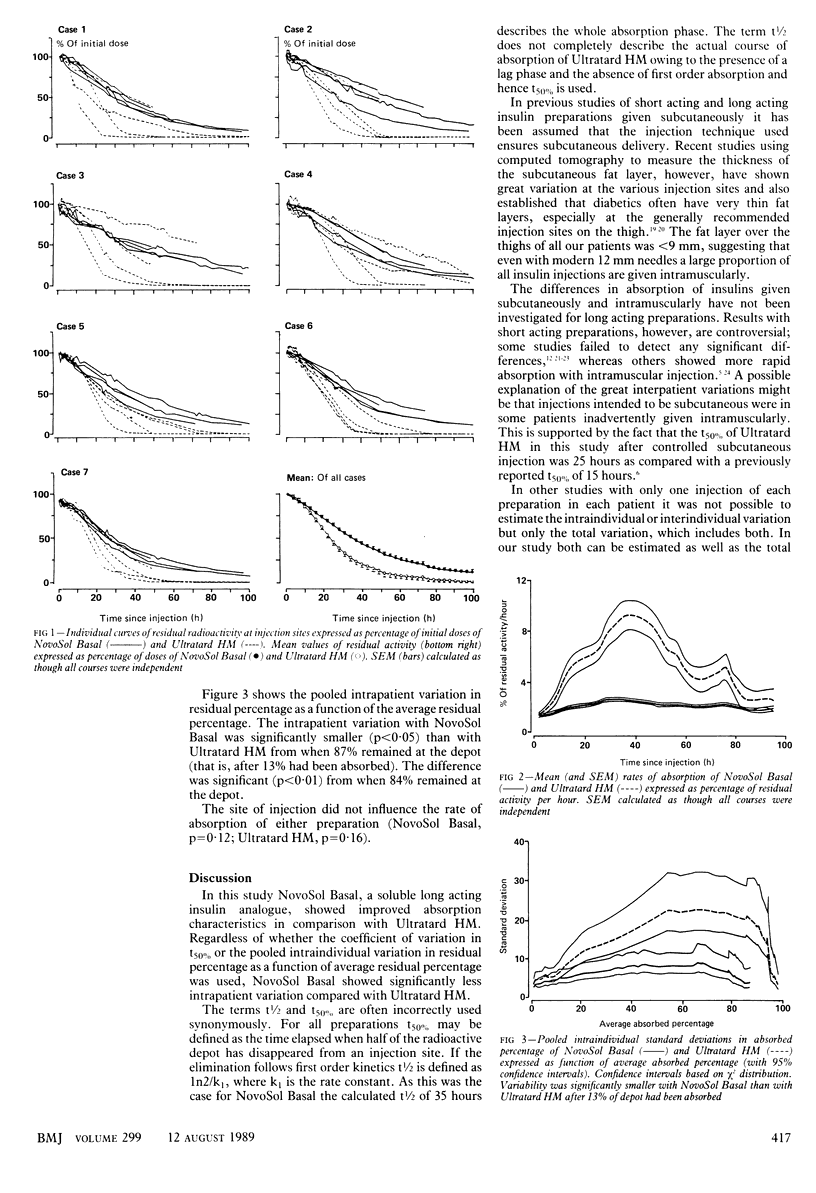
OBJECTIVE--To determine the courses of absorption and the interindividual and intraindividual variations in absorption of iodine-125 labelled Ultratard HM and NovoSol Basal injected subcutaneously. DESIGN--Open randomised crossover study. Each patient was tested during two study periods of five days each, during which he or she received a subcutaneous injection of either 125I-NovoSol Basal or 125I-Ultratard HM on four consecutive days. The aim was to detect a reduction in intraindividual standard deviation by a factor of two with a probability 0.95, taking 0.05 as the level of significance. This required 24 degrees of freedom and led to the choice of four courses in each of eight patients. SETTING--Referrals to the diabetes research centre in Hvidøre, Copenhagen. PATIENTS--Eight insulin dependent (type I) diabetics with low or undetectable C peptide concentrations who were receiving a multiple insulin injection regimen. One patient withdrew immediately after recruitment. INTERVENTIONS--After an overnight fast patients received 96 nmol (16 IU insulin) of either 125I-NovoSol Basal or 125I-Ultratard HM injected subcutaneously into the thigh. To ensure that the insulin entered the subcutaneous fat at the same depth, ultrasonography was performed on each patient before the first injection. A different injection site on the thigh was used each day for four days in order to facilitate monitoring of the disappearance of four different depots in each patient. MAIN OUTCOME MEASURE--Residual activity at the injection site was measured roughly every two hours throughout the day. No radioactivity measurements were performed overnight (10 pm till 8 am). The residual radioactivity after the injection on the first day (upper right thigh) was recorded for five days, that after the injection on the second day (upper left thigh) for four days, after the injection on the third day (lower right thigh) for three days, and after the last injection (lower left thigh) for two days. RESULTS--NovoSol Basal was absorbed according to first order kinetics with a mean t50% of 35.3 (SEM 1.4) hours; Ultratard HM was absorbed after a lag phase and the corresponding t50% was 25.5 (2.5) hours. The intraindividual variations in t50% were significantly smaller with NovoSol Basal than with Ultratard HM (18.4% v 44.5%; p less than 0.001). Interindividual variations, however, were not significantly different (25.2% v 36.9%; p = 0.38). The total variation in t50% was substantially smaller with NovoSol Basal than with Ultratard HM (20.3% v 42.8%). CONCLUSIONS--NovoSol Basal seems to be an appreciable advance over Ultratard HM as a soluble insulin preparation for obtaining reproducible 24 hour insulin concentrations in the blood
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger A. S., Saurbrey N., Kühl C., Villumsen J. Clinical experience with a new device that will simplify insulin injections. Diabetes Care. 1985 Jan-Feb;8(1):73–76. doi: 10.2337/diacare.8.1.73. [DOI] [PubMed] [Google Scholar]
- Berger M., Cüppers H. J., Hegner H., Jörgens V., Berchtold P. Absorption kinetics and biologic effects of subcutaneously injected insulin preparations. Diabetes Care. 1982 Mar-Apr;5(2):77–91. doi: 10.2337/diacare.5.2.77. [DOI] [PubMed] [Google Scholar]
- Berger M., Halban P. A., Girardier L., Seydoux J., Offord R. E., Renold A. E. Absorption kinetics of subcutaneously injected insulin. Evidence for degradation at the injection site. Diabetologia. 1979 Aug;17(2):97–99. doi: 10.1007/BF01222209. [DOI] [PubMed] [Google Scholar]
- Eaton R. P., Allen R. C., Schade D. S., Standefer J. C. "Normal" insulin secretion: the goal of artificial insulin delivery systems? Diabetes Care. 1980 Mar-Apr;3(2):270–273. [PubMed] [Google Scholar]
- Francis A. J., Hanning I., Alberti K. G. Human ultralente insulin: a comparison with porcine lente insulin as a twice-daily insulin in insulin-dependent diabetic patients with fasting hyperglycaemia. Diabetes Res. 1986 Jun;3(5):263–268. [PubMed] [Google Scholar]
- Frid A., Lindén B. Where do lean diabetics inject their insulin? A study using computed tomography. Br Med J (Clin Res Ed) 1986 Jun 21;292(6536):1638–1638. doi: 10.1136/bmj.292.6536.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J. A., Spradlin C. T., Nelson R. L., Wentworth S. M., Davidson J. A., Swarner J. L. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care. 1981 May-Jun;4(3):366–376. doi: 10.2337/diacare.4.3.366. [DOI] [PubMed] [Google Scholar]
- Hildebrandt P., Berger A., Vølund A., Kühl C. The subcutaneous absorption of human and bovine ultralente insulin formulations. Diabet Med. 1985 Sep;2(5):355–359. doi: 10.1111/j.1464-5491.1985.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Jefferson I. G., Marteau T. M., Smith M. A., Baum J. D. A multiple injection regimen using an insulin injection pen and pre-filled cartridged soluble human insulin in adolescents with diabetes. Diabet Med. 1985 Nov;2(6):493–495. doi: 10.1111/j.1464-5491.1985.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. H., Larsen U. D. Homogeneous mono-(125)i-insulins. Preparation and characterization of mono-(125)i-(tyr a14)-and mono-(125)i-(tyr a19)-insulin. Diabetologia. 1980;19(6):546–554. doi: 10.1007/BF00253183. [DOI] [PubMed] [Google Scholar]
- Kølendorf K., Aaby P., Westergaard S., Deckert T. Absorption, effectiveness and side effects of highly purified porcine NPH-insulin preparations (Leo). Eur J Clin Pharmacol. 1978 Nov 16;14(2):117–124. doi: 10.1007/BF00607442. [DOI] [PubMed] [Google Scholar]
- Lauritzen T., Faber O. K., Binder C. Variation in 125I-insulin absorption and blood glucose concentration. Diabetologia. 1979 Nov;17(5):291–295. doi: 10.1007/BF01235885. [DOI] [PubMed] [Google Scholar]
- Lauritzen T., Pramming S., Deckert T., Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983 May;24(5):326–329. doi: 10.1007/BF00251817. [DOI] [PubMed] [Google Scholar]
- Lauritzen T., Pramming S., Gale E. A., Deckert T., Binder C. Absorption of isophane (NPH) insulin and its clinical implications. Br Med J (Clin Res Ed) 1982 Jul 17;285(6336):159–162. doi: 10.1136/bmj.285.6336.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE E. W., MITCHELL M. L., CHALMERS T. C. Variability in absorption of insulin-I 131 in normal and diabetic subjects after subcutaneous and intramuscular injection. J Clin Invest. 1959 Jul;38(7):1222–1227. doi: 10.1172/JCI103897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar G. D., Reynolds C. Diurnal glucose variability and hormonal regulation. Horm Metab Res. 1977;Suppl 7:148–157. [PubMed] [Google Scholar]
- Saner B., Fankhauser S. Ultratard HM, ein neues Humaninsulin. Vorteile gegenüber den bisherigen Präparaten? Schweiz Med Wochenschr. 1986 Jan 25;116(4):116–119. [PubMed] [Google Scholar]
- Shahshahani M. N., Kitabchi Glucose-lowering effect of insulin by different routes in obese and lean nonketotic diabetic patients. J Clin Endocrinol Metab. 1978 Jul;47(1):34–40. doi: 10.1210/jcem-47-1-34. [DOI] [PubMed] [Google Scholar]


