Abstract
Individual Rel/NF-κB transcription factors, although dispensable for the development and maturation of most hemopoietic cells, are critical regulators of normal immune function. Redundancy among these proteins prompted us to examine the role of Rel and RelA in hemopoiesis by using mice that lack both subunits. Because of the death of double-mutant fetuses at day 13.5 of gestation (E13.5), E12 fetal liver hemopoietic progenitors were used for in vitro cultures and for repopulating stem cell studies in lethally irradiated normal recipient mice. Most striking, Rel/RelA-deficient hemopoietic precursors failed to promote the survival of myeloablated mice. This phenotype was associated with several defects including a reduction of spleen colony-forming unit progenitors, impaired erythropoiesis, and a deregulated expansion of granulocytes. In vitro progenitor assays also revealed that Rel or RelA serves an antiapoptotic role during monocyte differentiation. Despite the combined loss of Rel and RelA leading to these hemopoietic defects, c-rel−/−rela−/− stem cells contributed to the development of all lineages in mice engrafted with double-mutant fetal liver cells and normal bone marrow cells, albeit in a reduced fashion compared with controls. Collectively, these data indicate the loss of Rel and RelA does not appear to affect pluripotent stem cells; rather, Rel and RelA serve redundant functions in regulating differentiation and survival of committed progenitors in multiple hemopoietic lineages.
Rel/NF-κB transcription factors are homo- and heterodimeric proteins composed of related subunits that bind decameric sequences (κB elements) located within promoters and enhancers of cellular genes, particularly those encoding proteins involved in immune, acute phase and inflammatory responses (1). Each subunit shares a conserved N-terminal domain that encompasses sequences required for DNA binding, dimerization, and nuclear localization. In mammals, each of the five subunits, NF-κB1(p50), NF-κB2(p52), RelA (p65), RelB, and Rel is encoded by a different gene (1, 2). Rel, RelA, and RelB each possess distinct carboxyl-terminal transactivation domains, whereas NF-κB1 and NF-κB2, which only consist of the conserved N-terminal domain, lack intrinsic transcriptional transactivating properties (1, 2). In most cell types, the major proportion of Rel/NF-κB is retained in the cytoplasm in an inactive complex with regulatory IκB proteins (3, 4). Cytoplasmic Rel/NF-κB proteins are translocated to the nucleus in response to diverse stimuli that activate an IκB kinase complex, which, in turn, phosphorylates the IκB isoforms (5), targeting these proteins for ubiquitin-dependent, proteosome-mediated degradation (3, 4).
Mice homozygous for null mutations in genes encoding Rel/NF-κB subunits have revealed the unique roles of each transcription factor (6). RelA is essential for embryonic development, with rela−/− fetuses dying at day 15 (E15) of gestation because of apoptosis of fetal hepatocytes (7, 8). Rel, RelB, NF-κB1, or NF-κB2, although dispensable for embryogenesis, are important for the function and/or development of hemopoietic cells (9). Of relevance to this study, neither Rel nor RelA is essential for the generation or maturation of hemopoietic cells (7, 8, 10). Instead, the loss of either transcription factor leads to activation defects in mature B cells, T cells, and macrophages (9), indicating critical roles in regulating genes induced during immune responses.
Despite the unique roles ascribed to individual Rel/NF-κB subunits, similarities among these proteins may prevent certain phenotypes from being revealed in the single mutant mice because of compensation by other family members. This notion is supported by the finding that mice lacking two Rel/NF-κB subunits exhibit novel phenotypes or exaggerated versions of those seen in the single mutants. Examples include the blockage of lymphocyte development in the absence of NF-κB1 and RelA (11), greater severity of inflammatory disease in nfkb1−/−/relb−/− mice than observed in relb−/− animals (12), and impaired osteoclast and B cell development in nfkb1−/−nfkb2−/− mice (13, 14). Because Rel and RelA are the major transactivating Rel/NF-κB subunits expressed in hemopoietic cells (1, 2) and RelA/Rel heterodimers have been implicated in transcriptional regulation (15), we chose to examine the differentiation of hemopoietic cells that lack both subunits. Fetal liver c-rel−/−rela−/− hemopoietic precursors were used in clonal agar assays and transplantation studies to show that Rel/NF-κB transcription factors play a role in erythropoiesis, the regulation of granulopoiesis, and the survival of monocytic cells.
Materials and Methods
Fetal Liver Cell Engraftment.
The c-rel−/− (10) and rela−/− (7) mice used in this study were backcrossed for more than nine generations with C57BL/6-Ly5.2 mice. For hemopoietic cell reconstitutions, between 3 × 105 and 1 × 107 viable cells from E12 fetal liver were injected into the tail veins of 8- to 10-week-old C57BL/6-Ly5.1 mice that had received 1,100 rad of γ-irradiation. For coreconstitutions, irradiated C57BL/6-Ly5.1 mice received 1 × 106 to 1 × 107 E12 C57BL/6-Ly5.2 fetal liver cells and 2–7 × 105 adult C57BL/6-Ly5.1 bone marrow cells. Mice were maintained on autoclaved water containing 100 mg/ml neomycin sulfate. Fetuses of the desired genotype, confirmed by PCR, were derived from c-rel+/+rela+/− × c-rel+/+rela+/− and c-rel−/−rela+/− × c-rel−/−rela+/− matings.
Spleen Colony-Forming Unit (CFU-S) Assays.
CFU-S assays were performed essentially as described (16). Briefly, irradiated C57BL/6-Ly5.1 mice were injected with 1–3 × 105 E12 fetal liver cells and recipients were sacrificed 12 days postengraftment. Spleens were fixed in Carnoy’s solution (60% ethanol/30% chloroform/10% acetic acid), and the number of macroscopic colonies were counted.
Immunofluorescence Staining, Flow Cytometry, Cell Sorting, and Morphological Analysis of Cells.
Dispersed cells from the bone marrow, blood, thymus, and spleen of mice reconstituted with bone marrow and/or fetal liver hemopoietic precursors were stained with fluorochrome-labeled or biotinylated mAbs as described (17). Five thousand viable cells (negative for propidium iodide staining) then were analyzed by using a FACScan flow cytometer (Becton Dickinson). Fetal liver-derived macrophages were purified from the bone marrow of engrafted mice on a FACS Star II by three-color cell sorting by using anti-Mac-1, anti-Gr-1, and anti-Ly5.2 mAbs. The antibodies used in this study were: Ly5.2(5.430–15.2), Ly5.1(A201.7), Ter119, Gr-1(8CS), Mac-1(M1/76), F4/80, B220(RA3–6B2), Thy-1.2(30H12), Sca-1(E13.6.7), c-Kit(ACK-2), CD34(RAM34), CD44(IM781), and AA41. Cytospin preparations were stained with May Grunwald–Giemsa.
Fetal Liver Cultures.
Suspensions of fetal liver cells were cultured in 35-mm Petri dishes by using 10,000 cells in 1-ml volumes of DMEM containing 20% newborn calf serum and 0.3% agar. Cultures were stimulated by adding purified recombinant murine growth factors at the following concentrations: granulocyte/macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and IL-3 at 10 ng/ml, IL-6 at 500 ng/ml, stem cell factor at 100 ng/ml, erythropoietin at 20 units/ml, leukemia inhibiting factor at 10 ng/ml, and Flk ligand at 500 ng/ml. Cultures were incubated at 37°C in a fully humidified atmosphere of 10% CO2 in air for 7 days and then fixed and stained. All colonies (defined as >50 cells) were counted and typed at a magnification of ×400.
Results
Absence of Rel Accelerates the Onset of Embryonic Death in RelA-Deficient Mice.
Consistent with previous reports (7, 8), rela−/− fetuses developed normally until ≈E14.0, after which hepatic apoptosis, subsequent abdominal hemorrhage, and necrosis ensued. This phenotype was observed in c-rel−/−rela−/− fetuses, but occurred earlier in gestation, with fetal liver degeneration and hemorrhaging evident in most double-mutant fetuses by E13.5 (Table 1). Acceleration of the rela−/− phenotype also occurred in nfkb1−/−rela−/− mice (11). These findings suggest that Rel complexes partially compensate for RelA in fetal liver.
Table 1.
Accelerated prenatal death of c-rel−/−rela−/− fetuses
| Genotype | Embryonic day
|
|||||
|---|---|---|---|---|---|---|
| 12.0 | 13.0 | 13.5 | 14.0 | 14.5 | 15.0 | |
| c-rel+/+rela−/− | Normal morphology (4/4) | Normal morphology (7/7) | F.L. apoptosis abdominal hemorrhage (1/6) | F.L. apoptosis abdominal hemorrhage (5/7) | Necrotic embryo (5/5) | |
| c-rel−/−rela−/− | Normal morphology (10/10) | F.L. apoptosis abdominal hemorrhage (4/14) | F.L. apoptosis abdominal hemorrhage (4/6) | Necrotic embryo (4/4) | ||
Fetuses were timed such that day 0 is the day when morning plugs were identified. Saggital sections of fetuses were prepared and stained as described (7). Fetal liver apoptosis was determined by examination of the histological preparations. F.L., fetal liver. Numbers in parentheses refer to fetuses showing the specified phenotype vs. total number examined.
Hemopoietic Progenitor Cells from c-rel−/−rela−/− Fetal Liver Fail to Promote Survival of Lethally Irradiated Mice.
The combined roles of Rel and RelA in hemopoiesis first were assessed by injecting Ly5.2-positive fetal liver cells from E12 c-rel−/−rela−/− fetuses into lethally irradiated C57BL/6-Ly5.1 mice. Whereas ≈86% of lethally irradiated mice receiving control (wild-type, rela−/− or c-rel−/−) fetal liver cells survived for longer than 8 weeks, all animals engrafted with c-rel−/−rela−/− cells were sacrificed between 12 and 22 days postirradiation because of their moribund condition (Fig. 1). Whereas 3 × 105 control cells were sufficient to promote survival after lethal irradiation, up to 25-fold more c-rel−/−rela−/− cells failed to successfully engraft irradiated mice. Moreover, the period that recipients survived after c-rel−/−rela−/− fetal liver cell engraftment was in direct proportion to the number of donor cells, with those mice receiving more cells surviving longer. This failure of c-rel−/−rela−/− fetal liver cell engraftment did not simply reflect a reduction in the number and viability of cells from the E12 fetuses, because histology, propidium iodide exclusion, and flow cytometric analysis of hemopoietic precursors revealed no difference between E12 control and c-rel−/−rela−/− fetal liver cells (M.G., unpublished results).
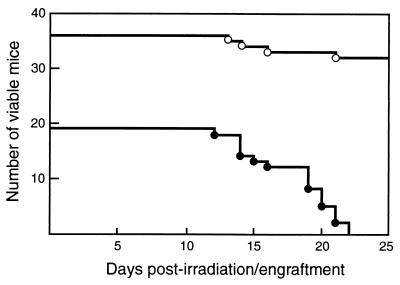
Figure 1.
Hemopoietic precursors in c-rel−/−rela−/− fetal liver fail to promote the survival of lethally irradiated mice. The period of survival for lethally irradiated C57BL/6-Ly5.1 mice engrafted with E12 c-rel−/−rela−/− (19 recipients, ●) or control (36 recipients: c-rel+/+rela+/+, n = 8; c-rel+/+rela+/−, n = 6; c-rel+/+rela−/−, n = 6; c-rel−/−rela+/+, n = 16, ○) fetal liver cells suspensions is presented as a Kaplan–Meyer plot. After engraftment, irradiated recipients were monitored daily and sacrificed for analysis before imminent death. Irradiated mice received a mean of 31 ± 17 × 105 (range 3–80 × 105) and 36 ± 20 × 105 (range 5–80 × 105) control or c-rel−/−rela−/− cells, respectively. Mice receiving between 3 × 105 and 2 × 106 c-rel−/−rela−/− cells had to be sacrificed after 12–16 days engraftment, whereas recipients of 4–8 × 106 double-mutant cells survived for 19–22 days.
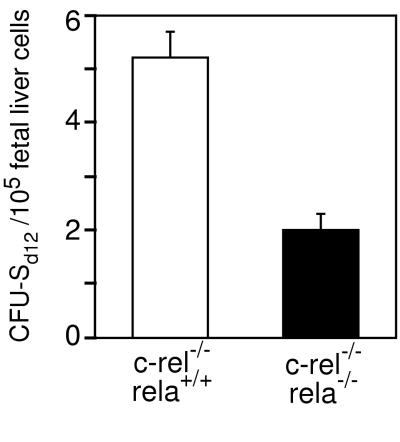
The successful engraftment of hemopoietic cells into lethally irradiated recipients encompasses two phases that utilize distinct precursor populations. The initial transient engraftment requires precursors that reside in the CFU-S compartment, whereas long-term reconstitution is dependent on more primitive stem cells (18). Because the rapid death of recipients injected with c-rel−/−rela−/− cells indicated a defect in transient engraftment, the frequency of CFU-S in E12 control and c-rel−/−rela−/− fetal liver was compared by enumerating macroscopic splenic hemopoietic nodules in irradiated mice injected with equivalent numbers of viable control or double-mutant cells. These experiments (Fig. 2) reveal a small, but consistent 2.5-fold reduction in CFU-S in E12 c-rel−/−rela−/− fetal liver, indicating that Rel or RelA is necessary for the establishment or maintenance of normal CFU-S numbers.
Figure 2.
Reduced numbers of CFU-S in c-rel−/−rela−/− fetal liver. Numbers of CFU-S (CFU-Sd12) are shown per 1 × 105 E12 control (c-rel−/−) and c-rel−/−rela−/− fetal liver cells transplanted into irradiated recipient mice. The data represent the mean ± SEM from five individual donor fetal livers of each genotype, each transplanted into four recipients, pooled from three independent experiments.
The Combined Absence of Rel and RelA Leads to Impaired Erythropoiesis.
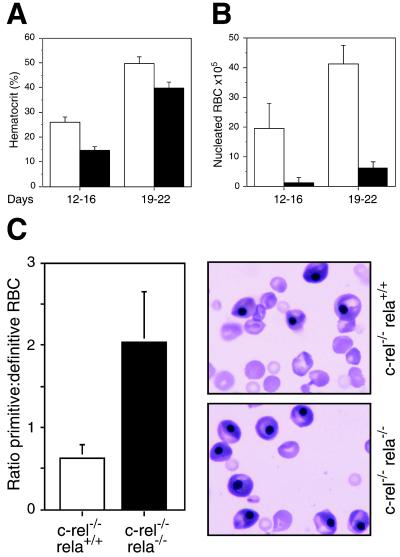
When sacrificed, all recipients of c-rel−/−rela−/− fetal liver cells presented with a reduced hematocrit compared with age-matched controls (Fig. 3A). Mice injected with up to 2 × 106 c-rel−/−rela−/− cells exhibited severe anemia, whereas those receiving 4–8 × 106 double-mutant cells were only mildly anemic. Consistent with the observed anemia, the bone marrow of all recipients of c-rel−/−rela−/− cells had markedly fewer erythroblasts than engrafted controls (Fig. 3B). A defect in erythropoiesis also was evident in the c-rel−/−rela−/− fetuses. When compared with control fetuses, a significantly higher proportion of E12 c-rel−/−rela−/− peripheral RBCs were nucleated embryonic cells (Fig. 3C). To determine whether this defect was associated with a reduction in erythroid progenitors, E12 control and c-rel−/−rela−/− fetal liver cells were cultured in vitro with optimal concentrations of erythropoietin, IL-3, and SCF. c-rel−/−rela−/− erythroid colonies were of similar number (Table 2), size, and appearance to controls. Collectively, these data indicate that although the absence of Rel and RelA does not reduce the frequency of committed erythroid progenitors, either transcription factor is necessary for normal erythrocyte differentiation or production in vivo.
Figure 3.
The absence of Rel and RelA impairs erythropoiesis. (A) Hematocrits of irradiated C57BL/6-Ly5.1 mice engrafted with E12 control (wild-type, c-rel−/−; open bars) or c-rel−/−rela−/− (solid bars) fetal liver cells before death. Recipients of c-rel−/−rela−/− fetal liver cells represented two distinct groups; mice surviving between 12 and 16 days postengraftment received fewer fetal liver cells than recipients surviving between 19 and 22 days. Hematocrits (normal range, 35–50%) were determined by flow cytometry by using the Technicon H1 system. Each data point represents the mean ± SEM from at least six mice of the appropriate genotypes. (B) The frequency of nucleated erythroid cells is reduced markedly in the marrow of mice transplanted with c-rel−/−rela−/− fetal liver cells. Control (c-rel−/−, open bars) and c-rel−/−rela−/− (solid bars) are shown. The data represent the mean ± SEM from at least five mice of each genotype. (C) Blood of E12 c-rel−/−rela−/− fetuses has a higher proportion of nucleated embryonic erythrocytes. The ratio of nucleated primitive to enucleated definitive RBCs in E12 control (c-rel−/−) and c-rel−/−rela−/− fetuses represents the mean ± SEM of this relative frequency in eight fetuses of each genotype. The blood smears presented here are typical for E12 c-rel−/− and c-rel−/−rela−/− fetuses.
Table 2.
Number of progenitor cells of various lineages in the fetal liver
| Genotype | Colonies
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | G | GM | M | Eo | Meg | E | E/Meg | Mix | Blast | |
| c-rel+/+rela+/+ | 113 ± 6 | 17 ± 3 | 18 ± 2 | 22 ± 3 | 2 ± 1 | 8 ± 1 | 26 ± 3 | 11 ± 2 | 2 ± 1 | 6 ± 1 |
| c-rel+/+rela−/− | 100 ± 17 | 16 ± 3 | 15 ± 3 | 16 ± 4 | 3 ± 1 | 8 ± 1 | 16 ± 3 | 13 ± 2 | 1 ± 1 | 11 ± 4 |
| c-rel−/−rela+/+ | 107 ± 13 | 13 ± 1 | 19 ± 3 | 19 ± 3 | 3 ± 1 | 10 ± 2 | 18 ± 3 | 13 ± 3 | 3 ± 1 | 9 ± 2 |
| c-rel−/−rela−/− | 91 ± 9 | 19 ± 1 | 9 ± 1 | 2 ± 1 | 2 ± 1 | 11 ± 2 | 21 ± 1 | 13 ± 2 | 2 ± 1 | 10 ± 3 |
Number of progenitor (colony-forming) cells in culture of 10,000 day 12 fetal liver cells stimulated by a combination of growth factors. G, granulocyte; GM, granulocyte–macrophage; M, macrophage; Eo, eosinophil; Meg, megakaryocyte; E, erythrocyte; E/Meg, erythrocyte–megakaryocyte; Mix, mixed cultures; blast, blast cell. Given values represent mean ± SEM from separate cultures from at least six individual fetal livers per genotype.
The Absence of Rel and RelA Leads to Granulocytosis.
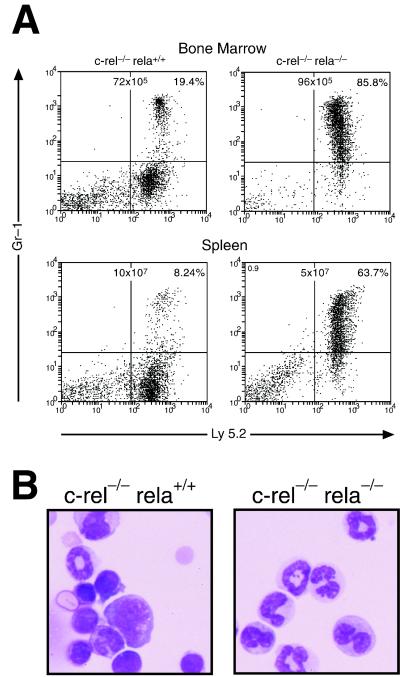
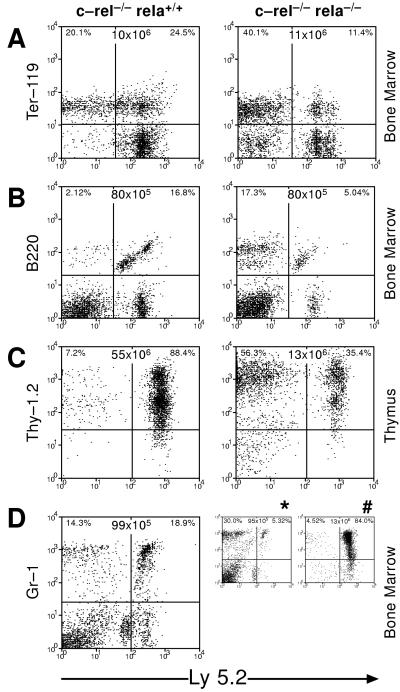
Recipients of c-rel−/−rela−/− fetal liver cells that survived between 19 and 22 days postengraftment were only mildly anemic, indicating other hemopoietic defects afflict these mice. A common feature of this group of mice, but not those of equivalent age receiving control cells, was an excess of differentiating granulocytes in hemopoietic organs (Fig. 4) and other tissues. The absence of a detectable infection in these mice (results not shown) indicated that this fetal liver-derived granulocytosis instead represented a deregulated expansion of these cells because of the combined loss of Rel and RelA. The strongest evidence for such a model came from mice reconstituted with c-rel−/−rela−/− fetal liver cells and normal bone marrow. Over a period of 5–26 weeks postengraftment, ≈40% of mice became moribund. Remarkably, in all cases this was accompanied by granulocytosis of fetal liver but not bone marrow origin, despite the presence of wild-type granulocytes in these mice (Fig. 5D). The close association between a selective expansion of c-rel−/−rela−/− granulocytes in coengrafted mice and the onset of sickness was reinforced with the finding in healthy mice that the number of c-rel−/−rela−/− fetal liver-derived granulocytes was always low (Fig. 5D). These data indicate that the loss of both Rel and RelA can lead to a deregulated granulocytosis.
Figure 4.
Granulocytosis in mice engrafted with c-rel−/−rela−/− fetal liver cells. (A) Bone marrow and splenic cells of irradiated C57BL/6-Ly5.1 mice 20 days after engraftment with control (c-rel−/−) or c-rel−/−rela−/− fetal liver cells were stained with mAbs specific to Ly5.2 and Gr-1 and analyzed by flow cytometry. The organ cellularity and distribution of Ly5.2 and Gr-1 among all viable bone marrow and splenic cells is shown. (B) Bone marrow cytospins from irradiated mice engrafted with control (c-rel−/−) or c-rel−/−rela−/− fetal liver cells 20 days after transplantation. These data are representative of five or more animals of each group.
Figure 5.
c-rel−/−rela−/− stem cells contribute to the development of various hemopoietic lineages. Cell suspensions from the bone marrow or thymus of irradiated C57BL/6-Ly5.1 mice engrafted 4–6 months earlier with normal Ly5.1 bone marrow and either E12 control (c-rel−/−) or c-rel−/−rela−/− fetal liver cells were stained with mAbs specific for fetal liver-derived erythroid (A), B lymphoid (B), T lymphoid (C), and granulocytic cells (D) and analyzed by flow cytometry. The symbols ∗ and # correspond to mice that were healthy and sick, respectively, 4 months after being engrafted with normal Ly5.1 bone marrow and c-rel−/−rela−/− fetal liver cells. Organ cellularity and distribution of normal and fetal liver-derived hemopoietic cells are shown. These data are representative of six or more animals analyzed in each group.
Lineage Commitment of c-rel−/−rela−/− Hemopoietic Stem Cells in Vivo.
Because of the failure of double-mutant fetal liver cells to successfully promote hemopoietic cell engraftment in lethally irradiated recipients, C57BL/6-Ly5.1 mice were coreconstituted with E12 c-rel−/−rela−/−(Ly5.2+) fetal liver cells and normal C57BL/6-Ly5.1 bone marrow. Typical flow cytometry profiles of the bone marrow and thymus from healthy mice not exhibiting granulocytosis 2–6 months after engraftment (Fig. 5) showed that erythroid, B and T lymphoid, and granulocytic cells of c-rel−/−rela−/− fetal liver origin are present, albeit in 2- to 10-fold lower numbers than control fetal liver derived cells in coreconstituted mice. The lower number of mature c-rel−/−rela−/− hemopoietic cells is not simply due to fewer stem cells, because an increase in the relative proportion of double-mutant fetal liver cells engrafted in these mice did not alter the frequency of mature c-rel−/−rela−/− hemopoietic cells (results not shown). These data show that the absence of RelA and Rel does not impair the developmental potential of primitive stem cells required for long-term reconstitution of the hemopoietic system.
The Absence of Rel and RelA Leads to Apoptosis During Macrophage Differentiation in Culture.
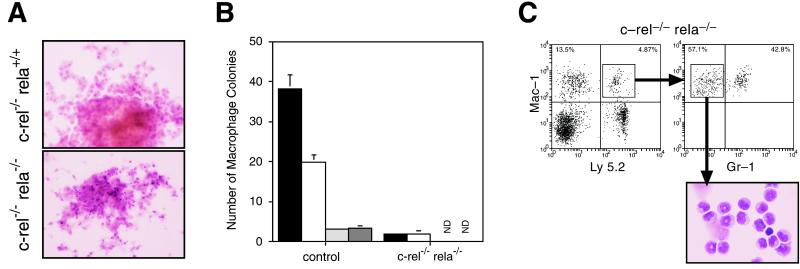
To quantify the frequency of various progenitors in c-rel−/−rela−/− fetal liver committed to specific hemopoietic lineages, colony-forming cell (CFC) assays were performed. Table 2 summarizes the frequency of CFCs for the various hemopoietic lineages. Although the number of granulocyte, eosinophil, megakaryocyte, and erythrocyte colonies in E12 c-rel−/−rela−/− fetal liver cultures were normal, the frequency of macrophage and granulocyte–macrophage colonies was reduced. Those c-rel−/−rela−/− macrophage colonies that did grow were smaller than control colonies, and microscopic examination revealed that the majority of mature macrophages in these colonies had undergone apoptosis (Fig. 6A). Because this cell death was independent of the type of hemopoietic growth factor stimulus (Fig. 6B), this suggested that the defect afflicted a common differentiation pathway. Despite monocyte death in cultures, preliminary flow cytometry indicated that mature monocytic cells could develop from c-rel−/−rela−/− fetal liver progenitors in vivo. This finding, however, was complicated by the knowledge that granulocytes and macrophages share many cell surface markers, including those recognized by commonly used mAbs. To clarify the issue of c-rel−/−rela−/− monocyte differentiation in vivo, Ly5.2+Mac-1+Gr-1lo cells isolated by cell sorting from the bone marrow of coreconstituted C57BL/6-Ly5.1 mice appear to be typical monocytic cells (Fig. 6C). These data indicate that apoptosis resulting from the absence of Rel and RelA during monocyte differentiation in culture could be bypassed in vivo.
Figure 6.
c-rel−/−rela−/− macrophages undergo apoptosis during maturation in culture. (A) Representative control (c-rel−/−) and c-rel−/−rela−/− macrophage colonies were grown for 7 days in the presence of macrophage colony-stimulating factor (M-CSF). Cultures were fixed and stained as described in Materials and Methods. (B) The reduction in c-rel−/−rela−/− macrophage colonies is independent of the growth factor stimulus. Equivalent numbers of viable E12 control (wild-type, n = 6; c-rel−/−, n = 7; and rela−/−, n = 6) and c-rel−/−rela−/− fetal liver cells (n = 7) were grown in agar for 7 days in the presence of optimal concentrations of M-CSF (solid bars), stem cell factor SCF/IL-3/erythropoietin (open bars), SCF (lightly shaded bars), or Flk ligand/leukemia inhibiting factor (darkly shaded bars). N.D., no colonies detected. (C) c-rel−/−rela−/− progenitors can differentiate into monocytes in vivo. Cells in the bone marrow of irradiated C57BL/6-Ly5.1 mice engrafted 4 months earlier with normal Ly5.1 bone marrow and E12 c-rel−/−rela−/− fetal liver cells that stained positive with mAbs specific for Ly5.2 and Mac-1 but were negative for Gr-1 were sorted by flow cytometry and cytospins were prepared.
Discussion
Here we describe the phenotype of mice that lack Rel and RelA, two Rel/NF-κB subunits that possess transcriptional transactivating properties. Development in c-rel−/−rela−/− fetuses appeared normal until E13, at which point fetal liver degeneration resulting from apoptosis occurred. This phenotype was similar to that seen in rela−/− fetuses (7, 8) except it occurred earlier in gestation, suggesting that Rel, in part, compensates for the absence of RelA. This accelerated embryonic death coincides with the overlapping expression of c-rel and rela in fetal liver during development (19). Because embryonic death resulting from the loss of RelA can be overcome by inactivating TNFR1 (20), these combined findings suggest that Rel participates in preventing tumor necrosis factor-α toxicity in vivo.
Previous studies had shown that the loss of either Rel or RelA did not impair hemopoiesis; rather, it led to defects in the immune responsiveness of mature hemopoietic cells (7–10). This is supported by our present observations that the behavior of both rela−/− and c-rel−/−fetal liver cells was similar to wild-type fetal liver cells in all experiments (see Results; data not shown). Engraftment of mouse c-rel−/−rela−/− fetal liver hemopoietic progenitors into irradiated recipients, combined with the use of in vitro cultures, revealed that Rel and RelA serve redundant roles in regulating cellular maturation and survival during hemopoiesis. Most profound was the inability of c-rel−/−rela−/− fetal liver hemopoietic precursors to promote transient engraftment essential for survival after myeloablative irradiation. Although stem cells crucial for primary engraftment reside in the CFU-S compartment (21), the moderate reduction in the frequency of CFU-S in E12 c-rel−/−rela−/− fetal liver cannot account for the failure of transplanted fetal liver cells to rescue irradiated recipients. Instead, impaired erythropoiesis and deregulated granulopoiesis appear to responsible for the death of engrafted mice. Although the loss of Rel and RelA affected erythropoiesis, the severity of the resultant anemia in recipient mice depended on the number of engrafted c-rel−/−rela−/− fetal liver cells. This observation is consistent with a defect in which erythropoiesis was not blocked but, instead, was ineffective. Such an in vivo defect could involve a delay in differentiation or an abnormality in mature RBCs. This conclusion is supported indirectly by the finding that the frequency of erythroid progenitors and their capacity to differentiate in culture appear to be normal. The blood of E12 c-rel−/−rela−/− fetuses also had a higher than normal number of nucleated embryonic RBCs. The persistence of embryonic erythrocytes in midgestation also has been documented in rb−/− (22, 23) and nrf-1−/− (24) fetuses, where it reflects a defect or retardation in the switch to definitive erythropoiesis. Similar to our results with c-rel−/−rela−/− fetal liver cells, the capacity of rb−/− and nrf-1−/− fetal liver-derived progenitors to form erythroid colonies in vitro also was normal, emphasizing the importance of the hemopoietic microenvironment for effective erythropoiesis in vivo (22–24). Our findings establish a role for Rel and RelA in erythroid cells. Furthermore, they suggest that these transcription factors serve redundant functions in erythroid cells, a conclusion that is consistent with the observation that a significant proportion of fetal liver cells expressing Rel and RelA are erythroid precursors (19).
The loss of Rel and RelA also leads to the development of granulocytosis. Interestingly, granulocytosis occurs in recipients of nfkb1−/−rela−/− fetal liver cells (11), ikba−/− mice (25), and mutant mice lacking the regulatory carboxyl terminus of the NF-κB2 precursor protein (6). Collectively, these findings suggest that a deregulation or imbalance of Rel/NF-κB subunits interferes with the transcription of genes that are required for normal granulocyte differentiation or activation. The basis of this excessive granulocyte production is not known. Although in vitro assays indicate the number and differentiative capacity of granulocyte precursors in c-rel−/−rela−/− fetal liver appear normal, because granulocytes and macrophages can share a common progenitor, the maturation defect afflicting c-rel−/−rela−/− monocytes, in turn, could deregulate granulopoiesis. Because granulocytosis is not always pathogenic (26), if the death of c-rel−/−rela−/− fetal liver engrafted mice is a result of this condition, it would indicate that these cells are functioning aberrantly and may be producing cytokines or toxic factors.
The capacity of c-rel−/−rela−/− fetal liver cells to contribute to all hemopoietic lineages in long-term, coreconstituted mice indicates that Rel and RelA are not essential for the differentiation of pluripotent stem cells. However, the lower than expected frequency of all mature c-rel−/−rela−/− hemopoietic cells in these mice indicates that hemopoiesis is not normal. This difference could reflect a reduced capacity of mutant stem cells to seed or compete with wild-type bone marrow in engrafted mice, a decreased ability of c-rel−/−rela−/− stem cells to respond to proliferation and differentiation inducing stimuli, or a shortened life span of mature c-rel−/−rela−/− hemopoietic cells in vivo. The latter is consistent with a role for Rel/NF-κB factors in cell survival (27) and supported by the observation that apoptosis is higher in c-rel−/−rela−/− monocytes (see below) and lymphocytes (M.G., unpublished results).
Apoptosis of maturing c-rel−/−rela−/− macrophages in culture shows that Rel or RelA is required for monocyte survival during the response of progenitors to proliferation- and differentiation-inducing stimuli. Although RelA is necessary to protect mature macrophages against tumor necrosis factor-α-induced apoptosis (28), this evidence demonstrates an antiapoptotic role for Rel/NF-κB during monocyte differentiation. Despite these in vitro findings, the presence of c-rel−/−rela−/− fetal liver-derived monocytes in the bone marrow and peripheral blood of long-term coreconstituted mice indicates that compensatory signals or factors critical for monocyte differentiation and survival operate in vivo. It remains to be determined which gene(s) regulated by Rel and RelA are required for survival during monocyte differentiation. A1, a Bcl-2-like prosurvival homolog induced in lymphocytes by Rel during mitogenic stimulation (29), also is up-regulated during macrophage differentiation (30). Although Rel-dependent A1 expression is important in protecting B cells from activation-induced apoptosis (29), a protective role in macrophages remains unclear, because A1−/− mice exhibit enhanced neutrophil but not macrophage death (31). Ultimately, the identification of Rel/NF-κB-regulated genes essential for monocyte survival should offer insights into the mechanisms by which these transcription factors control this process in response to growth and differentiation signals.
Acknowledgments
We thank Drs. Andreas Strasser and Andrew Elefanty for antibodies and technical advice, Dr. Frank Battye and colleagues for assistance with cell sorting, and Drs. Warren Alexander, Yukio Nakamura, and Andreas Strasser for critical discussions and comments on the manuscript. This work was supported by the National Health and Medical Research Council (Australia), the Anti-Cancer Council of Victoria, Commonwealth AIDS Research Grants (no. 971274), and the International Association for Cancer Research (St. Andrew, U.K.).
Abbreviations
- E
embryonic day
- CFU-S
spleen colony-forming unit
References
- 1.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.Gilmore T D, Koedood M, Piffat K A, White D W. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 3.Finco T S, Baldwin A S. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 4.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 5.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 6.Attar R M, Caamano J, Carrasco D, Iotsova V, Ishikawa H, Ryseck R P, Weih F, Bravo R. Semin Cancer Biol. 1997;8:93–101. doi: 10.1006/scbi.1997.0060. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Nature (London) 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 8.Doi T S, Takahashi T, Taguchi O, Azuma T, Obata Y. J Exp Med. 1997;185:953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerondakis S, Grumont R, Rourke I, Grossmann M. Curr Opin Immunol. 1998;10:353–359. doi: 10.1016/s0952-7915(98)80175-1. [DOI] [PubMed] [Google Scholar]
- 10.Kontgen F, Grumont R J, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz B H, Scott M L, Cherry S R, Bronson R T, Baltimore D. Immunity. 1997;6:765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- 12.Weih F, Durham S K, Barton D S, Sha W C, Baltimore D, Bravo R. J Exp Med. 1997;185:1359–1370. doi: 10.1084/jem.185.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzoso G, Carlson L, Xing L, Poljak L, Shores E W, Brown K D, Leonardi A, Tran T, Boyce B F, Siebenlist U. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 15.Hansen S K, Nerlov C, Zabel U, Verde P, Johnsen M, Baeuerle P A, Blasi F. EMBO J. 1992;11:205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura S, Roberts A W, Metcalf D, Alexander W S. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grumont R J, Rourke I J, O’Reilly L A, Strasser A, Miyake K, Sha W, Gerondakis S. J Exp Med. 1998;187:663–674. doi: 10.1084/jem.187.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright E G, Lord B I. Baillieres Clin Haematol. 1992;5:499–507. doi: 10.1016/s0950-3536(11)80004-1. [DOI] [PubMed] [Google Scholar]
- 19.Carrasco D, Weih F, Bravo R. Development. 1994;120:2991–3004. doi: 10.1242/dev.120.10.2991. [DOI] [PubMed] [Google Scholar]
- 20.Doi T S, Marino M W, Takahashi T, Yoshida T, Sakakura T, Old L J, Obata Y. Proc Natl Acad Sci USA. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones R J, Celano P, Sharkis S J, Sensenbrenner L L. Blood. 1989;73:397–401. [PubMed] [Google Scholar]
- 22.Clarke A R, Maandag E R, van Roon M, van der Lugt N M, van der Valk M, Hooper M L, Berns A, te Riele H. Nature (London) 1992;359:328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 23.Jacks T, Fazeli A, Schmitt E M, Bronson R T, Goodell M A, Weinberg R A. Nature (London) 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 24.Chan J Y, Kwong M, Lu R, Chang J, Wang B, Yen T S, Kan Y W. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg A A, Sha W C, Bronson R T, Baltimore D. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 26.Lindeman G J, Adams J M, Cory S, Harris A W. Immunity. 1994;1:517–527. doi: 10.1016/1074-7613(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 27.Sonenshein G E. Semin Cancer Biol. 1997;8:113–119. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- 28.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 29.Grumont R J, Rourke I J, Gerondakis S. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera L P, Waldmann T A. Proc Natl Acad Sci USA. 1998;95:14308–14313. doi: 10.1073/pnas.95.24.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, Hatakeyama S. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]