Abstract
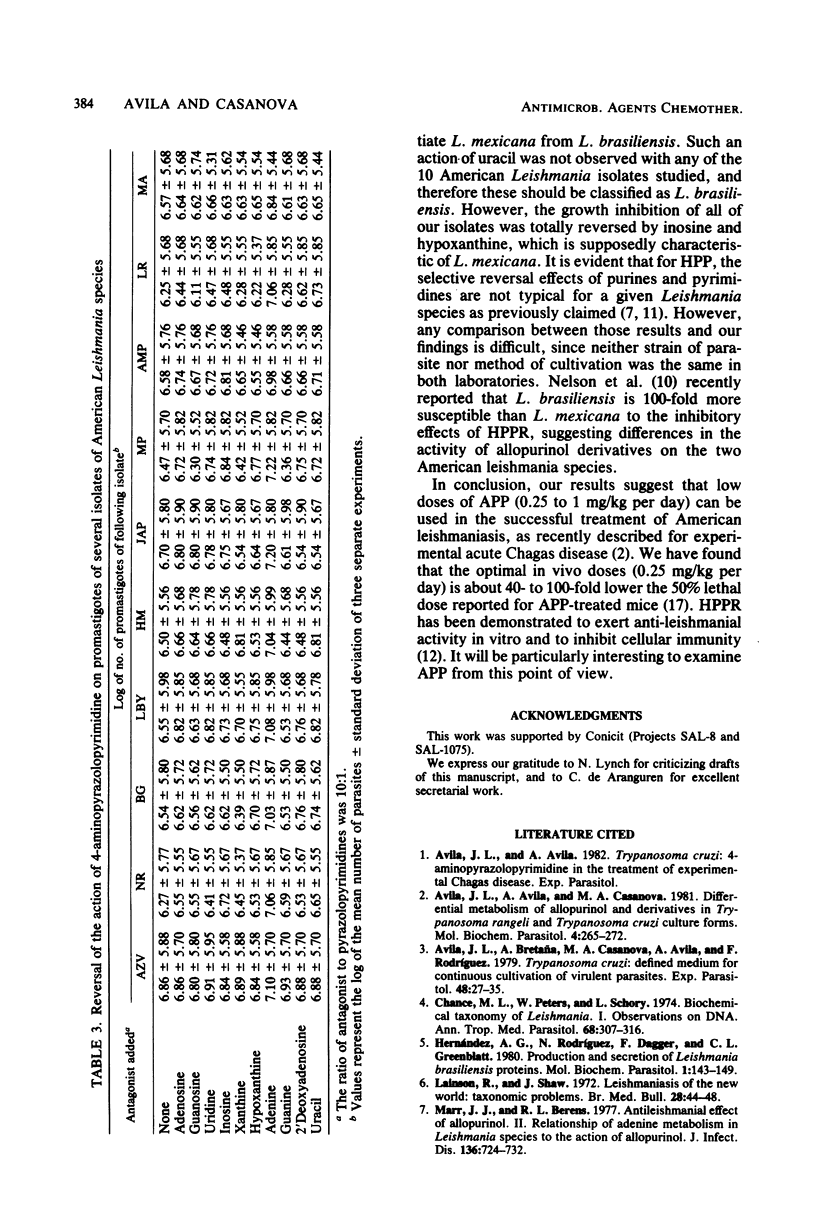
When assayed on promastigotes of 10 American Leishmania isolates (including Leishmania brasiliensis and mexicana species), 4-aminopyrazolopyrimidine (AAP) was severalfold more active than allopurinol as a leishmanistatic drug. There were some intraspecific and interspecific differences among the isolates in their susceptibility to the inhibitory effects of APP and allopurinol. APP-2'-deoxyriboside did not affect the 10 isolates tested. This was surprising, because allopurinol riboside has previously been shown to be more active than allopurinol. In all of the American Leishmania isolates tested, the metabolism of [14C]6-APP resulted in a high level of HPP-ribose-5'-P and lower levels of APP-ribose-5'-P, APP-ribose-5'-PP, and APP-ribose-5'-PPP. MP, LR, LBY, and JAP isolates strongly converted APP into APP derivatives, thus perhaps explaining their greater susceptibility to the inhibitory effects of APP. With the 10 American Leishmania isolates tested, several purines reversed the inhibitory effects of allopurinol, but only adenine countered the inhibitory effects of APP. This suggests biochemical differences in the mechanisms of action of allopurinol and APP. Finally, and contrary to previous claims, the reversal by purines of the allopurinol-induced growth inhibition was not a Leishmania species-specific effect.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Avila A., de Casanova M. A. Differential metabolism of allopurinol and derivatives in Trypanosoma rangeli and T. cruzi culture forms. Mol Biochem Parasitol. 1981 Dec 31;4(5-6):265–272. doi: 10.1016/0166-6851(81)90059-1. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Bretaña A., Casanova M. A., Avila A., Rodríguez F. Trypanosoma cruzi: defined medium for continuous cultivation of virulent parasites. Exp Parasitol. 1979 Aug;48(1):27–35. doi: 10.1016/0014-4894(79)90051-1. [DOI] [PubMed] [Google Scholar]
- Chance M. L., Peters W., Shchory L. Biochemical taxonomy of Leishmania. I. Observations on DNA. Ann Trop Med Parasitol. 1974 Sep;68(3):307–316. [PubMed] [Google Scholar]
- Hernandez A. G., Rodriguez N., Dagger F., Greenblatt C. L. Production and secretion of Leishmania braziliensis proteins. Mol Biochem Parasitol. 1980 Jun;1(3):143–149. doi: 10.1016/0166-6851(80)90013-4. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Berens R. L. Antileishmanial effect of allopurinol. II. Relationship of adenine metabolism in Leishmania species to the action of allopurinol. J Infect Dis. 1977 Dec;136(6):724–732. doi: 10.1093/infdis/136.6.724. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Berens R. L., Nelson D. J. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978 Dec 1;544(2):360–371. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Bugge C. J., Elion G. B., Berens R. L., Marr J. J. Metabolism of pyrazolo(3,4-d)pyrimidines in Leishmania braziliensis and Leishmania donovani. Allopurinol, oxipurinol, and 4-aminopyrazolo(3,4-d)pyrimidine. J Biol Chem. 1979 May 25;254(10):3959–3964. [PubMed] [Google Scholar]
- Nelson D. J., LaFon S. W., Elion G. B., Marr J. J., Berens R. L. Comparative metabolism of a new antileishmanial agent, allopurinol riboside, in the parasite and the host cell. Adv Exp Med Biol. 1979;122B:7–12. doi: 10.1007/978-1-4684-8559-2_2. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., LaFon S. W., Tuttle J. V., Miller W. H., Miller R. L., Krenitsky T. A., Elion G. B., Berens R. L., Marr J. J. Allopurinol ribonucleoside as an antileishmanial agent. Biological effects, metabolism, and enzymatic phosphorylation. J Biol Chem. 1979 Nov 25;254(22):11544–11549. [PubMed] [Google Scholar]
- Nishida Y., Kamatani N., Tanimoto K., Akaoka I. Suppression of cellular immunity due to inhibition of purine nucleoside phosphorylase by allopurinol-riboside. Adv Exp Med Biol. 1979;122B:309–313. doi: 10.1007/978-1-4684-8559-2_50. [DOI] [PubMed] [Google Scholar]
- PHILIPS F. S., SCHOLLER J., STERNBERG S. S. Production of fatty livers by 4-aminopyrazolo-(3, 4-d)-pyrimidine; toxicological and pathological studies. Proc Soc Exp Biol Med. 1956 Nov;93(2):398–402. doi: 10.3181/00379727-93-22769. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Marr J. J. Antileishmanial effect of allopurinol. Antimicrob Agents Chemother. 1974 May;5(5):469–472. doi: 10.1128/aac.5.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Arredondo B., González M. Comparative study of American cutaneous leishmaniasis and diffuse cutaneous leishmaniasis in two strains of inbred mice. Infect Immun. 1978 Nov;22(2):301–307. doi: 10.1128/iai.22.2.301-307.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Labrador F., Torrealba J. W. Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol. 1979 Feb;9(1):27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Scorza J. V., Valera M., de Scorza C., Carnevali M., Moreno E., Lugo-Hernandez A. A new species of Leishmania parasite from the Venezuelan Andes region. Trans R Soc Trop Med Hyg. 1979;73(3):293–298. doi: 10.1016/0035-9203(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Tuttle J. V., Krenitsky T. A. Purine phosphoribosyltransferases from Leishmania donovani. J Biol Chem. 1980 Feb 10;255(3):909–916. [PubMed] [Google Scholar]



