Abstract
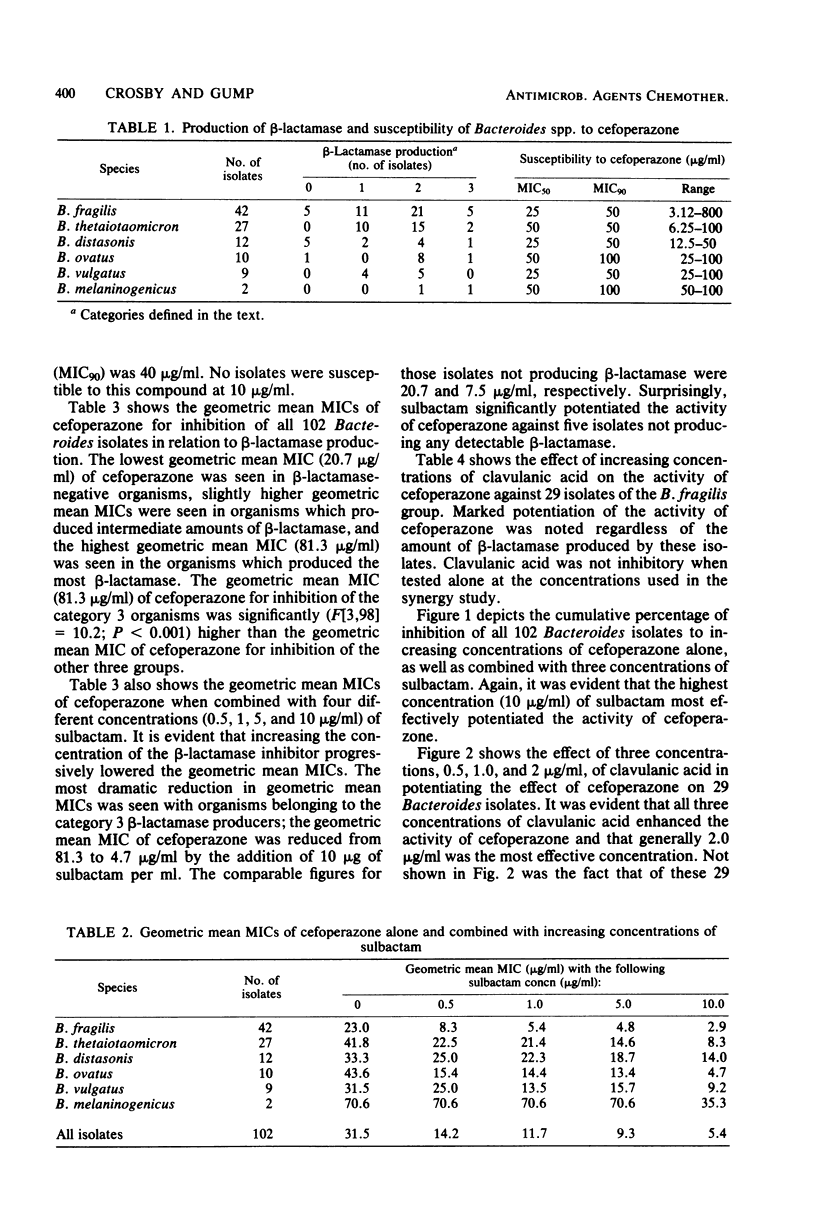
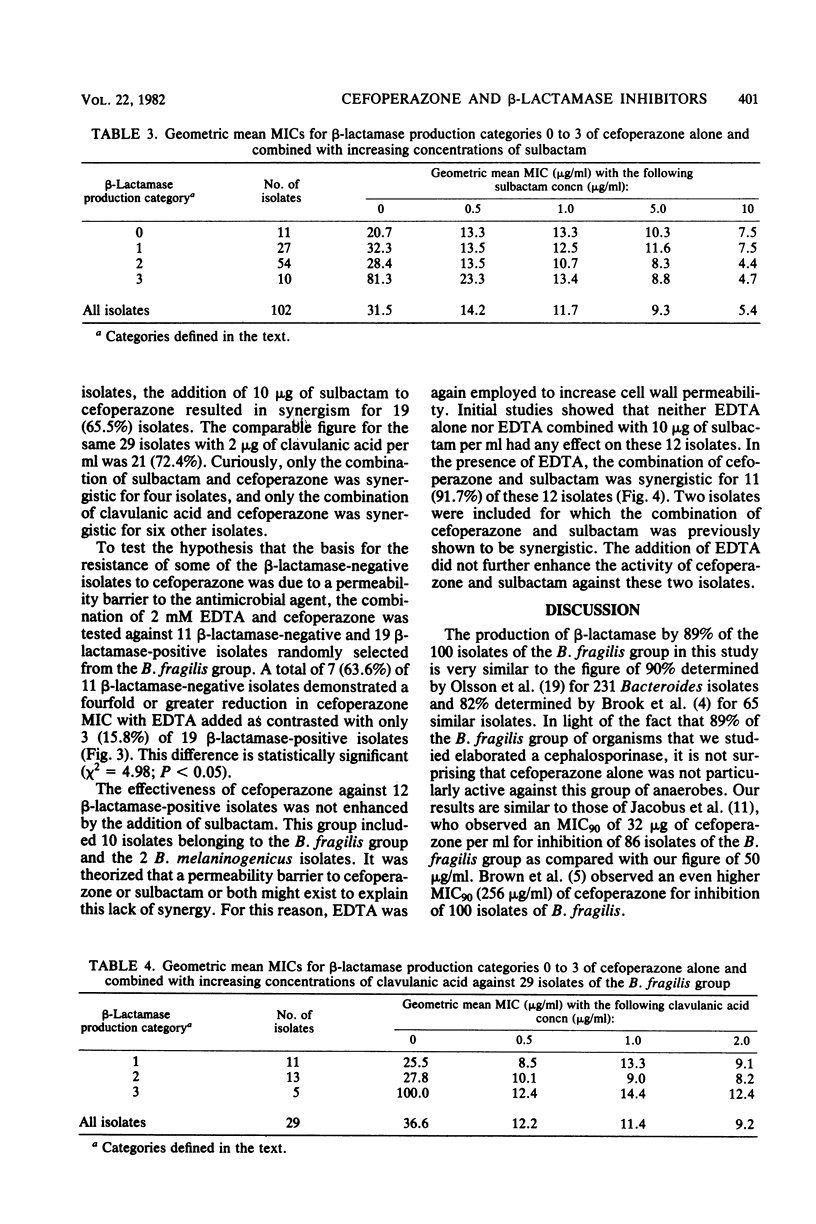
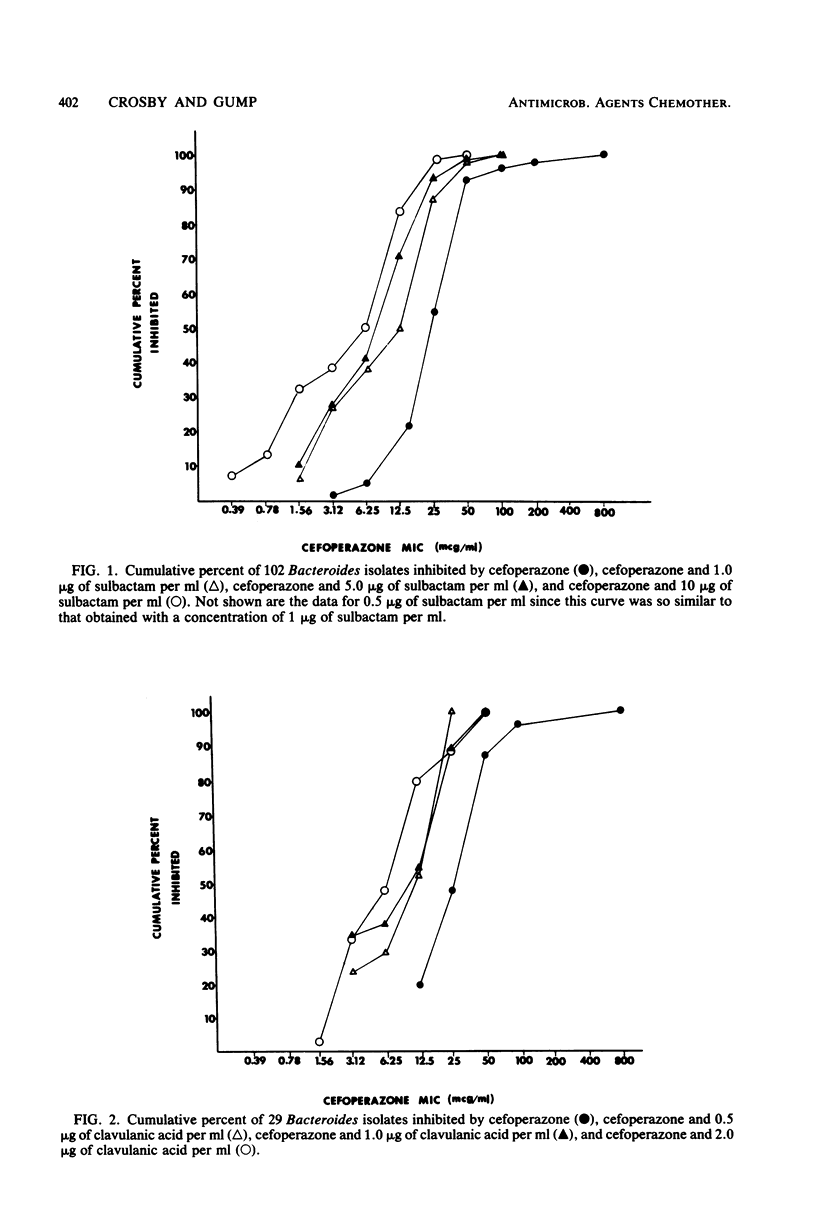
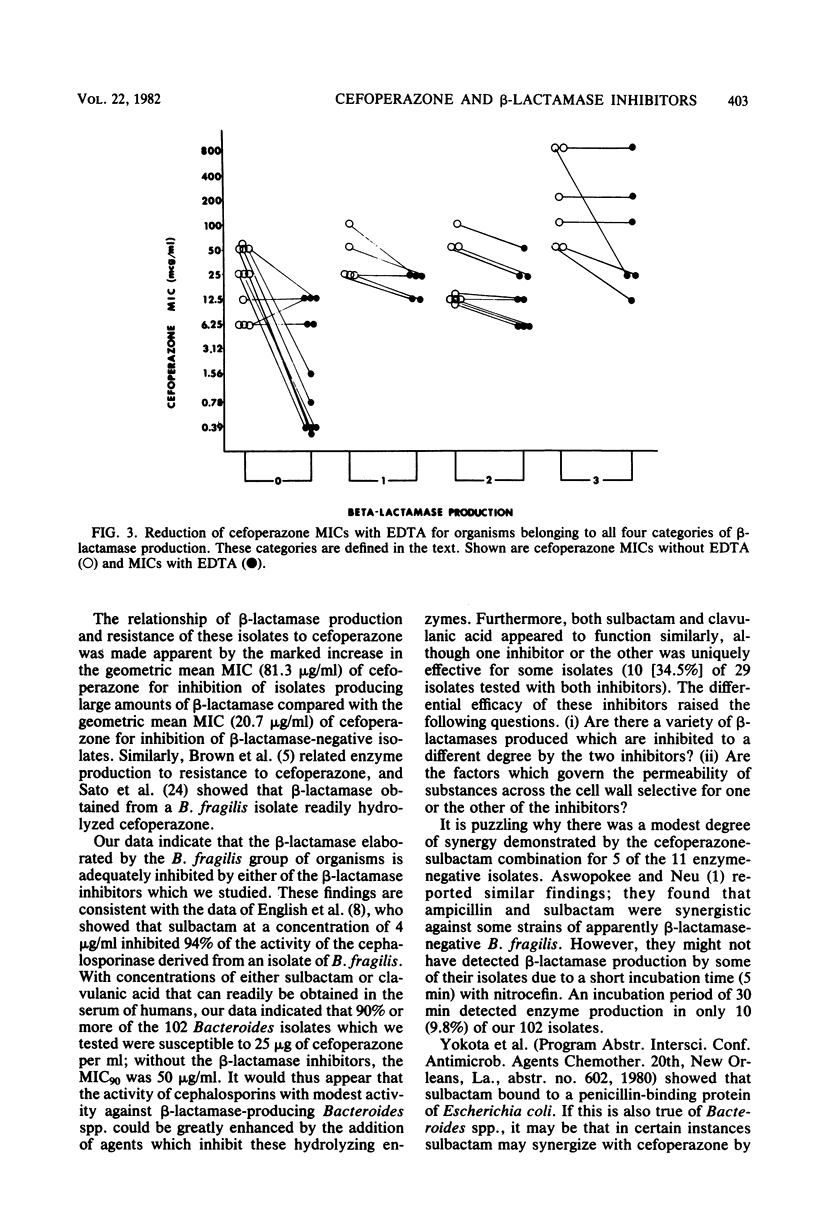
A total of 102 isolates of Bacteroides spp. were studied for beta-lactamase production and susceptibility to cefoperazone alone or in combination with either of the beta-lactamase inhibitors sulbactam and clavulanic acid. The geometric mean minimal inhibitory concentration of cefoperazone alone was 31.5 micrograms/ml and when combined with 10 micrograms of sulbactam per ml or 2 micrograms of clavulanic acid per ml was reduced to 5.4 and 9.2 micrograms/ml, respectively. When bacterial suspensions were tested for beta-lactamase production with nitrocefin, 91 (89.2%) of these isolates produced the enzyme. The geometric mean minimal inhibitory concentrations of cefoperazone rose only slightly for isolates with low or intermediate enzyme activity but rose significantly for those with high activity. The addition of EDTA to cefoperazone significantly more frequently enhanced the activity of cefoperazone against beta-lactamase-negative as opposed to beta-lactamase-positive isolates. Furthermore, EDTA resulted in synergistic activity of the cefoperazone-sulbactam combination on beta-lactamase-positive isolates for which the combination had previously not shown a synergistic effect. This study demonstrates the relationship between beta-lactamase production and the resistance of Bacteroides spp. to cefoperazone and shows that inhibition of these enzymes can reverse this resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswapokee N., Neu H. C. A sulfone beta-lactam compound which acts as a beta-lactamase inhibitor. J Antibiot (Tokyo) 1978 Dec;31(12):1238–1244. doi: 10.7164/antibiotics.31.1238. [DOI] [PubMed] [Google Scholar]
- Bradley T. J., Wyatt J. Penicillin resistance in Staphylococcus aureus 893 not mediated by -lactamase. J Pharm Pharmacol. 1972 Dec;24(Suppl):25P–29P. [PubMed] [Google Scholar]
- Brook I., Calhoun L., Yocum P. Beta-lactamase-producing isolates of Bacteroides species from children. Antimicrob Agents Chemother. 1980 Jul;18(1):164–166. doi: 10.1128/aac.18.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Del Bene V. E., Collins C. D. In vitro activity of N-formimidoyl thienamycin, moxalactam, and other new beta-lactam agents against Bacteroides fragilis: contribution of beta-lactamase to resistance. Antimicrob Agents Chemother. 1981 Feb;19(2):248–252. doi: 10.1128/aac.19.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. 'Beta-lactams' as beta-lactamase inhibitors. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):207–223. doi: 10.1098/rstb.1980.0039. [DOI] [PubMed] [Google Scholar]
- Del Bene V. E., Farrar W. E., Jr Cephalosporinase activity in Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Mar;3(3):369–372. doi: 10.1128/aac.3.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Girard A. E., Lynch J. E., Barth W. E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978 Sep;14(3):414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay E. L., Sutter V. L., Finegold S. M. Rapid beta-lactamase testing in bacteroides. J Antimicrob Chemother. 1981 Nov;8(5):413–416. doi: 10.1093/jac/8.5.413. [DOI] [PubMed] [Google Scholar]
- Jacobus N. V., Tally F. P., Barza M., Gorbach S. L. Susceptibility of anaerobic bacteria to cefoperazone and other beta-lactam antibiotics. Clin Ther. 1980;3(Spec Issue):34–38. [PubMed] [Google Scholar]
- Kammer R. B., Preston D. A., Turner J. R., Hawley L. C. Rapid detection of ampicillin-resistant Haemophilus influenzae and their susceptibility to sixteen antibiotics. Antimicrob Agents Chemother. 1975 Jul;8(1):91–94. doi: 10.1128/aac.8.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Nord C. E., Olsson B., Dornbusch K. beta-lactamases in bacteroides. Scand J Infect Dis Suppl. 1978;(13):27–32. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson-Liljequist B., Dornbusch K., Nord C. E. Characterization of three different beta-lactamases from the Bacteroides fragilis group. Antimicrob Agents Chemother. 1980 Aug;18(2):220–225. doi: 10.1128/aac.18.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Dornbusch K., Nord C. E. Factors contributing to resistance to beta-lactam antibiotics in Bacteroides fragilis. Antimicrob Agents Chemother. 1979 Feb;15(2):263–268. doi: 10.1128/aac.15.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Dornbusch K., Nord C. E. Susceptibility to beta-lactam antibiotics and production of beta-lactamase in Bacteroides fragilis. Med Microbiol Immunol. 1977 Oct 7;163(3):183–194. doi: 10.1007/BF02126677. [DOI] [PubMed] [Google Scholar]
- Pechère J. C., Guay R., Dubois J., Letarte R. Hydrolysis of Cefotaxime by a beta-lactamase from Bacteroides fragilis. Antimicrob Agents Chemother. 1980 Jun;17(6):1001–1003. doi: 10.1128/aac.17.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Factors influencing the antibacterial action of beta-lactam antibiotics. J Antimicrob Chemother. 1978 Jul;4(B):1–14. doi: 10.1093/jac/4.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Sato K., Inoue M., Mitsuhashi S. Activity of beta-lactamase produced by Bacteroides fragilis against newly introduced cephalosporins. Antimicrob Agents Chemother. 1980 Apr;17(4):736–737. doi: 10.1128/aac.17.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timewell R., Taylor E., Phillips I. The beta-lactamases of Bacteroides species. J Antimicrob Chemother. 1981 Feb;7(2):137–146. doi: 10.1093/jac/7.2.137. [DOI] [PubMed] [Google Scholar]
- Voll M. J., Leive L. Release of lipopolysaccharide in Escherichia coli resistant to the permeability increase induced by ethylenediaminetetraacetate. J Biol Chem. 1970 Mar 10;245(5):1108–1114. [PubMed] [Google Scholar]
- Weiser R., Asscher A. W., Wimpenny J. In vitro reversal of antibiotic resistance by ethylenediamine tetraacetic acid. Nature. 1968 Sep 28;219(5161):1365–1366. doi: 10.1038/2191365a0. [DOI] [PubMed] [Google Scholar]



