Abstract
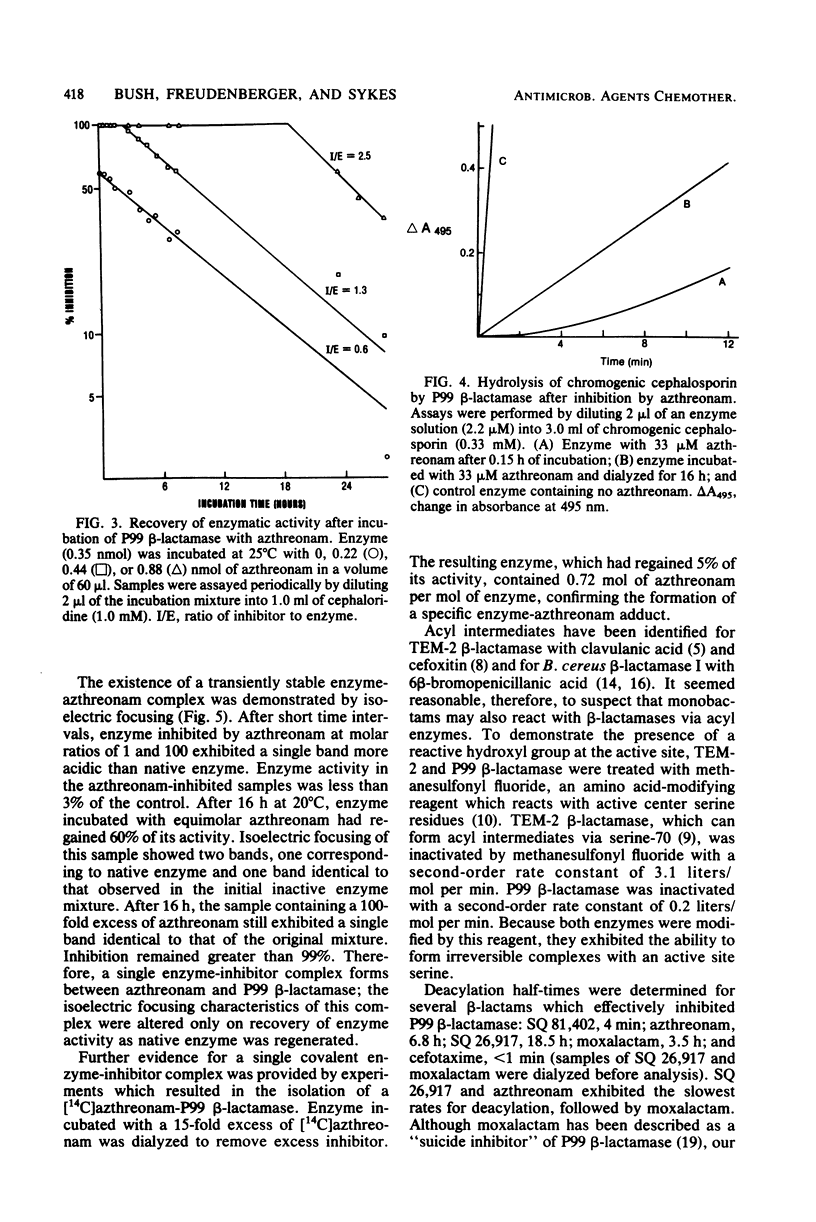
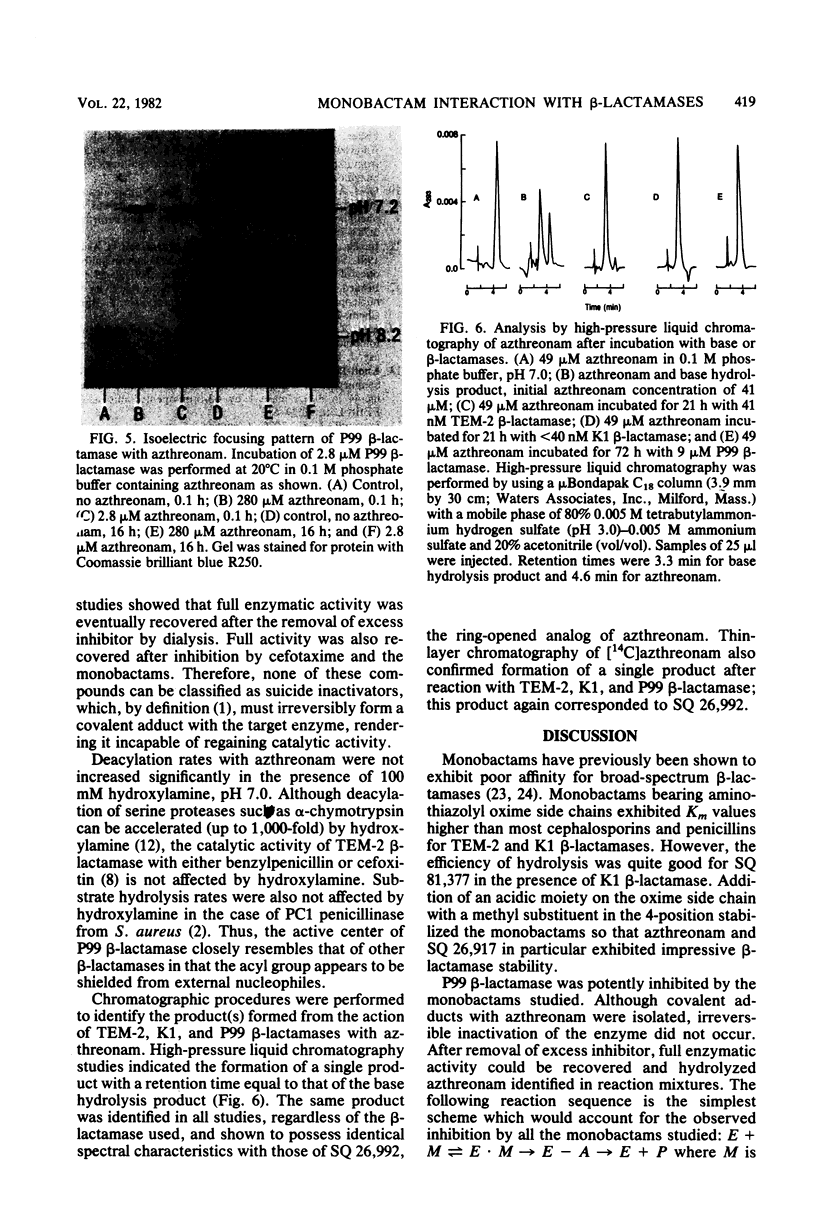
Monobactams containing 3 beta-aminothiazolyl oxime side chains (SQ 81,377, SQ 81,402, azthreonam, and SQ 26,917) have poor affinities for the broad-spectrum beta-lactamases TEM-2 and K1. Addition of a 4-methyl substituent significantly increased stability to hydrolysis by these enzymes. P99 cephalosporinase from Enterobacter cloacae was strongly inhibited by the monobactams. Interaction of azthreonam with the P99 enzyme in equimolar concentrations resulted in a single covalent complex which retained less than 3% catalytic activity. On incubation, enzymatic activity was slowly regained. Chromatographic studies of the incubation mixtures revealed the presence of a single ring-opened product. It is concluded that monobactams act as poor substrates for broad-spectrum beta-lactamases and tight-binding competitive substrates for the P99 beta-lactamase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. G., Pratt R. F. Pre-steady state beta-lactamase kinetics. Observation of a covalent intermediate during turnover of a fluorescent cephalosporin by the beta-lactamase of STaphylococcus aureus PC1. J Biol Chem. 1981 Nov 25;256(22):11401–11404. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruderlein H., Daniel R., Perras D., DiPaola A., Fideliá A., Belleau B. An investigation of the destruction of the beta-lactam ring of penems by the albumin drug-binding site. Can J Biochem. 1981 Oct;59(10):857–866. doi: 10.1139/o81-118. [DOI] [PubMed] [Google Scholar]
- Charnas R. L., Knowles J. R. Inactivation of RTEM beta-lactamase from Escherichia coli by clavulanic acid and 9-deoxyclavulanic acid. Biochemistry. 1981 May 26;20(11):3214–3219. doi: 10.1021/bi00514a035. [DOI] [PubMed] [Google Scholar]
- Dixon M. The graphical determination of K m and K i . Biochem J. 1972 Aug;129(1):197–202. doi: 10.1042/bj1290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Bradley S. M., Knowles J. R. Inactivation of the RTEM beta-lactamase from Escherichia coli. Interaction of penam sulfones with enzyme. Biochemistry. 1981 May 12;20(10):2726–2731. doi: 10.1021/bi00513a004. [DOI] [PubMed] [Google Scholar]
- GOLD A. M., FAHRNEY D. SULFONYL FLUORIDES AS INHIBITORS OF ESTERASES. II. FORMATION AND REACTIONS OF PHENYLMETHANESULFONYL ALPHA-CHYMOTRYPSIN. Biochemistry. 1964 Jun;3:783–791. doi: 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]
- INWARD P. W., JENCKS W. P. THE REACTIVITY OF NUCLEOPHILIC REAGENTS WITH FUROYL-CHYMOTRYPSIN. J Biol Chem. 1965 May;240:1986–1996. [PubMed] [Google Scholar]
- Imada A., Kitano K., Kintaka K., Muroi M., Asai M. Sulfazecin and isosulfazecin, novel beta-lactam antibiotics of bacterial origin. Nature. 1981 Feb 12;289(5798):590–591. doi: 10.1038/289590a0. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loosemore M. J., Cohen S. A., Pratt R. F. Inactivation of Bacillus cereus beta-lactamase I by 6 beta-bromopenicillanic acid: kinetics. Biochemistry. 1980 Aug 19;19(17):3990–3995. doi: 10.1021/bi00558a016. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Pratt R. F., Anderson E. G., Odeh I. Certain monocyclic beta-lactams are beta-lactamase substrates: nocardicin A and desthiobenzylpenicillin. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1266–1273. doi: 10.1016/0006-291x(80)90626-9. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. The beta-lactamase stability of a novel beta-lactam antibiotic containing a 7 alpha-methoxyoxacephem nucleus. J Antimicrob Chemother. 1980 Jul;6(4):445–453. doi: 10.1093/jac/6.4.445. [DOI] [PubMed] [Google Scholar]
- Ross G. W., Boulton M. G. Purification of beta-lactamases on QAE-sephadex. Biochim Biophys Acta. 1973 Jun 6;309(2):430–439. doi: 10.1016/0005-2744(73)90041-7. [DOI] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H., Wells J. S. Monobactams--monocyclic beta-lactam antibiotics produced by bacteria. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):1–16. doi: 10.1093/jac/8.suppl_e.1. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Cimarusti C. M., Bonner D. P., Bush K., Floyd D. M., Georgopapadakou N. H., Koster W. M., Liu W. C., Parker W. L., Principe P. A. Monocyclic beta-lactam antibiotics produced by bacteria. Nature. 1981 Jun 11;291(5815):489–491. doi: 10.1038/291489a0. [DOI] [PubMed] [Google Scholar]
- Wells J. S., Trejo W. H., Principe P. A., Bush K., Georgopapadakou N., Bonner D. P., Sykes R. B. EM5400, a family of monobactam antibiotics produced by Agrobacterium radiobacter. I. Taxonomy, fermentation and biological properties. J Antibiot (Tokyo) 1982 Mar;35(3):295–299. doi: 10.7164/antibiotics.35.295. [DOI] [PubMed] [Google Scholar]