Abstract
The members of the chimaerin family of Rac-GTPase-activating proteins possess a single C1 domain with high homology to those present in protein kinase C (PKC) isozymes. This domain in PKCs is involved in phorbol ester and diacylglycerol (DAG) binding. We previously have demonstrated that one of the chimaerin isoforms, β2-chimaerin, binds phorbol esters with high affinity. In this study we analyzed the properties of β2-chimaerin as a DAG receptor by using a series of conformationally constrained cyclic DAG analogues (DAG lactones) as probes. We identified analogs that bind to β2-chimaerin with more than 100-fold higher affinity than 1-oleoyl-2-acetylglycerol. The potencies of these analogs approach those of the potent phorbol ester tumor promoters. The different DAG lactones show some selectivity for this novel receptor compared with PKCα. Cellular studies revealed that these DAG analogs induce translocation of β2-chimaerin from cytosolic (soluble) to particulate fractions. Using green fluorescent protein-fusion proteins for β2-chimaerin we determined that this novel receptor translocates to the perinuclear region after treatment with DAG lactones. Binding and translocation were prevented by mutation of the conserved Cys-246 in the C1 domain. The structural homology between the C1 domain of β2-chimaerin and the C1b domain of PKCδ also was confirmed by modeling analysis. Our results demonstrate that β2-chimaerin is a high affinity receptor for DAG through binding to its C1 domain and supports the emerging concept that multiple pathways transduce signaling through DAG and the phorbol esters.
Signaling in response to the second messenger diacylgycerol (DAG) is thought to proceed through the activation of protein kinase C (PKC) isozymes (1, 2). Binding of this lipid second messenger and its related analogs, the phorbol esters, occurs at the C1 domains (also called cysteine-rich regions or zinc fingers) present in the classical PKCs (PKCα, βI, βII, and γ) and novel PKCs (PKCδ, ɛ, η, and θ). This 50- to 51-aa domain, which is present in tandem in these PKC isozymes, possesses the motif HX12CX2CXnCX2CX4HX2CX7C, where H is histidine, C is cysteine, X is any other amino acid, and n is 13–14 (3–6). The phorbol ester receptor family has expanded with the discovery of the chimaerins. Unlike PKCs, the chimaerins do not possess a functional kinase domain but they are GTPase-activating proteins for Rac, a small GTP binding protein of the Ras superfamily (7). Four chimaerin isoforms (α1- or n-, α2-, β1-, and β2-chimaerin) have been identified to date, all of them possessing a single C1 domain with approximately 40% homology to those present in PKCs (7–10). It is therefore predictable that the biological responses of the phorbol esters and those mediated by DAG signaling could involve the activation of PKC-independent pathways.
We previously have reported that α1- and β2-chimaerin are indeed high affinity receptors for the phorbol esters and also for the bryostatins, macrocyclic lactones with antitumor properties (11, 12). Like PKC isozymes, β2-chimaerin expressed in Sf9 cells binds [3H]phorbol 12,13-dibutyrate ([3H]PDBu) with high affinity in a phospholipid-dependent manner. Interestingly, structure-activity analysis using a series of phorbol ester analogs revealed a unique pattern of ligand recognition for β2-chimaerin. While the tumor promoter thymeleatoxin (a mezerein analog) is approximately 60 times less potent for binding to β2-chimaerin than to PKCα, the indolactam analogs did not show significant differences in binding between the two receptor classes (12). It is likely that different residues within the C1 domains are involved in ligand binding interaction in each receptor class, and that other structural elements within the receptors should further modify these interactions. Although the pharmacological interaction of DAGs with PKC isozymes has been widely studied, the properties of chimaerins as receptors for DAGs have not been examined to date.
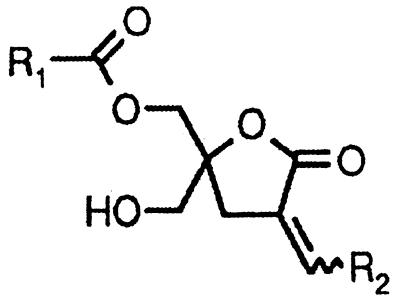
DAGs possess substantially lower potency for binding to PKCs and reduced metabolic stability compared with their corresponding phorbol ester analogs (13). A strategy that has generated novel, potent DAG analogs is to impose conformational rigidity of the glycerol backbone by constraining it into a lactone ring (14). The concept is to identify rigid rotamers that would approximate the actual conformation of the physiologically active DAG. Cyclic pentonolactones represent the most suitable structures generated so far and have proved to be potent PKC ligands and activators (15–18). Structural analysis of isolated C1 domains of PKCs using NMR techniques and x-ray crystallography, together with extensive mutagenesis studies, have provided essential information on the receptor-ligand interaction (19–21). According to modeling studies (22), two energetically equivalent binding modes (sn-1 and sn-2) for cyclic DAG lactones‡‡ have been determined. In addition to the importance of hydrogen bonds, modeling studies have revealed potential hydrophobic contacts (unpublished work), which may include Met-239, Phe-243, Leu-250, Trp-252, and Val-255 in the C1b domain of PKCδ (positions 9, 13, 20, 22, and 25 in the motif, respectively). Analysis of the sn-1 and sn-2 binding modes to optimize those hydrophobic interactions provided evidence that the addition of branched chains attached to the carbonyl group (acyl branching) or to the lactone group (α-alkylidene branching) would improve such hydrophobic contacts (see structure in Table 1). In fact, these branched DAG analogs show further enhancement in potency as PKC ligands and as PKC activators in vitro and in cellular systems (unpublished work).
Table 1.
Binding affinities of DAG lactones to β2-chimaerin
A fixed concentration of [3H]PDBu (5 nM) and increasing concentrations (in triplicate) of the competing ligand were used in each binding assay. The ID50 values were determined from the competition curves and the corresponding Ki values were calculated as described in Materials and Methods. Values are expressed as the mean ± SE of the number of experiments in parentheses. ND, not determined.
In this study we took advantage of these branched DAG lactones to demonstrate that β2-chimaerin is a high affinity DAG receptor. These compounds were as potent as the phorbol esters for binding to β2-chimaerin, with affinities in the low nM range. The observation that DAG lactones also induce translocation of β2-chimaerin in cells strongly suggests that this Rac-GTPase-activating protein is a novel cellular receptor for DAG.
Materials and Methods
Materials.
[3H]PDBu was obtained from NEN Life Science Products. Phorbol 12-myristate 13-acetate (PMA), 4α-PMA, and GF 109203X were purchased from Alexis (San Diego, CA). Cell culture reagents and media were obtained from Life Technologies (Grand Island, NY).
Expression and Purification of Recombinant β2-Chimaerin.
β2-chimaerin was expressed in Sf9 insect cells, as described (12). Coomassie blue staining of purified protein revealed >90% purity. PKCα was expressed in Sf9 insect cells and purified as described (23).
Cell Culture.
COS-1 cells were cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere.
[3H]PDBu Binding and Competition Assays.
[3H]PDBu binding was measured by using the polyethylene glycol precipitation assay developed by Sharkey and Blumberg (24), using 1 mM EGTA and 100 μg/ml phosphatidylserine. The assay was carried out at 18°C for 30 min. To measure competition of [3H]PDBu binding by different analogs, we used a fixed concentration of [3H]PDBu (5 nM) and 5–7 increasing concentrations of the competing nonradioactive ligand. ID50 values were determined from the competition curve, and the Ki for the competing ligand was calculated from the ID50 by using the relationship Ki = ID50/(1 + L/Kd), where L is the concentration of free [3H]PDBu at the ID50. Binding using total lysates was carried out by using 15 nM [3H]PDBu. COS-1 cells were lysed in 1 ml of lysis buffer (see below). Fifty microliters per tube of cell lysate (in triplicate) was used in the assay, as described (12).
Expression of β2-Chimaerin in COS-1 Cells and Subcellular Fractionation.
A mammalian expression vector for β2-chimaerin in pCR3ɛ (a modified version of pCR3, Invitrogen) was generated (12). The resulting vector, pCR3ɛ-β2-chimaerin, encodes for an epitope-tagged (ɛ-tag) protein that can be detected with an anti-PKCɛ antibody (Life Technologies). pCR3ɛ-β2-chimaerin (1 μg) was transfected into COS-1 cells in 6-well plates by using Lipofectamine (Life Technologies) according to the manufacturer’s protocol. Forty-eight hours after transfection, cells were treated with DAG analogs for different times. Experiments were performed in the presence of the PKC inhibitor GF 109203X (5 μM), added 30 min before and during the incubation with DAGs, as described (12). DAG analogs were dissolved in DMSO, and the final concentration of DMSO in the medium was 1%. This concentration of DMSO did not affect expression and/or localization of β2-chimaerin or PKC isozymes.
After treatment with DAG analogs, cells were harvested into lysis buffer (20 mM Tris⋅HCl, pH 7.4/5 mM EGTA/5 μg/ml 4-(2-aminoethyl)benzenesulfonyl fluoride/5 μg/ml leupeptin/5 μg/ml aprotinin/1 μg/ml pepstatin A) and lysed by sonication. Separation of cytosolic (soluble) and particulate fractions was performed by ultracentrifugation as described (12, 25). Equal amounts of protein for each fraction were subjected to SDS/PAGE and transferred to nitrocellulose membranes. Membranes were immunostained with the anti-ɛ-tag antibody (12), an anti-β2-chimaerin antibody, or an anti-PKCα antibody (Upstate Biotechnology). Densitometric analysis was performed under conditions that yielded a linear response and analyzed with a Scanner Control, version 1.00 (Molecular Dynamics).
Generation of a C1 Domain Mutated Form of β2-Chimaerin.
The C1 domain mutant C246A-β2-chimaerin was constructed by site-directed mutagenesis using the U.S.E. mutagenesis system from Amersham Pharmacia Biotech. pCR3ɛ-β2-chimaerin was used as a template. Replacement of Cys-246 by Ala was achieved with the primer CGGTGCTCAGACGCTGGATTGAACGTA (codon underlined in the primer). The resulting plasmid (pCR3ɛ-C246A-β2-chimaerin) was sequenced and the mutation was confirmed.
Generation of Green Fluorescence Protein (GFP) Constructs and Analysis of β2-Chimaerin Localization.
A 1.4-kb EcoRI–EcoRI fragment was isolated from pCRII-β2-chimaerin (12) and subcloned in-frame into the GFP plasmid pEGFP-C3 (CLONTECH). For C246A-β2-chimaerin, a 1.4-kb XhoI–EcoRI fragment was isolated from pCR3ɛ-C246-β2-chimaerin and subcloned in-frame into pEGFP-C2 (CLONTECH). The resulting pEGFP-β2-chimaerin and pEGFP-C246A-β2-chimaerin constructs were confirmed by sequencing. COS-1 cells were transfected with the GFP-expression vectors (1 μg) by using FuGENE (Boehringer Mannheim), according to the manufacturer’s protocol. After 48 h, cells were treated with DAG analogs and fixed with 3.7% formaldehyde. Photomicrographs were taken with an Olympus fluorescent microscope.
Modeling Studies.
The initial three-dimensional structure of the C1 domain of β2-chimaerin was modeled by using a homology modeling program modeller (26), based on the x-ray structure of the C1b domain of PKCδ (20). The structure was further refined by using the charmm program (27) through energy minimization and 200-ps molecular dynamics simulation in water, which was used in computational docking studies. For the docking studies of PKC ligands, the autodock program (28) was used. A total of 100 autodock runs were performed for each ligand to obtain statistically significant binding modes. In the docking experiments, the flexibility of the ligand was fully taken into account while the receptor was kept rigid.
Results
Binding of DAG Analogs to β2-Chimaerin.
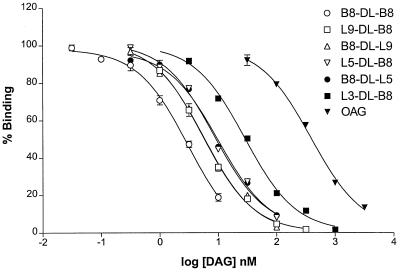
Using β2-chimaerin expressed and purified from Sf9 insect cells, we previously have determined that this protein is a high affinity phorbol ester receptor in vitro. Scatchard plot analysis using [3H]PDBu as a radioligand revealed a Kd of 1.9 ± 0.2 nM for β2-chimaerin in the presence of phosphatidylserine vesicles (12). To analyze the properties of β2-chimaerin as a receptor for DAGs, we used two series of conformationally constrained DAG lactones. The first series has an acyl chain of variable length in R1 and a branched 4-methyl-3-(methylethyl)pentylidene [(CH2CH(i-Pr)2] chain at R2. The second series has the side chains at R1 and R2 reversed (Table 1). Design and synthesis of the branched lactones will be described elsewhere (unpublished work). Competition analysis using [3H]PDBu and increasing concentrations of the branched DAG lactones shows that these compounds are high affinity ligands for β2-chimaerin (Table 1 and Fig. 1). As was previously observed for PKCα, lower Ki values (better affinities) were achieved with increasing length of the acyl chain, although binding affinities reach a plateau beyond an optimal chain length. Interchange of R1 and R2 did not substantially affect the binding affinities for β2-chimaerin. Interestingly, a DAG lactone with branched chains at both positions (B8-DL-B8) proved to be the most potent compound (Ki = 0.9 ± 0.1 nM, n = 10), which is approximately 160 times more potent than the widely used 1-oleoyl-2-acetylglycerol, an acyclic DAG. It is remarkable that the potencies of these compounds are in the same range as those of the phorbol esters and that the most potent of the compounds achieved a potency below 1 nM. Comparison of the Ki values of the cyclic DAG lactones for β2-chimaerin and PKCα reveals up to 6-fold higher affinities for β2-chimaerin. This finding is in marked contrast with the binding properties of phorbol ester and mezerein analogs, which show up to 60-fold higher affinities for PKCα than for β2-chimaerin (12).
Figure 1.
Binding of DAG analogs to β2-chimaerin. Binding was performed by using a fixed concentration of [3H]PDBu (5 nM) in the presence of 100 μg/ml phosphatidylserine and 1 mM EGTA, and increasing concentrations of nonradioactive DAGs. A representative experiment is shown. The Ki value and the number of experiments for each analog are presented in Table 1. Each point represents the mean of three experimental values, generally with a SE of < 2%. OAG, 1-oleoyl-2-acetylglycerol.
DAG Analogs Induce Translocation of β2-Chimaerin.
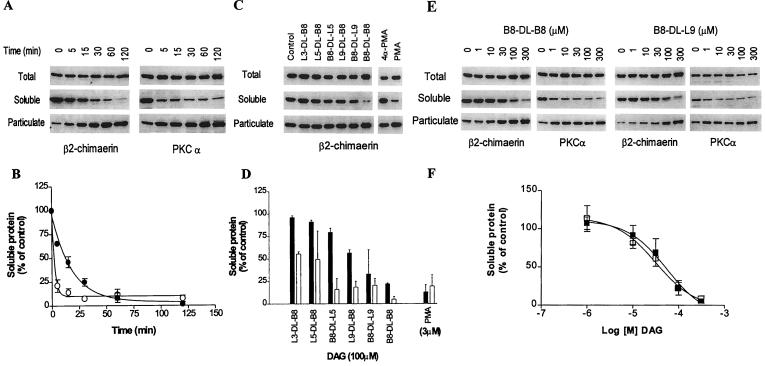
It is well established that phorbol ester treatment or elevation of DAG levels results in the redistribution of classical and novel PKCs from cytosolic to particulate (membrane and cytoskeletal) fractions. We evaluated whether β2-chimaerin also can be translocated by the constrained DAG analogs by using COS-1 cells as a model. To rule out involvement of PKC isozymes in the effect of the DAGs, experiments were performed in the presence of the PKC inhibitor GF 109203X (5 μM), added 30 min before and during the incubation with the DAGs, as described (12). Transfection of COS-1 cells with pCR3ɛ-β2-chimaerin results in a significant increase in the levels of [3H]PDBu binding in cell lysates (12). Treatment of COS-1 cells with the DAG B8-DL-B8 results in a time-dependent translocation of β2-chimaerin from the soluble (cytosolic) to the particulate fraction, with approximately 50% of the protein translocated after 15 min of treatment, as determined by densitometric analysis (Fig. 2 A and B). Similarly, B8-DL-B8 induces translocation of endogenous PKCα from cytosolic to particulate fractions. Studies with the different DAG analogs (100 μM) show a marked difference between potencies for in vitro binding and translocation. Despite the similar binding affinities in vitro, not all the DAG lactones induce translocation of β2-chimaerin in COS-1 cells (Fig. 2 C and D). The most lipophilic ligands seem to be more potent in inducing translocation. A dose-dependence analysis for B8-DL-B8 and B8-DL-L9 revealed ED50 values for translocation of β2-chimaerin of 40 ± 12 μM (n = 3) and 37 ± 11 μM (n = 3), respectively, as determined by densitometry (Fig. 2 E and F). Interestingly, the cyclic DAGs are more potent for inducing translocation of PKCα than β2-chimaerin (Fig. 2 C–F), as was previously observed with phorbol esters (12).
Figure 2.
Translocation of β2-chimaerin by DAG lactones. COS-1 cells were transfected with pCR3ɛ-β2-chimaerin and 48 h later were treated with cyclic DAGs. Soluble and particulate fractions were separated by ultracentrifugation and subjected to Western blot analysis using the anti-ɛ-tag antibody (for ɛ-tagged β2-chimaerin) or anti-PKCα antibody, as indicated. (A, C, and E) Representative Western blots. The molecular mass of β2-chimaerin is 50 kDa and that of PKCα is 80 kDa. (B, D, and F) The densitometric analysis of the immunoreactivity in the soluble fraction for A, C, and E, respectively. Results are expressed as percentage of the values observed in control cells and represent the mean ± SE of three independent experiments. (A and B) Time course of translocation after treatment of COS-1-transfected cells with B8-DL-B8 (100 μM); β2-chimaerin (●); PKCα (○). (C and D) Translocation of β2-chimaerin by different cyclic DAGs (100 μM) after 1 h treatment; β2-chimaerin (solid bars); PKCα (open bars). (E and F) Concentration-dependence of β2-chimaerin translocation after 1 h incubation with B8-DL-B8 (■) or B8-DL-L9 (□).
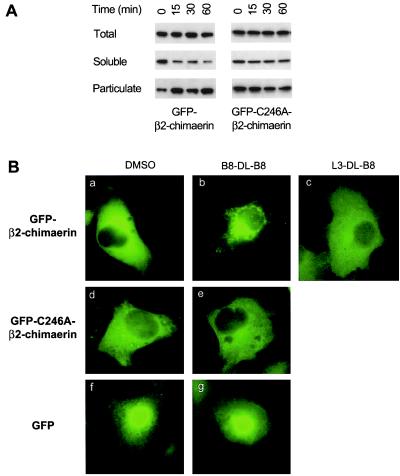
Evaluation of Translocation Using GFP-Tagged β2-Chimaerin.
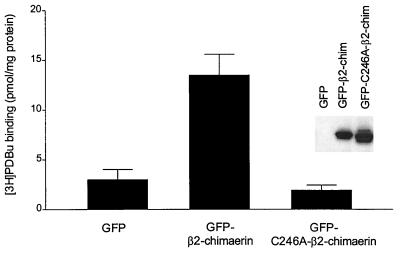
The use of GFP fusion proteins is a valuable tool to study intracellular localization of various proteins, including PKC isozymes (29, 30). To monitor the subcellular distribution of β2-chimaerin, a GFP-tagged vector for β2-chimaerin was constructed. Expression of GFP-β2-chimaerin in COS-1 cells resulted in a marked increase in [3H]PDBu binding levels in total lysates (see Fig. 4), as previously observed with a nonfusion β2-chimaerin protein (12). Western blot analysis using an anti-β2-chimaerin antibody revealed that B8-DL-B8 induced translocation of GFP-β2-chimaerin in COS-1 cells (Fig. 3A, Left), suggesting that, as reported for PKCs and many other proteins, the fusion protein retains the biological functionality and localization of the native protein. Analysis of subcellular localization of GFP-β2-chimaerin by fluorescence microscopy revealed an even cytoplasmic distribution without nuclear staining (Fig. 3Ba). Interestingly, B8-DL-B8 treatment redistributed GFP-β2-chimaerin to a perinuclear region, with the consequent loss of cytoplasmic staining (Fig. 3Bb). A similar subcellular localization was observed after PMA treatment (unpublished work). In agreement with the results by Western blot (Fig. 2C), no translocation of GFP-β2-chimaerin was observed by the DAG L3-DL-B8 (Fig. 3Bc). Immunofluorescence analysis of cells transfected with pCR3,-β2-chimaerin by using the ɛ-tag antibody revealed a similar pattern of translocation as the GFP-fused protein (data not shown), confirming that the GFP tag did not affect localization. Localization of the nonfused GFP protein was not affected by B8-DL-B8 treatment (Fig. 3B f and g).
Figure 4.
Cys246Ala mutation in the C1 domain of β2-chimaerin abolishes [3H]PDBu binding. COS-1 cells were transfected with pEGFP, pEGFP-β2-chimaerin, or pEGFP-C246A-β2-chimaerin. After 48 h binding of [3H]PDBu was measured in total cellular lysates by using 15 nM radioligand, as described in Materials and Methods. Two additional experiments gave similar results. (Inset) Representative Western blot analysis using an anti-β2-chimaerin antibody.
Figure 3.
Effect of cyclic DAG on the subcellular redistribution of GFP-β2-chimaerin and GFP-C246A-β2-chimaerin. (A) COS-1 cells were transfected with pEGFP-β2-chimaerin or pEGFP-C246A-β2-chimaerin. Forty-eight hours later cells were treated with B8-DL-B8 (100 μM) for different times and subjected to subcellular fractionation. Western blot analysis using an anti-β2-chimaerin antibody was performed on soluble and particulate fractions. Two additional experiments gave similar results. (B) COS-1 cells were transfected with pEGFP-β2-chimaerin (a–c), pEGFP-C246A-β2-chimaerin (d and e), or pEGFP (f and g) and treated with the DAG lactones (100 μM, 30 min) or vehicle (DMSO), as indicated in each case. Cells were fixed with 3.7% formaldehyde and visualized by fluorescent microscopy. Similar results were observed in two additional independent experiments.
Mutation in Cys-246 Prevents Translocation of β2-Chimaerin.
Based on structural studies and mutational analysis of the C1 domains in PKC isozymes (19–21), it is predictable that mutation of conserved cysteines in the C1 domain of β2-chimaerin will abrogate phorbol ester/DAG binding and translocation. We generated a GFP-fusion construct for a mutated form of β2-chimaerin, in which Cys in position 246 was replaced by Ala (C246A). Cys-246 in β2-chimaerin corresponds to the third Cys in the second C1 domain of PKCδ, which is involved in the coordination of Zn2+ (20, 21). Expression of the GFP-C246A-β2-chimaerin mutant in COS-1 cells did not result in any measurable increase in [3H]PDBu binding in cellular lysates, as compared with the natural GFP-β2-chimaerin (Fig. 4). Similar results were observed when ɛ-tagged C246A-β2-chimaerin was transfected into COS-1 cells (data not shown). Analysis of translocation by Western blot revealed that GFP-C246A-β2-chimaerin is unresponsive to B8-DL-B8 (Fig. 3A, Right). Likewise, no translocation of GFP-C246A-β2-chimaerin was observed by fluorescence microscopy after B8-DL-B8 treatment (Fig. 3B d and e). This mutated protein is also unresponsive to PMA (unpublished work). Therefore, the C1 domain of β2-chimaerin mediates binding of DAG/phorbol esters and translocation.
Modeling of the C1 Domain of β2-Chimaerin.
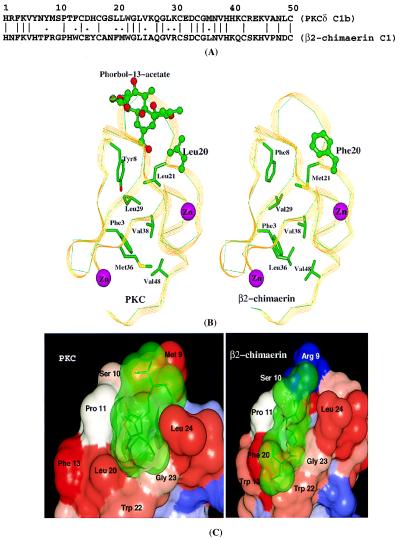
The C1 domain of β2-chimaerin shares 46% amino acid identity and 56% similarity with the C1b domain of PKCδ (Fig. 5A). Our modeling studies using modeller and charmm revealed a quite similar three-dimensional structure for β2-chimaerin C1 and PKCδ C1b domains (Fig. 5B). The Cys and His residues involved in the coordination of the two zinc atoms are absolutely conserved in β2-chimaerin. The residues forming the two hydrophobic cores are highly conserved, with either identical or similar corresponding residues. In PKCδ C1b, residues 7–13 and 21–27 form the phorbol ester binding site (20, 21). Residues in close contact with phorbol esters in PKCδ C1b, namely 8–12, 20–24, and 27 are highly conserved in β2-chimaerin. The importance of several of these amino acids (Pro-11, Leu-20, Leu-21, Trp-22, Leu-24, and Gly-27) in ligand binding was confirmed in our analysis by site-directed mutagenesis of the PKCδ C1b domain (21). Of these amino acids, only one is different in β2-chimaerin (Phe-20). In the C1b domain of PKCδ, Leu-20 interacts directly with the five-member ring of phorbol 13-acetate. It may be possible that the difference in size and shape between these two amino acids is critical in conferring selectivity for different classes of ligands.
Figure 5.
Modeling of the β2-chimaerin C1 domain. (A) Alignment of PKCδ C1b and β2-chimaerin C1 domains. (B) Three-dimensional structure of PKCδ C1b and β2-chimaerin C1 domains. (C) Docking of B8-DL-B8 to the PKCδ C1b and β2-chimaerin C1 domains.
Computational docking studies using autodock were performed for the DAG B8-DL-B8. To validate the docking method used in this study, we first performed docking studies using phorbol 13-acetate and found that the binding mode predicted for this phorbol ester was identical to that determined by x-ray crystallography by Hurley and coworkers (20) (data not shown). Two favorable binding modes, termed sn-1 and sn-2 (22), were predicted for B8-DL-B8. The sn-1 binding mode, which is energetically more favorable, is shown in Fig. 5C. Our docking studies indicate that B8-DL-B8 has strong interactions both with PKCδ C1b and β2-chimaerin C1. Moreover, the binding modes of the DAG lactone to PKCδ C1b and to β2-chimaerin are similar in terms of hydrogen bonding network and hydrophobic interactions.
Discussion
PKC isozymes generally have been viewed as the only receptors for the phorbol esters and the second messenger DAG. The presence of a C1 domain in the chimaerins, with high homology to those present in PKCs, suggested the possibility that these proteins also may bind phorbol esters and DAG. Our pharmacological approach using recombinant β2-chimaerin purified from Sf9 insect cells has unequivocally established that this protein is indeed a high affinity receptor for phorbol esters and DAG. Moreover, like PKC isozymes, β2-chimaerin is subject to intracellular translocation by DAGs and phorbol esters.
Although the overall homology between the C1 domain of β2-chimaerin and PKCs is approximately 40%, molecular modeling reveals that the topology of the β2-chimaerin C1 domain resembles those of PKC isozymes. Mutation of Cys-246 in β2-chimaerin abolishes binding and prevents translocation of β2-chimaerin. According to our modeling analysis, Cys-246 is involved in coordination of Zn2+. Two Zn2+ ions are integral parts of the C1 domain, and mutations in conserved Cys and His residues disrupt the conformation necessary for phorbol ester/DAG binding (19–21, 31, 32).
The successful design of the five-member ring γ-lactone template represents an important step in the rational synthesis of novel DAG analogs (14, 15). An extensive set of studies led to the generation of DAG mimetics with affinities in the μM and nM range (14–18). These minimal structures were refined to optimize their interactions with conserved hydrophobic amino acids within the C1 domains. Previous work highlighted the importance of acyl chains in DAG lactones in the interaction with PKC (18). Substitutions with branched acyl chains in DAG lactones drive binding affinities into the low nM range. Therefore, these simple chemical structures with affinities comparable to those of phorbol esters represent potent pharmacological tools for the study of phorbol ester/DAG receptors. Interestingly, our computational docking studies revealed that DAG lactones have a similar pattern of interaction with both the C1b domain of PKCδ and the C1 domain of β2-chimaerin.
Although the C1 domain of β2-chimaerin undoubtedly fulfills the requirements necessary for ligand binding, it is evident from this and our previous studies that structural differences between β2-chimaerin and other receptor classes confer unique binding properties in each case (5, 11, 12, 23). Marked differences in ligand binding affinities for structurally unrelated analogs exist between PKC isozymes, chimaerin isoforms, and the Caenorhabditis elegans protein Unc-13. Although it is likely that ligands can spatially accommodate into the binding groove of each C1 domain, the basis for specificity probably is related to unique interactions with specific residues within each particular domain. On the other hand, several related proteins having similar C1 motifs (e.g., atypical PKCζ or the protooncogenes Vav and c-Raf), do not bind phorbol esters/DAGs, which suggests that critical residues for the interaction are missing in these proteins (33). It is interesting that, unlike phorbol esters and mezerein analogs, which are selective for PKCα, the DAG lactones were all at least modestly selective for β2-chimaerin. Modeling analysis and mutational studies will be necessary to establish the molecular basis for the interaction of different ligands with each receptor.
The cyclic branched DAG lactone structures represent useful probes for studying phorbol ester receptors in cells. These compounds activate and translocate PKC. Likewise, branched DAG lactones are potent inhibitors of EGF binding to its receptor in NIH 3T3 cells, a PKC-mediated event. Moreover, the B8-DL-B8 displays significant antitumor activity in the NIH 60-cell drug screen, showing a pattern of activity resembling that of the 12-deoxyphorbol ester prostratin (unpublished work). In addition to the PKC effects, cyclic DAGs induce translocation of β2-chimaerin, suggesting that this protein is a cellular DAG receptor. Despite the similar in vitro binding potency, translocation of β2-chimaerin requires higher concentrations of DAGs than does translocation of PKCα. This finding is not surprising because differences in translocation for individual PKC isozymes have been reported in several cellular systems (34). One likely explanation for such differences may be a differential lipid cofactor requirement regulating their association to membranes. The importance of phospholipids or other lipid regulators (such as fatty acids) in controlling the activation and localization of individual PKC isozymes has been demonstrated previously (35, 36). A second factor that might account for the differential translocation of β2-chimaerin and PKCs is the requirement of interacting proteins that regulate subcellular compartmentalization. Individual PKC isozymes are translocated to unique intracellular compartments by phorbol esters (37, 38), and specificity may be driven by their association with isozyme-specific intracellular receptors (e.g., RACKs and others). PKCɛ, for example, specifically translocates to the Golgi apparatus after phorbol ester treatment in cardiac myocytes. Association of PKCɛ to the Golgi is regulated by the coatomer protein β′-COP, which acts as a receptor for this PKC isozyme (38). The unique localization observed for β2-chimaerin after DAG treatment strongly suggests that a similar mechanism may operate for this novel phorbol ester receptor. Interestingly, preliminary experiments show that β2-chimaerin is not translocated to the plasma membrane after stimulation of receptors that increase membrane DAG levels (unpublished data). An attractive possibility is that the localization of this novel phorbol ester receptor is regulated by DAG and/or other lipids generated in intracellular compartments other than the plasma membrane, as also may be the case for PKC isozymes. An alternative explanation for the differential translocation is the nonequivalence among the C1 domains for binding and redistribution (39–41).
In summary, by using a series of cyclic DAG lactones, we established that β2-chimaerin is a novel receptor for DAG. The expansion of the family of DAG/phorbol ester receptors with the discovery of the chimaerins, Unc-13, and Ras-GRP (a novel Ras exchange factor with a single C1 domain), strongly suggests a high degree of complexity in the signaling pathways regulated by DAG signaling. The rational design of selective agonists and antagonists for each receptor class is therefore of critical importance for discerning their cellular functions.
Acknowledgments
This work is supported by Grants RPG-97-092-01-CNE (American Cancer Society) and RO1-CA74197-01 (National Institutes of Health) (to M.G.K.). M.J.C. is a recipient of a fellowship from Ministerio de Educación y Ciencia (Spain). M.L.G.-B. is a recipient of a fellowship from Fundación Ramón Areces (Spain).
Abbreviations
- PKC
protein kinase C
- DAG
diacylglycerol
- PDBu
phorbol 12,13-dibutyrate
- PMA
phorbol 12-myristate 13-acetate
- GFP
green fluorescence protein
Footnotes
Nomenclature of DAG lactones is as follows: (Z)-{1-(hydroxymethyl)-4-[4-methyl-3-(methylethyl)pentylidene]-3-oxo-2-oxolanyl}methyl butanoate (L3-DL-B8), hexanoate (L5-DL-B8) and decanoate (L9-DL-B8); (Z)-[4-X-1-(hydroxymethyl)-3-oxo-2-oxolanyl]methyl 4 methyl-3-(methylethyl)pentanoate (X = Butylidene, B8-DL-L3; X = Hexylidene, B8-DL-L5; X = decylidene, B8-DL-L9); (Z)-{1-(hydroxymethyl)-4-[4-methyl-3-(methylethyl)pentylidene]-3-oxo-2-oxolanyl}methyl 4-methyl-3-(methylethyl)pentanoate (B8-DL-B8).
References
- 1.Nishizuka Y. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 2.Newton A C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 3.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. Proc Natl Acad Sci USA. 1989;86:4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quest A F, Bardes E S, Bell R M. J Biol Chem. 1994;269:2961–2970. [PubMed] [Google Scholar]
- 5.Kazanietz M G, Barchi J J, Jr, Omichinski J G, Blumberg P M. J Biol Chem. 1995;270:14679–14684. doi: 10.1074/jbc.270.24.14679. [DOI] [PubMed] [Google Scholar]
- 6.Hurley J H, Newton A C, Parker P J, Blumberg P M, Nishizuka Y. Protein Sci. 1997;6:477–480. doi: 10.1002/pro.5560060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S, Kozma R, Monfries C, Hall C, Lim H H, Smith P, Lim L. Biochem J. 1990;272:767–773. doi: 10.1042/bj2720767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall C, Sin W C, Teo M, Michael G J, Smith P, Dong J M, Lim H H, Manser E, Spurr N K, Jones T A, Lim L. Mol Cell Biol. 1993;13:4986–4998. doi: 10.1128/mcb.13.8.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung T, How B-E, Manser E, Lim L. J Biol Chem. 1993;268:3813–3816. [PubMed] [Google Scholar]
- 10.Leung T, How B-E, Manser E, Lim L. J Biol Chem. 1994;269:12888–12892. [PubMed] [Google Scholar]
- 11.Areces L B, Kazanietz M G, Blumberg P M. J Biol Chem. 1994;269:19553–19558. [PubMed] [Google Scholar]
- 12.Caloca M J, Fernandez N, Lewin N E, Ching D, Modali R, Blumberg P M, Kazanietz M G. J Biol Chem. 1997;272:26488–26496. doi: 10.1074/jbc.272.42.26488. [DOI] [PubMed] [Google Scholar]
- 13.Nishizuka Y. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 14.Teng K, Marquez V E, Milne G W A, Barchi J J, Kazanietz M G, Lewin N E, Blumberg P M, Abushanab E. J Am Chem Soc. 1992;114:1059–1070. [Google Scholar]
- 15.Lee J, Marquez V E, Blumberg P M, Kazanietz M G. Biorg Med Chem. 1993;1:119–123. doi: 10.1016/s0968-0896(00)82109-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Marquez V E, Lewin N E, Kazanietz M G, Bahador A, Blumberg P M. Biorg Med Chem Lett. 1993;3:1101–1106. [Google Scholar]
- 17.Lee J, Marquez V E, Lewin N E, Bahador A, Kazanietz M G, Blumberg P M. Biorg Med Chem Lett. 1993;3:1107–1110. [Google Scholar]
- 18.Marquez V E, Lee J, Sharma R, Teng K, Wang S, Lewin N E, Bahador A, Kazanietz M G, Blumberg P M. Biorg Med Chem Lett. 1994;4:355–360. [Google Scholar]
- 19.Hommel U, Zurini M, Luyten M. Nat Struct Biol. 1994;1:383–387. doi: 10.1038/nsb0694-383. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Kazanietz M G, Blumberg P M, Hurley J H. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 21.Kazanietz M G, Wang S, Milne G W A, Lewin N E, Liu H L, Blumberg P M. J Biol Chem. 1995;270:21852–21859. doi: 10.1074/jbc.270.37.21852. [DOI] [PubMed] [Google Scholar]
- 22.Benzaria S, Bienfait B, Nacro K, Wang S, Lewin N E, Beheshti M, Blumberg P M, Marquez V E. Biorg Med Chem Lett. 1998;8:3403–3408. doi: 10.1016/s0960-894x(98)00614-3. [DOI] [PubMed] [Google Scholar]
- 23.Kazanietz M G, Areces L B, Bahador A, Mischak H, Goodnight J, Mushinski J F, Blumberg P M. Mol Pharmacol. 1993;44:298–307. [PubMed] [Google Scholar]
- 24.Sharkey N A, Blumberg P M. Cancer Res. 1985;45:19–24. [PubMed] [Google Scholar]
- 25.Szallasi Z, Smith C B, Pettit G R, Blumberg P M. J Biol Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- 26.Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Proteins Struct Funct Genet. 1995;23:318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- 27.Brooks B R, Bruccoleri R E, Olafson B D, States D J, Swaminathan S, Karplus M. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 28.Goodsell D S, Morris G M, Olson A J. J Mol Recognit. 1996;9:1–5. doi: 10.1002/(sici)1099-1352(199601)9:1<1::aid-jmr241>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Ohmori S, Shirai Y, Sakai N, Fujii M, Konishi H, Kikkawa U, Saito N. Mol Cell Biol. 1998;18:5263–5271. doi: 10.1128/mcb.18.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng X, Zhang J, Barak L S, Meyer T, Caron M G, Hannun Y A. J Biol Chem. 1998;273:10755–10762. doi: 10.1074/jbc.273.17.10755. [DOI] [PubMed] [Google Scholar]
- 31.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. Proc Natl Acad Sci USA. 1989;86:4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichikawa S, Hatanaka H, Takeuchi Y, Ohno S, Inagaki F. J Biochem. 1995;117:566–574. doi: 10.1093/oxfordjournals.jbchem.a124745. [DOI] [PubMed] [Google Scholar]
- 33.Kazanietz M G, Bustelo X R, Barbacid M, Mischak H, Kolch W, Wong G, Pettit G R, Bruns J D, Blumberg P M. J Biol Chem. 1994;269:11590–11594. [PubMed] [Google Scholar]
- 34.Szallasi Z, Kosa K, Smith C B, Dlugosz A A, Williams E K, Yuspa S H, Blumberg P M. Mol Pharmacol. 1995;47:258–265. [PubMed] [Google Scholar]
- 35.Shinomura T, Asaoka Y, Oka M, Yoshida K, Nishizuka Y. Proc Natl Acad Sci USA. 1991;88:5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barry O P, Kazanietz M G, Pratico D, FitzGerald G A. J Biol Chem. 1999;274:7545–7556. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- 37.Murray N R, Burns D J, Fields A P. J Biol Chem. 1994;269:21385–21390. [PubMed] [Google Scholar]
- 38.Csukai M, Chen C H, De Matteis M A, Mochly-Rosen D. J Biol Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- 39.Slater S J, Ho C, Kelly M B, Larkin J D, Taddeo F J, Yeager M D, Stubbs C D. J Biol Chem. 1996;271:4627–4631. doi: 10.1074/jbc.271.9.4627. [DOI] [PubMed] [Google Scholar]
- 40.Irie K, Yanai Y, Oie K, Ishizawa J, Nakagawa Y, Ohigashi H, Wender P A, Kikkawa U. Bioorg Med Chem. 1997;5:1725–1737. doi: 10.1016/s0968-0896(97)00116-8. [DOI] [PubMed] [Google Scholar]
- 41.Szallasi Z, Bogi K, Gohari S, Biro T, Acs P, Blumberg P M. J Biol Chem. 1996;271:8299–8301. doi: 10.1074/jbc.271.31.18299. [DOI] [PubMed] [Google Scholar]