Abstract
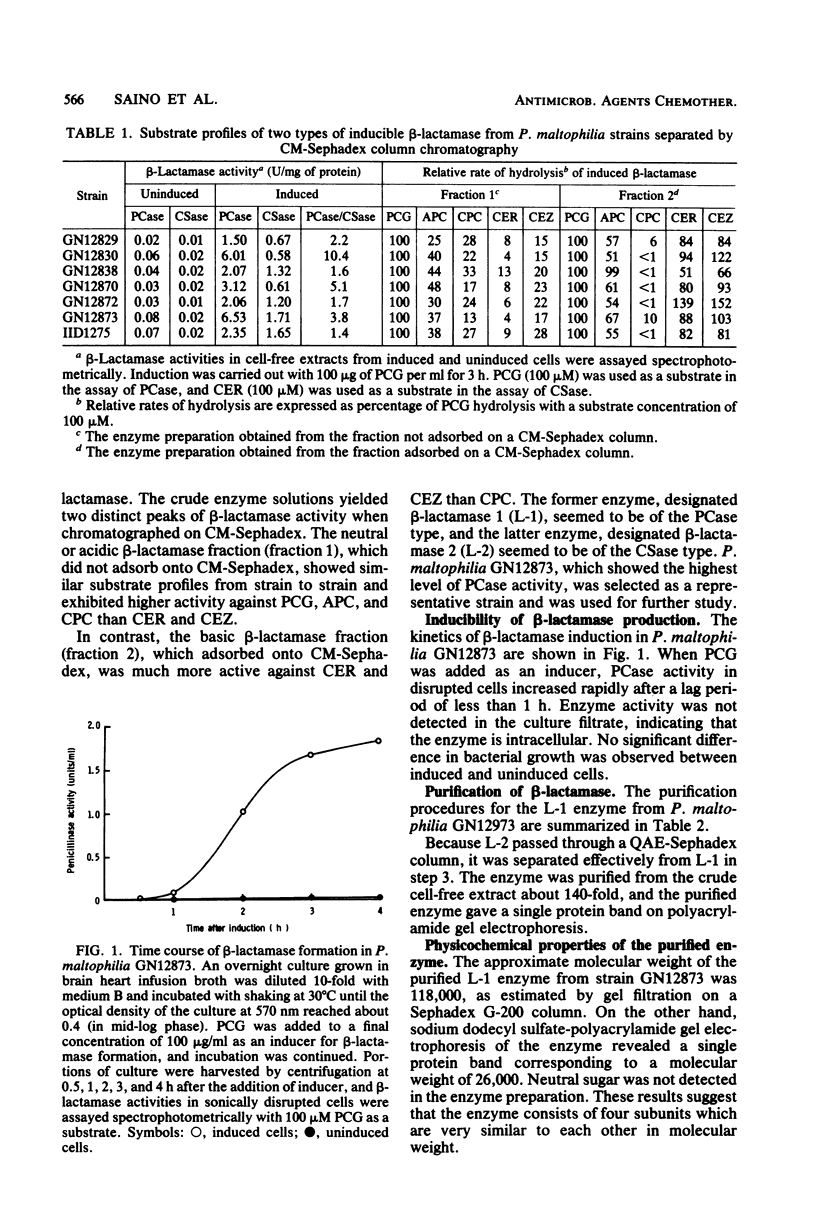
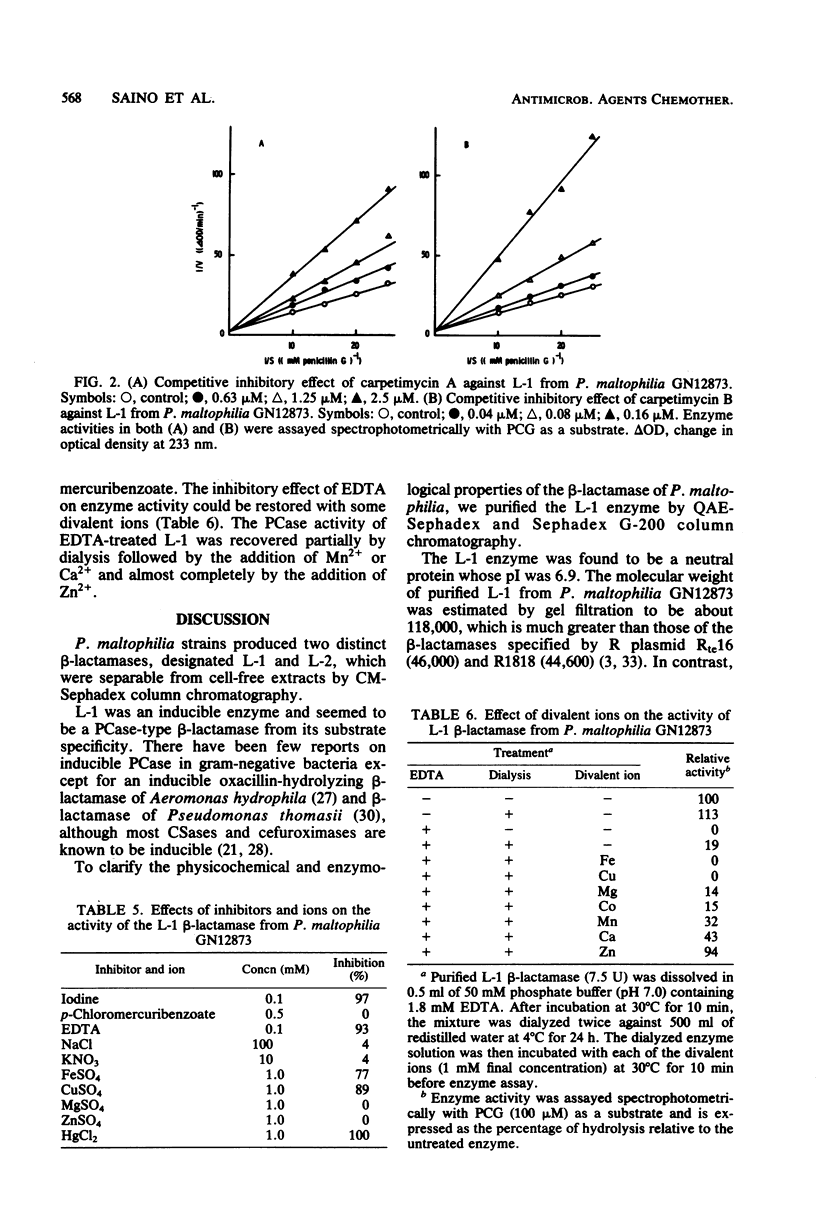
Two types of beta-lactamase were found in the cell-free extract from Pseudomonas maltophilia GN12873. One was an inducible penicillin beta-lactamase, and the other was an inducible cephalosporin beta-lactamase. The purified penicillin beta-lactamase gave a single protein band on polyacrylamide gel electrophoresis. The isoelectric point was 6.9, and the approximate molecular weight was 118,000 by gel filtration and 26,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, suggesting that this enzyme consisted of four subunits. For the hydrolysis of penicillin G, the optimal pH was 8.0 and the optimal temperature was 35 degrees C. The enzyme activity was inhibited by cephamycin derivatives, carpetimycins A and B, iodine, and HgCl2, but not by clavulanic acid. Furthermore, beta-lactamase activity was almost completely inhibited by EDTA but was recovered by the addition of zinc ion. The enzyme showed a unique substrate profile, hydrolyzing N-formimidoyl thienamycin at a significant rate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The dimeric nature of an R-factor mediated beta-lactamase. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1000–1005. doi: 10.1016/0006-291x(76)91245-6. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Barnishan J. Effect of divalent cation concentrations on the antibiotic susceptibilities of nonfermenters other than Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1979 Oct;16(4):434–438. doi: 10.1128/aac.16.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felegie T. P., Yu V. L., Rumans L. W., Yee R. B. Susceptibility of Pseudomonas maltophilia to antimicrobial agents, singly and in combination. Antimicrob Agents Chemother. 1979 Dec;16(6):833–837. doi: 10.1128/aac.16.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. J. Pseudomonas maltophilia endocarditis after replacement of the mitral valve: a case study. J Infect Dis. 1973 Nov;128(Suppl):771–773. doi: 10.1093/infdis/128.supplement_3.s771. [DOI] [PubMed] [Google Scholar]
- Gardner P., Griffin W. B., Swartz M. N., Kunz L. J. Nonfermentative gram-negative bacilli of nosocomial interest. Am J Med. 1970 Jun;48(6):735–749. doi: 10.1016/s0002-9343(70)80009-2. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Pseudomonas maltophilia infections in man. Am J Clin Pathol. 1969 Jan;51(1):58–61. doi: 10.1093/ajcp/51.1.58. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Iyobe S., Inoue M., Mitsuhashi S. Purification and properties of a new beta-lactamase from Pseudomonas cepacia. Antimicrob Agents Chemother. 1980 Mar;17(3):355–358. doi: 10.1128/aac.17.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Lapage S. P., Easterling B. G. Distribution in clinical material and identification of Pseudomonas maltophilia. J Clin Pathol. 1979 Jan;32(1):66–72. doi: 10.1136/jcp.32.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesado T., Hashizume T., Asahi Y. Antibacterial activities of a new stabilized thienamycin, N-formimidoyl thienamycin, in comparison with other antibiotics. Antimicrob Agents Chemother. 1980 Jun;17(6):912–917. doi: 10.1128/aac.17.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi F., Saino Y., Koshi T., Hattori Y., Nakayama M., Iwasaki A., Mori T., Mitsuhashi S. Antimicrobial and beta-lactamase inhibitory activities of carpetimycins A and B, new carbapenem antibiotics. Antimicrob Agents Chemother. 1982 Apr;21(4):536–544. doi: 10.1128/aac.21.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minami S., Inoue M., Mitsuhashi S. Purification and properties of cephalosporinase in Escherichia coli. Antimicrob Agents Chemother. 1980 Jul;18(1):77–80. doi: 10.1128/aac.18.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonogi K., Kuno M., Kida M., Mitsuhashi S. Beta-lactamase stability and antibacterial activity of cefmenoxime (SCE-1365), a novel cephalosporin. Antimicrob Agents Chemother. 1981 Aug;20(2):171–175. doi: 10.1128/aac.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Ross G. W., Chanter K. V., Harris A. M., Kirby S. M., Marshall M. J., O'Callaghan C. H. Comparison of assay techniques for beta-lactamase activity. Anal Biochem. 1973 Jul;54(1):9–16. doi: 10.1016/0003-2697(73)90241-8. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J. 1966 Jan;98(1):11C–13C. doi: 10.1042/bj0980011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Morioka K., Ogawa M., Yamagishi S. Inducible oxacillin-hydrolyzing penicillinase in Aeromonas hydrophila isolated from fish. Antimicrob Agents Chemother. 1976 Aug;10(2):191–195. doi: 10.1128/aac.10.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel J. D., Trenholme G. M., Harris A. A., Jupa J. E., Levin S. Pseudomonas maltophilia pseudosepticemia. Am J Med. 1978 Mar;64(3):403–406. doi: 10.1016/0002-9343(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Toda M., Sato K., Nakazawa H., Inoue M., Mitsuhashi S. Effect of N-formimidoyl thienamycin (MK0787) on beta-lactamases and activity against beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Nov;18(5):837–838. doi: 10.1128/aac.18.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Yeggy J. P., Field C. C., Markovetz A. J. Resistance plasmids in Pseudomonas cepacia 4G9. J Bacteriol. 1979 Dec;140(3):1017–1022. doi: 10.1128/jb.140.3.1017-1022.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma S., Terakado N., Mitsuhashi S. Biochemical properties of a penicillin beta-lactamase mediated by R factor from Bordetella bronchiseptica. Antimicrob Agents Chemother. 1975 Sep;8(3):238–242. doi: 10.1128/aac.8.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]


