Abstract
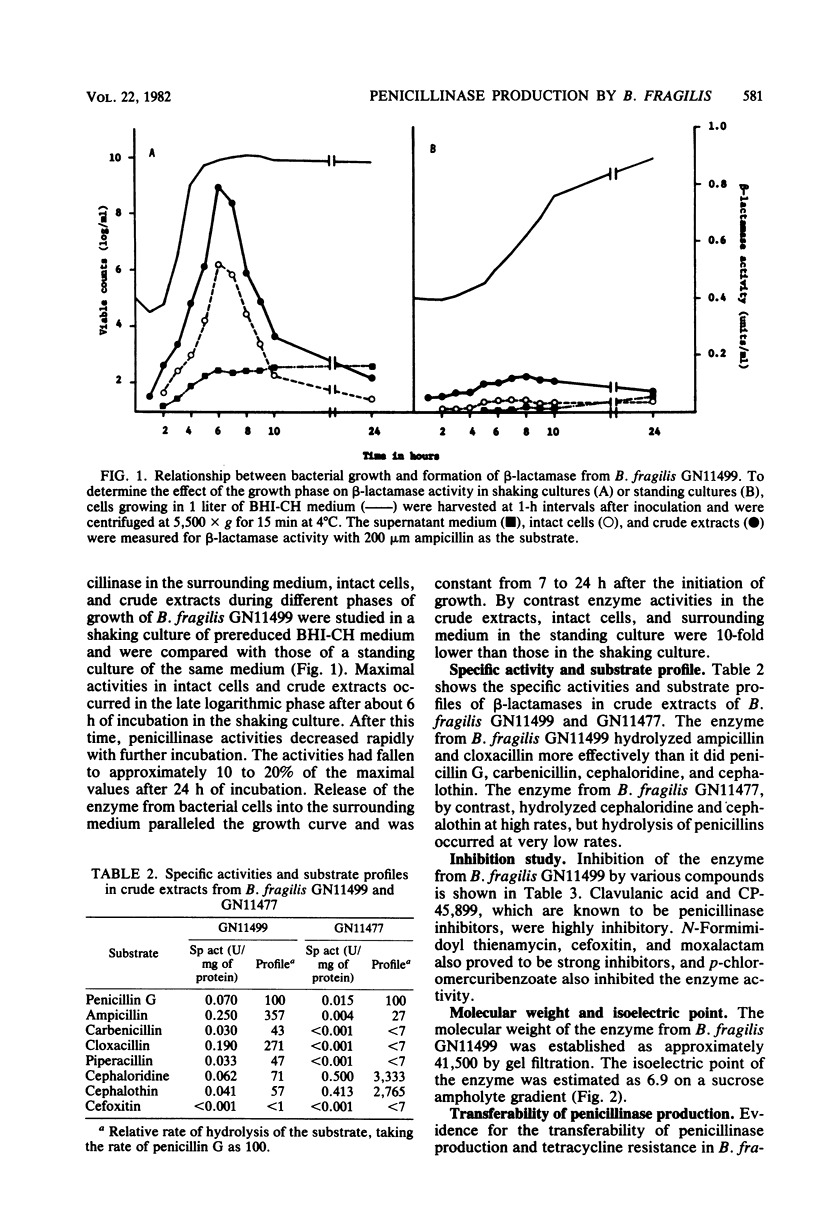
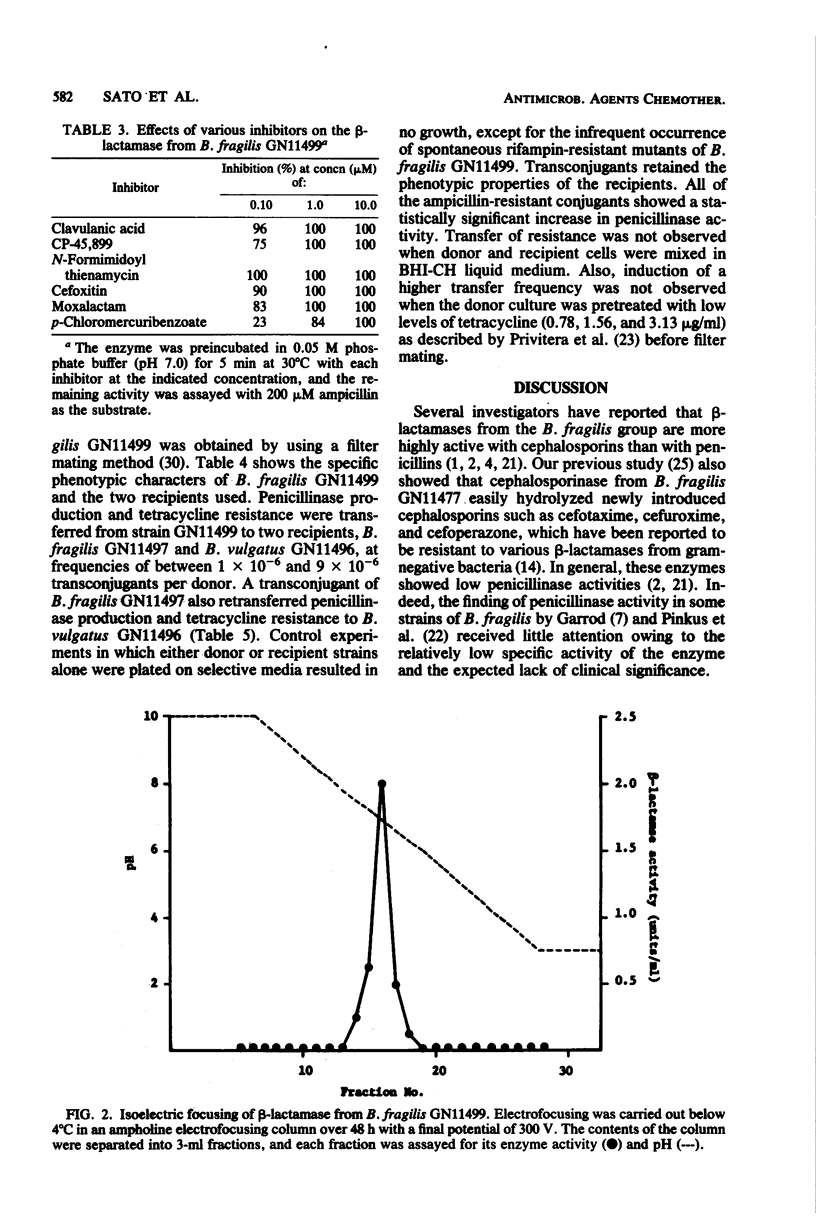
A highly penicillin-resistant strain of Bacteroides fragilis, strain GN11499, was found among 80 clinical isolates of the B.fragilis group and appears to produce a new type of penicillinase. Penicillinase activity was detected in crude extracts and had a specific activity of 0.25 U/mg of protein. About 20% of the enzyme was released into the surrounding medium during growth. The enzyme hydrolyzed ampicillin and cloxacillin more rapidly than it did penicillin G, carbenicillin, and cephaloridine. Relative rates in a crude extract with penicillin G as 100 were ampicillin, 357; carbenicillin, 57; cloxacillin, 271; and cephaloridine, 71. Enzyme activity was inhibited by clavulanic acid, CP-45, 899, N-formimidoyl thienamycin, cefoxitin, moxalactam, and p-chloromercuribenzoate. The enzyme had a molecular weight of approximately 41,500 and an isoelectric point of 6.9. Penicillinase production and tetracycline resistance were transferred from B.fragilis GN11499 to two susceptible strains of B.fragilis and Bacteroides vulgatus by filter mating.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Sykes R. B. Characterisation of a -lactamase obtained from a strain of Bacteroides fragilis resistant to -lactam antibiotics. J Med Microbiol. 1973 May;6(2):201–206. doi: 10.1099/00222615-6-2-201. [DOI] [PubMed] [Google Scholar]
- Britz M. L., Wilkinson R. G. Purification and properties of beta-lactamase from Bacteroides fragilis. Antimicrob Agents Chemother. 1978 Mar;13(3):373–382. doi: 10.1128/aac.13.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., Farrar W. E., Jr Cephalosporinase activity in Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Mar;3(3):369–372. doi: 10.1128/aac.3.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Nord C. E., Olsson B. Antibiotic susceptibility testing of anaerobic bacteria by the standardized disc diffusion method with special reference to bacteroides fragilis. Scand J Infect Dis. 1975;7(1):59–66. doi: 10.3109/inf.1975.7.issue-1.11. [DOI] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Girard A. E., Lynch J. E., Barth W. E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978 Sep;14(3):414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARROD L. P. Sensitivity of four species of bacteroides to antibiotics. Br Med J. 1955 Dec 24;2(4955):1529–1531. doi: 10.1136/bmj.2.4955.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of two extracellular beta-lactamases from Bacillus cereus 569-H. Biochem J. 1970 Jul;118(3):457–465. doi: 10.1042/bj1180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsubara N., Minami S., Muraoka T., Saikawa I., Mitsuhashi S. In vitro antibacterial activity of cefoperazone (T-1551), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1979 Dec;16(6):731–735. doi: 10.1128/aac.16.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Fu K. P., Aswapokee P. Antibacterial activity of a new 1-oxa cephalosporin compared with that of other beta-lactam compounds. Antimicrob Agents Chemother. 1979 Aug;16(2):141–149. doi: 10.1128/aac.16.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. Clavulanic acid, a novel inhibitor of beta-lactamases. Antimicrob Agents Chemother. 1978 Nov;14(5):650–655. doi: 10.1128/aac.14.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G., Veo G., Braude A. I. Bacteroides penicillinase. J Bacteriol. 1968 Oct;96(4):1437–1438. doi: 10.1128/jb.96.4.1437-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sato K., Inoue M., Mitsuhashi S. Activity of beta-lactamase produced by Bacteroides fragilis against newly introduced cephalosporins. Antimicrob Agents Chemother. 1980 Apr;17(4):736–737. doi: 10.1128/aac.17.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976 Oct;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Goldin B. R., Sullivan N., Johnston J., Gorbach S. L. Antimicrobial activity of metronidazole in anaerobic bacteria. Antimicrob Agents Chemother. 1978 Mar;13(3):460–465. doi: 10.1128/aac.13.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M., Sato K., Nakazawa H., Inoue M., Mitsuhashi S. Effect of N-formimidoyl thienamycin (MK0787) on beta-lactamases and activity against beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Nov;18(5):837–838. doi: 10.1128/aac.18.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]


