Abstract
One function of N-linked glycans is to assist in the folding of glycoproteins by mediating interactions of the lectin-like chaperone proteins calnexin and calreticulin with nascent glycoproteins. These interactions can be prevented by inhibitors of the α-glucosidases, such as N-butyl-deoxynojirimycin (NB-DNJ) and N-nonyl-DNJ (NN-DNJ), and this causes some proteins to be misfolded and retained within the endoplasmic reticulum (ER). We have shown previously that the NN-DNJ-induced misfolding of one of the hepatitis B virus (HBV) envelope glycoproteins prevents the formation and secretion of virus in vitro and that this inhibitor alters glycosylation and reduces the viral levels in an animal model of chronic HBV infection. This led us to investigate the effect of glucosidase inhibitors on another ER-budding virus, bovine viral diarrhea virus, a tissue culture surrogate of human hepatitis C virus (HCV). Here we show that in MDBK cells α-glucosidase inhibitors prevented the formation and secretion of infectious bovine viral diarrhea virus. Data also are presented showing that NN-DNJ, compared with NB-DNJ, exhibits a prolonged retention in liver in vivo. Because viral secretion is selectively hypersensitive to glucosidase inhibition relative to the secretion of cellular proteins, the possibility that glucosidase inhibitors could be used as broad-based antiviral hepatitis agents is discussed. A single drug against HBV, HCV, and, possibly, HDV, which together chronically infect more than 400 million people worldwide, would be of great therapeutic value.
Worldwide, more than 100 million people are chronically infected with the hepatitis C virus (HCV) (1, 2). In the absence of a vaccine this represents one of the most serious threats to the public health of developed nations. With an estimated 3.9 million North Americans chronically infected, hepatitis C is now the leading reason for liver transplantation in the United States. It causes about 8,000 U.S. deaths annually, a number that is expected to triple in the next 20 years in the absence of effective intervention.
HCV is an RNA virus belonging to the Flaviviridae family (3). Individual isolates consist of closely related, yet heterologous populations of viral genomes. This genetic diversity is thought to be a way to enable the virus to escape the immune system of the host, leading to a high rate of chronic infection. Persistent infection develops in as many as 85% of HCV patients, and in at least 20% of these patients the chronic infection leads to cirrhosis within 20 years of onset of infection. Chronic HCV also increases the risk of liver cancer (4). At present, the only specific treatment for chronic hepatitis C is IFN-α therapy, either on its own or in combination with the guanosine analogue ribavirin. However, only half of the patients respond to interferon, and relapse is common when treatment is stopped (2). Clearly, alternatives and complements to current therapies are necessary.
We have shown previously that hepatitis B virus (HBV) secretion from human hepatoblastoma cells in tissue culture is sensitive to inhibitors of endoplasmic reticulum (ER) α-glucosidase under conditions that do not compromise cell viability (5, 6), and recently we demonstrated the antiviral effect of glucosidase inhibitors in a woodchuck animal model of HBV infection. In woodchucks chronically infected with woodchuck hepatitis virus, treatment with ER α-glucosidase inhibitors results in the disruption of the proper folding and transport of viral envelope glycoproteins and prevents the secretion of infectious enveloped virus (7).
ER α-glucosidases are responsible for the stepwise removal of terminal glucose residues from N-glycan chains attached to nascent glycoproteins. This enables the glycoproteins to interact with the ER chaperones calnexin and calreticulin, which bind exclusively to monoglucosylated glycoproteins. Interaction with calnexin is crucial for the correct folding of some but not all glycoproteins, and inhibitors of the glucosidases can be used to specifically target proteins that depend on it (8, 9).
The two HCV envelope glycoproteins E1 and E2, which contain 5 or 6 and 11 N-linked glycosylation sites, respectively, both interact with calnexin during productive folding (10). Because of the lack of an efficient cell culture replication system the understanding of HCV particle assembly is very limited. However, the absence of complex glycans on HCV envelope glycoproteins expressed by recombinant Vaccinia and Sindbis viruses, the localization of these glycoproteins in the ER, and their absence on the cell surface (11) suggest that initial virion morphogenesis occurs by budding into intracellular vesicles from the ER. Additionally, mature E1–E2 heterodimers do not leave the ER, and ER retention signals have been identified in the C-terminal regions of both E1 and E2.
In the absence of a suitable cell culture system able to support the replication of human HCV, bovine viral diarrhea virus (BVDV) serves as a model organism for HCV (12), because both share a significant degree of local protein region homology (3), common replication strategies, and probably the same subcellular location for viral envelopment.
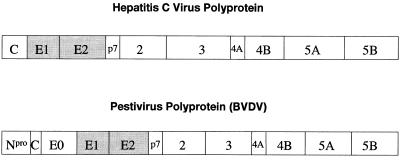
BVDV, like HCV, is a small, enveloped, positive-stranded RNA virus and, like all viruses within the Flaviviridae, encodes all of its proteins in a single, long ORF, with the structural proteins in the N-terminal portion of the polyprotein and the nonstructural or replicative proteins at the C-terminal end (Fig. 1) (14). The BVDV polyprotein has six potential N-glycosylation sites in the region encoding for the two heterodimer-forming envelope proteins gp25 (E1) and gp53 (E2) and eight potential N-glycosylation sites in the region encoding for gp48 (E0), a hydrophilic secreted protein of unknown function. The structures of the oligosaccharides attached to any of these glycoproteins remain to be determined.
Figure 1.
Polyproteins of the Flaviviridae. Viruses within the Flaviviridae (the flavi-, pesti-, and hepatitis C viruses) encode all of their proteins in a single, long ORF with the structural proteins in the N-terminal portion and the replicative nonstructural proteins in the C-terminal portion of the polyprotein (13). The polyproteins subsequently are processed by a combination of viral and host proteinases.
In this paper we describe the sensitivity of BVDV to α-glucosidase inhibitors and discuss the possible reasons for the select sensitivity of ER-budding viruses upon glycan processing mediated by ER α-glucosidases and the implications for a possible therapy.
Methods
Cells, Virus, and Inhibitors.
Noncytopathic (ncp) BVDV-free MDBK cells (European Collection of Animal Cell Cultures, Porton Down, U.K.) and cytopathic (cp) BVDV virus (strain NADL) were used in these studies. MDBK cells were monitored for BVDV contamination and shown to be negative by immunostaining with polyclonal bovine anti-BVDV serum. MDBK and HepG2 cells were maintained in RPMI 1640 medium (GIBCO/BRL) containing 10% FBS (PAA Laboratories, Teddington, U.K.), which had been screened and found negative for the presence of BVDV and BVDV-specific antibodies. N-Butyldeoxynojirimycin (NB-DNJ) and N-nonyldeoxynojirimycin (NN-DNJ) were provided by Monsanto. NB-DNJ was dissolved in medium and filtered just before use. NN-DNJ was made up as a 13-mg/ml stock solution in ethanol and diluted with medium before use. N-Butyldeoxygalactojirimycin (NB-DGJ) and deoxymannojirimycin (DMJ) were purchased from Boehringer Mannheim and made up as 200 mM and 100 mM stock solutions in water, respectively. They were diluted with medium and filtered just before use. NB-[U-14C]deoxynojirimycin (specific activity, 4.4 mCi/mmol) and deoxynojirimycin (DNJ) were a gift of Searle.
Toxicity and Cell Viability Assays (Propidium Iodide Dye Exclusion Assay).
BVDV-infected and uninfected control cells were grown in the absence or presence of varying concentrations of NB-DNJ and NN-DNJ. After 3 days, the cells were harvested by using trypsin/EDTA and combined with the free-floating cells recovered from the respective supernatants by a low-speed spin. After two PBS washes, the cells were washed once with FACS buffer (0.1 M PBS/0.02 M NaN3/0.1% BSA), resuspended in FACS buffer containing 1 μg/ml propidium iodide, and analyzed by using a FACScan Cytometer (Becton Dickinson) to determine the percentage of dead cells present.
Plaque Reduction and Yield Assays.
MDBK cells were grown in six-well plates in the presence or absence of inhibitor (see figure legends), infected with cp BVDV [multiplicity of infection (moi) = 0.005; 500 plaque-forming units (pfu)/well] for 1 hr at 37°, and incubated for 2 or 3 days in the presence or absence of inhibitor (plaque-reduction assay). After counting the plaques by eye under the microscope, the supernatant containing secreted virus was removed from the wells and used to infect a fresh monolayer of MDBK cells in six-well plates. After 3 days the resulting plaques were counted under the microscope (yield assay).
Assay for the Inhibition of Ceramide-Specific Glucosyltransferase by NB-DGJ.
MDBK cells were grown to confluency in [14C]palmitate containing medium (0.25 μCi/ml) for 3 days in the presence of up to 680 μM NB-DGJ. The cells were washed three times with PBS, scraped off the flasks, and extracted by using CHCl3/MeOH (2:1, vol/vol) overnight at 4°C. The first extract was kept and another 0.5 ml of CHCl3/MeOH (2:1, vol/vol) was added for 3 hr at room temperature. The extracts were combined and aliquots were scintillation-counted. Samples were adjusted to 200,000 cpm, dried under nitrogen, resuspended in 10 μl of CHCl3/MeOH (2:1, vol/vol), and separated by TLC (CHCl3/MeOH; 2:1, vol/vol). Radiolabeled lipids were detected by fluorography, and the dose-dependent decrease of Glc-ceramide and gangliosides observed (data not shown) showed that the ceramide-specific glucosyltransferase was inhibited at concentrations that had no antiviral effect.
Assay for the Inhibition of Complex Sugar Formation by DMJ.
MDBK cells were grown for 3 days in the absence/presence of 300 μg/ml DMJ. The cells were stained with Erythrina cristagalli (ECA) lectin (Vector; 28 μg/ml and 280 μg/ml), which recognizes the Gal α1,4GalNAc epitope, and analyzed by FACS. At the lower lectin concentration, a shift in the staining intensity marked the decrease in binding sites (i.e., complex glycans) available for the lectin. At the higher lectin concentration, the presence of DMJ protected cells from being killed by lectin binding (data not shown).
BVDV RNA Isolation.
Plaque assays (moi = 0.014; 7,000 pfu/well) and yield assays were performed as described above. The viral RNA was isolated from the culture medium supernatants of NB-DNJ-treated and untreated, BVDV-infected MDBK cells. Briefly, the supernatants were harvested, clarified by a slow-speed spin, and concentrated 8-fold by using 10-kDa cutoff Centricons (Amicon). Viral RNA was purified from 25% of the concentrates by using the Qiagen Viral RNA Purification kit following the manufacturer’s instructions. Reverse transcription–PCR (RT-PCR) was performed by using the Titan One Tube RT-PCR System (Boehringer Mannheim). Primers P1 and P2 and conditions used were as described in Sullivan and Akkina (15), with minor modifications. The samples were analyzed by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Preparation of N-[3H]N-DNJ.
DNJ (61 μmol) was aminated reductively with nonylaldehyde (1.2 mol equivalents) in the presence of 1 mol equivalent of sodium cyanoboro[3H]hydride (10 Ci/mmol; Amersham) for 3 hr at room temperature. Tritium-labeled NN-DNJ was purified from the reaction mixture by cation-exchange and reverse-phase HPLC. The product was greater than 95% radioactively pure by HPLC, and the compound structure was verified by mass spectrometry and 1H-NMR. The specific activity was 145 mCi/mmol.
Organ Distribution of Radiolabeled Imino Sugars.
Radiolabeled NN-DNJ and NB-DNJ were dried under vacuum, resuspended in whole mouse serum (Becton Dickinson), and sonicated on ice for 1 min. The suspension was filtered by using a 0.2-μm filter, and the radioactivity was recovered in the filtrate with typical recovery rates of 78–95%. The filtrates were administered to BALB/c mice by oral gavage (1–3 μCi per mouse), and after 30, 60, and 90 min the mice were sacrificed by cervical dislocation and the organs were removed. The organs were weighed and homogenized in water at 0.2–0.4 g/ml by using an Ultra-Turrax homogenizer (Janke & Kunkel, Staufen, Germany). Aliquots were taken for radioactivity determinations.
Results
Glucosidase Inhibitors Prevent the Secretion of Infectious BVDV.
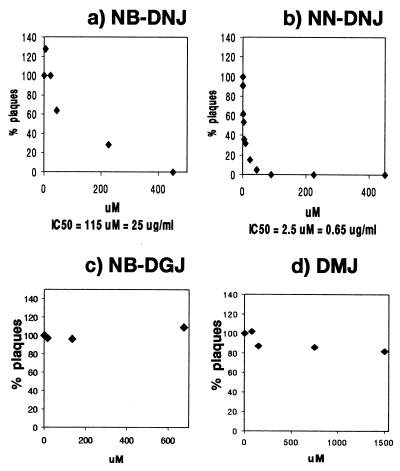
MDBK cells were infected with BVDV under plaquing conditions (moi = 0.005), and 1 hr after infection the inoculum was removed and replaced with either fresh medium or medium containing varying concentrations of α-glucosidase inhibitors. Two days later the amount of infectious virus present in the culture medium was determined by yield assay. As shown in Fig. 2, the imino sugar derivatives NB-DNJ and NN-DNJ strongly inhibit the cytopathic effect of BVDV on MDBK cells in a dose-dependent manner. The nonyl compound (IC50 = 2.5 μM) was shown to be 46 times more potent than the butyl compound (IC50 = 115 μM) (Figs. 2 a and b). Cell viability was not compromised at any of the NB-DNJ concentrations used, as determined by FACS analysis (data not shown). Also, even using 90 μM NN-DNJ, i.e., a concentration that is 100% antiviral (Fig. 2b), the number of dead cells increased by only 3.7% compared with the infected, untreated control, though higher concentrations of this compound caused more pronounced cytotoxicity. Because both compounds inhibit not only the ER α-glucosidases, but also the ceramide-specific glucosyltransferase, which catalyses the first step in glycosphingolipid biosynthesis (16), it was necessary to establish via which pathway these drugs exert their antiviral effect. The two pathways were pharmacologically dissected by using NB-DGJ, an inhibitor that targets only the glucosyltransferase (6). In the plaque-reduction assay, NB-DGJ had no effect on BVDV plaque formation (Fig. 2c). The higher NB-DGJ concentrations used were sufficient to completely inhibit the glucosyltransferase, as shown by the dose-dependent decrease of Glc-ceramide and gangliosides in [14C]palmitate-labeled, NB-DGJ-treated MDBK cells (data not shown). This implies that the antiviral effect observed with NB-DNJ and NN-DNJ can be attributed to the inhibition of the ER α-glucosidases involved in N-glycan processing. Pretreatment with glucosidase inhibitors did not prevent viral entry (data not shown), showing that the viral receptor is functional in glucosidase-inhibited cells. Consistent with another report about HBV secretion (6), inhibiting complex glycan formation with DMJ (Fig. 2d) did not prevent BVDV plaque formation either. This indicates that the antiviral effect of NB-DNJ and NN-DNJ is mediated by a step before complex N-glycan formation and highlights the important role of glucosidase processing in the formation of BVDV.
Figure 2.
Secretion of infectious BVDV in the presence of N-butyl-DNJ (a), N-nonyl-DNJ (b), N-butyl-DGJ (c), and DMJ (d). (a and b) MDBK cells were grown to confluency in six-well plates and infected with 500 pfu of cp BVDV (NADL strain) per well for 1 hr at 37°. The inoculum then was replaced with medium containing the indicated concentrations of either NB-DNJ, NN-DNJ, NB-DGJ, or DMJ (plaque assay). After 3 days the supernatants were removed and used to infect fresh MDBK monolayers in six-well plates. The presence of inhibitor during the 1-hr infection does not have an effect (data not shown). After 1 hr the inoculum was removed and the cells were washed thoroughly and incubated with inhibitor-free medium. After 3 days the plaques were counted (yield assay) and the results were expressed as a percentage of the number of plaques resulting from infection with the inhibitor-free plaque assay supernatant (=100%) (x axis). The y axis indicates the inhibitor concentrations used in the plaque assay. The IC50 is indicated at the bottom. (c and d) Plaque assay results are shown. NB-DGJ was used at concentrations of up to 680 μM, an inhibitor concentration sufficient to inhibit completely the ceramide-specific glucosyltransferase (data not shown). DMJ was used at concentrations of up to 1.5 mM, an inhibitor concentration sufficient to protect treated cells from killing by a complex sugar-binding lectin (ECA) (data not shown). All experiments were done at least in duplicate, and each data point represents the average of two wells counted. Experiment 2 (b) was done in triplicate (n = 3 ± 0.13), and each data point represents the average of two wells counted.
Glucosidase Inhibitors Prevent the Secretion of Viral RNA into the Culture Medium.
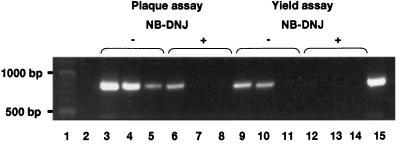
Although inhibition of glucosidases by NN-DNJ and NB-DNJ prevented the appearance of infectious BVDV in the culture medium, it was possible that BVDV virus-like particles were still secreted, but in a noninfectious state. The possibility that noninfectious BVDV virions were secreted from glucosidase-inhibited cells was examined by RT-PCR to determine the amount of viral RNA in the culture medium of MDBK cells after infection with BVDV as a function of NB-DNJ treatment. Briefly, MDBK cells maintained as monolayers were infected with BVDV and left untreated or incubated with various concentrations of NB-DNJ. After 3 days, the culture medium was collected and concentrated, and the amount of viral RNA was determined by RT-PCR amplification. Some of the culture medium from the plaque assay also was used to perform a virus yield assay. The resulting culture medium was collected and concentrated after 3 days, and the amount of viral RNA was determined as above. The results are shown in Fig. 3. Lane 3 shows that the expected 826-nt PCR product is detected in the culture medium of infected cells that have not been incubated with NB-DNJ (virus/no drug). Lane 6 contains a sample from the culture medium of cells that were infected with BVDV and incubated for 3 days in the presence of 4.5 mM NB-DNJ. Lane 9 contains a sample from the culture medium of the yield assay from untreated cells, and lane 12 contains a sample from NB-DNJ-treated cells. Compared with lane 9 there is a greater than 90% reduction in the product represented in lane 12.
Figure 3.
RT-PCR of viral RNA extracted from the supernatants of NB-DNJ-treated MDBK cells. MDBK cells were grown to confluency in six-well plates, infected with cytopathic BVDV, and incubated for 3 days in the absence or presence of 4.5 mM (1,000 μg/ml) NB-DNJ. Yield assays were performed with the resulting culture medium supernatants. Viral RNA was purified from the culture medium supernatants of both plaque and yield assays, and RT-PCR was performed as described in Methods. The samples were analyzed by electrophoresis in a 1.5% agarose gel and stained with ethidium bromide. Lanes: 1, 100-bp DNA ladder; 2, culture medium from uninfected cells; 3, plaque assay culture medium from infected cells, no NB-DNJ; 4 and 5, products of a PCR on 1:10 and 1:100 dilutions, respectively, of the sample used in lane 3; 6, plaque assay culture medium from infected cells maintained in 4.5 mM NB-DNJ; 7 and 8, products of a PCR on 1:10 and 1:100 dilutions, respectively, of the sample used in lane 6; 9, yield assay culture medium from cells incubated with plaque assay supernatant from untreated cells; 10 and 11, products of a PCR on 1:10 and 1:100 dilutions, respectively, of the sample used in lane 9; 12, yield assay culture medium from cells incubated with plaque assay supernatant from NB-DNJ-treated cells; 13 and 14, products of a PCR on 1:10 and 1:100 dilutions, respectively, of the sample used in lane 12; 15, RT-PCR product using a cDNA control.
PCR analysis also was performed on dilutions of the samples to quantify the PCR. Lanes 4 and 5 (Fig. 3), for example, show PCR amplifications of serial dilutions of the material used in lane 3. Clearly, the PCR used here was quantitative and could detect at least 10-fold differences in the amount of material in the samples.
Infectious Virus Does Not Accumulate in α-Glucosidase-Inhibited Cells.
In HepG2.2.15 cells, an HBV-secreting cell line (17), secretion of enveloped virus is prevented and viral DNA accumulates within the cells when they are treated with α-glucosidase inhibitors (18). However, it is unclear whether the accumulated DNA is contained within infectious particles, because it is difficult to determine the infectivity of HBV particles in tissue culture.
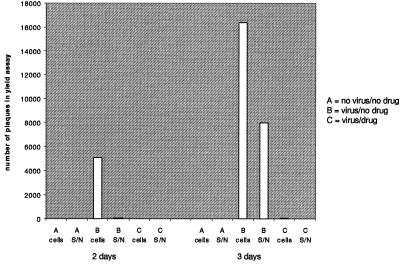
Prevention of BVDV secretion may also cause the accumulation of viral material inside the cells, and because cell culture growth systems for BVDV are readily available, it is easier to test whether this material is infectious. BVDV-infected cells were either left untreated or were grown in the presence of 1 mg/ml NB-DNJ for 2 or 3 days before they were washed thoroughly and lysed by freeze-thawing. Virus yield assays were performed to determine the number of pfus in the cell lysates as well as in the supernatants. As shown in Fig. 4, for untreated cells most of the infectious viral material (more than 97%) was recovered from inside the cells after 2 days, and after 3 days a third of the infectious viral material could be detected in the supernatant. Significantly, the intracellular compartment of BVDV-infected and NB-DNJ-treated cells contained no detectable infectious material after 2 days and almost no infectious material after 3 days after infection. At no time after infection did the culture medium (supernatant) of BVDV-infected cells contain infectious virus. Thus, at a time when untreated BVDV-infected cells contained large amounts of progeny virus within the cells, NB-DNJ-treated cells were virtually devoid of infectious material. These data show that BVDV does not accumulate inside inhibitor-treated cells in an infectious state.
Figure 4.
Infectivity of viral material inside and outside of NB-DNJ-treated cells 2 and 3 days after infection. Noninfected (A) and BVDV-infected (B and C) MDBK cells were grown in the absence (A and B) or presence (C) of 1 mg/ml NB-DNJ for either 2 or 3 days. The supernatants were saved and the cells were washed and lysed by freeze-thawing. Yield assays were performed to determine the number of pfus in the supernatants (S/N) and cell lysates (cells).
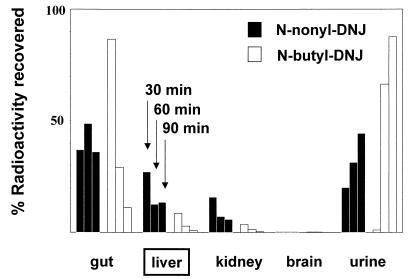
In Vivo, NN-DNJ Associates with the Liver to a Greater Extent than NB-DNJ.
It was of interest to determine whether NN-DNJ showed liver selectivity in vivo. Therefore, mice were dosed orally with either radiolabeled NB-DNJ or NN-DNJ. At the indicated time points they were sacrificed and the percentage of radioactive counts associated with the specified organs were determined as described in methods. As Fig. 5 shows, a substantial proportion of NN-DNJ is associated with the liver after 30 min and remains over the 90-min period of testing. This is consistent with the antiviral activity observed with this compound in the woodchuck animal model of chronic HBV (7). In contrast, by 90 min, most of the NB-DNJ has passed through the gut and liver and is secreted (Fig. 5). Indeed, after 90 min there is more than 15–20 times more NN-DNJ associated with the liver than NB-DNJ. This prolonged association with the liver may be the result of NN-DNJ’s preferred uptake into cells of hepatic origin, which may be a result of its longer fatty acid side chain, which probably plays an important role in uptake and retention.
Figure 5.
In vivo tissue distribution of radioactively labeled compounds. Radioactively labeled imino sugars were administered to BALB/c mice as described in Methods. After 30, 60, and 90 min, the mice were sacrificed and the organs were removed. The organs were weighed and homogenized, and aliquots were taken for radioactivity determination. Each column represents at least three animals, with cpm normalized for varying organ sizes by expressing as cpm per gram of organ homogenate.
Discussion
HBV is exquisitely sensitive to α-glucosidase inhibition (5, 7). Because it appears unnecessary to inhibit the enzyme to any great extent to achieve an antiviral effect in vivo (7), this led us to speculate that the sensitivity of the virus may be due to a requirement to oligomerize and assemble the envelope in the ER, where protein folding takes place. A few misfolded envelope proteins may be sufficient to disrupt the proper envelopment process and amplify the effect of the inhibitor on virus assembly as compared with the effect on host cell proteins, which do not seem to be impaired at antiviral inhibitor concentrations. Our studies led us to propose that other viruses that acquire their envelopes from intracellular membranes such as the ER would be equally sensitive to ER α-glucosidase inhibition, provided one or more of their glycoproteins depended on calnexin-mediated folding.
Although HBV and HCV have completely different genomes and life cycles, they share at least three things in common: liver targeting, budding from the ER (or other intracellular compartments), and specification of envelope glycoproteins that fold via a calnexin-dependent pathway (10, 19, 20). We demonstrate here how these similarities can be exploited with a single compound that shows preferential uptake by the liver and disrupts the folding of the viral envelope glycoproteins by preventing interactions with the ER chaperone calnexin.
Because it is not possible to grow HCV in cell culture, BVDV has been used as a substitute. As Fig. 2 shows, BVDV is extremely sensitive to α-glucosidase inhibitors. Preliminary data suggest that the concentrations of α-glucosidase inhibitors required to achieve an antiviral effect with both BVDV and HBV inhibit the enzyme by only 10–20% (unpublished data). This may explain the lack of toxicity of these compounds observed in vivo (7).
NN-DNJ is a more potent antiviral agent in vitro. This may be due to increased cellular uptake and/or retention or better ER targeting. The in vivo studies also suggest a better bioselectivity for the nonyl compound. The entry into cells of NB-DNJ, as well as other imino sugar derivatives with shorter alkyl chains, is thought to occur by passive diffusion (21). The longer alkyl chain (and subsequent increase in hydrophobicity) may cause NN-DNJ to imbed into intracellular membranes better, leading to its retention. This also may add to the prolonged retention within the liver in vivo (Fig. 5).
The notion that the same drug may work against both HBV and HCV has additional appeal, because many individuals are infected with both pathogens. The emergence of glucosidase inhibitor-resistant mutants is unlikely, because the viruses rely heavily on the host enzyme-mediated glycosylation process. Glycosylation of at least the HBV M protein has been shown to be essential for virus secretion (18). Although targeting a host function such as the ER α-glucosidase must be carefully considered in terms of toxicity, the approach would provide a useful complement to more conventional antiviral strategies, which commonly lead to the emergence of drug-resistant mutants. Clearly, a single drug against HBV and HCV (and possibly hepatitis delta virus, which depends on the HBV glycoproteins), which together infect more than 400 million people worldwide, would be of great therapeutic value.
Acknowledgments
We thank Drs. Robert Jordan, Taj Mattu, and Laura Steel for their helpful comments. This work was supported by The Hepatitis B Foundation and The IgX-Oxford Corp. B.S.B. is supported by National Institutes of Health Grant CA 06927 and an appropriation from the Commonwealth of Pennsylvania. T.M.B., R.A.D., and B.S.B. own stock in IgX-Oxford Corp., which is majority-owned by Synergy Pharmaceuticals, Inc., in which B.S.B. and R.A.D. own stock. R.A.D. and B.S.B. are on the Board of Directors of Synergy Pharmaceuticals, Inc.
Abbreviations
- HCV and HBV
hepatitis C and B virus, respectively
- ER
endoplasmic reticulum
- BVDV
bovine viral diarrhea virus
- NB-DNJ
N-butyl-DNJ
- NN-DNJ
N-nonyl-DNJ
- NB-DGJ
N-butyldeoxygalactojirimycin
- DMJ
deoxymannojirimycin
- DNJ
deoxynojirimycin
- RT-PCR
reverse transcription–PCR
References
- 1.Alter M J. Hepatology. 1997;26, Suppl. 1:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle J H, Di Bisceglie A M. New Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 3.Miller R H, Purcell R H. Proc Natl Acad Sci USA. 1990;87:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Bisceglie A M. Lancet. 1998;351:351–355. doi: 10.1016/S0140-6736(97)07361-3. [DOI] [PubMed] [Google Scholar]
- 5.Block T M, Platt F, Lu X, Gerlich W, Foster G, Blumberg B S, Dwek R A. Proc Natl Acad Sci USA. 1994;91:2235–2239. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Mehta A, Dwek R, Butters T, Block T. Virology. 1995;213:660–665. doi: 10.1006/viro.1995.0038. [DOI] [PubMed] [Google Scholar]
- 7.Block T M, Lu X, Mehta A S, Blumberg B S, Tennant B, Ebling M, Korba B, Lansky D M, Jacob G S, Dwek R A. Nat Med. 1998;4:610–614. doi: 10.1038/nm0598-610. [DOI] [PubMed] [Google Scholar]
- 8.Rudd P M, Dwek R A. Crit Rev Biochem Mol Biol. 1997;32:1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 9.Mehta A, Zitzmann N, Rudd P M, Block T M, Dwek R A. FEBS Lett. 1998;430:17–22. doi: 10.1016/s0014-5793(98)00525-0. [DOI] [PubMed] [Google Scholar]
- 10.Choukhi A, Ung S, Wychowski C, Dubuisson J. J Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubuisson J, Hsu H H, Cheung R C, Greenberg H B, Russell D G, Rice C M. J Virol. 1994;68:6147–6160. doi: 10.1128/jvi.68.10.6147-6160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henzler H-J, Kaiser K. Nat Biotechnol. 1998;16:1077–1079. doi: 10.1038/3538. [DOI] [PubMed] [Google Scholar]
- 13.Chambers T J, Grakoui A, Rice C M. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 14.Houghton M, Han J, Kuo G, Choo Q-L, Weiner A J. In: Viral Hepatitis, Scientific Basis and Clinical Management. Zuckerman A J, Thomas H C, editors. Edinburgh: Churchill Livingstone; 1993. pp. 229–237. [Google Scholar]
- 15.Sullivan D G, Akkina R K. Virus Res. 1995;38:231–239. doi: 10.1016/0168-1702(95)00065-x. [DOI] [PubMed] [Google Scholar]
- 16.Platt F M, Neises G R, Dwek R A, Butters T D. J Biol Chem. 1994;269:8362–8365. [PubMed] [Google Scholar]
- 17.Sells M A, Chen M L, Acs G. Proc Natl Acad Sci USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta A, Lu X, Block T M, Blumberg B S, Dwek R A. Proc Natl Acad Sci USA. 1997;94:1822–1827. doi: 10.1073/pnas.94.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werr M, Prange R. J Virol. 1998;72:778–782. doi: 10.1128/jvi.72.1.778-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubuisson J, Rice C M. J Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan A, van den Broeck L, van Boeckel C, Ploegh H, Bolscher J. J Biol Chem. 1991;266:14504–14510. [PubMed] [Google Scholar]