Abstract
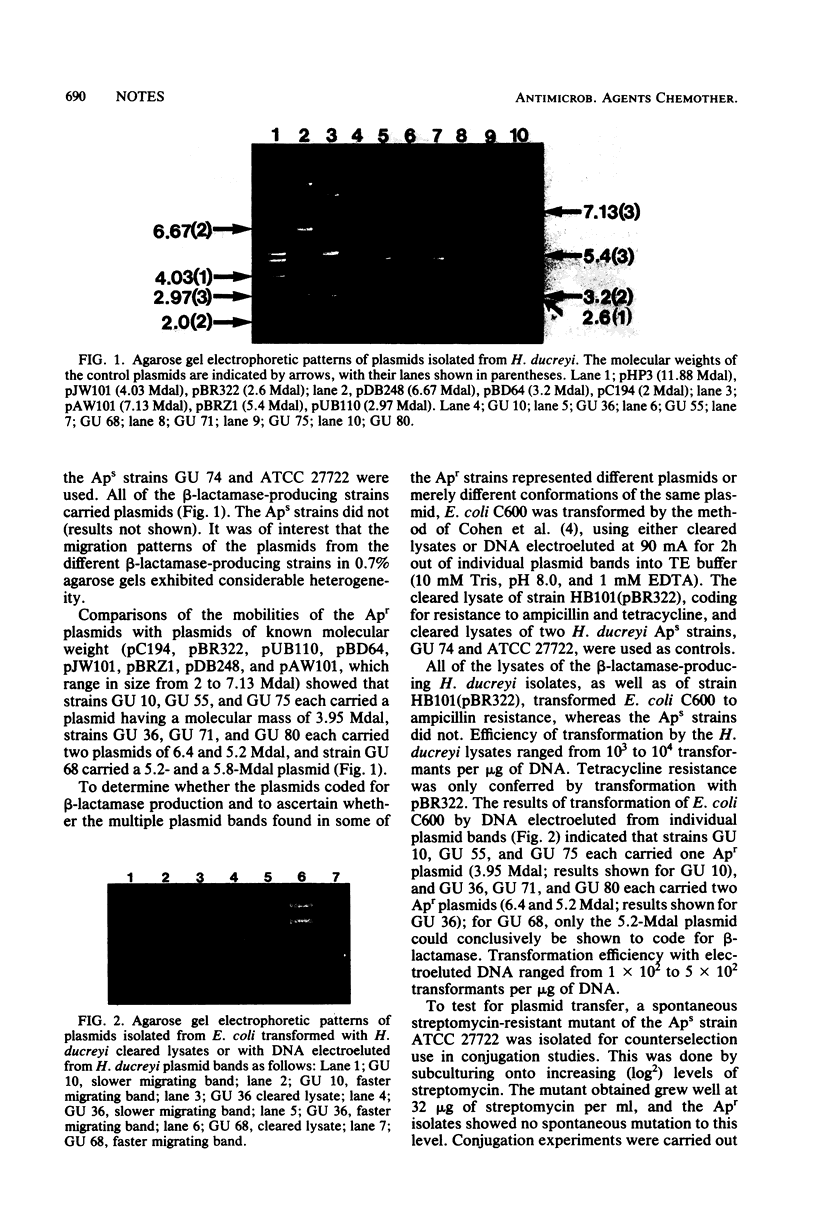
Seven of the 96 ampicillin-resistant isolates of Haemophilus ducreyi reported in the preceding article (Bilgeri et al., Antimicrob. Agents Chemother. 22:686-688, 1982) were investigated and found to harbor plasmids of 3.95, 5.2, 5.8, and 6.4 megadaltons. All except the 5.8-megadalton plasmid have been shown to code for beta-lactamase. The 6.4- and 5.2-megadalton plasmids of three isolates were conjugally transferable to a streptomycin-resistant mutant of H. ducreyi at high frequencies, perhaps due to the presence in these strains of a high-molecular-weight plasmid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilgeri Y. R., Ballard R. C., Duncan M. O., Mauff A. C., Koornhof H. J. Antimicrobial susceptibility of 103 strains of Haemophilus ducreyi isolated in Johannesburg. Antimicrob Agents Chemother. 1982 Oct;22(4):686–688. doi: 10.1128/aac.22.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brunton J. L., Maclean I., Ronald A. R., Albritton W. L. Plasmid-mediated ampicillin resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1979 Feb;15(2):294–299. doi: 10.1128/aac.15.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Slaney L., Maclean I. W., Albritton W. L. Mobilization of nonconjugative antibiotic resistance plasmids in Haemophilus ducreyi. J Bacteriol. 1982 Feb;149(2):726–732. doi: 10.1128/jb.149.2.726-732.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handsfield H. H., Totten P. A., Fennel C. L., Falkow S., Holmes K. K. Molecular epidemiology of Haemophilus ducreyi infections. Ann Intern Med. 1981 Sep;95(3):315–318. doi: 10.7326/0003-4819-95-3-315. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne G. M., Farrar W. E., Jr Transfer of ampicillin resistance between strains of Haemophilus influenzae type B. J Infect Dis. 1975 Sep;132(3):276–281. doi: 10.1093/infdis/132.3.276. [DOI] [PubMed] [Google Scholar]





