Abstract
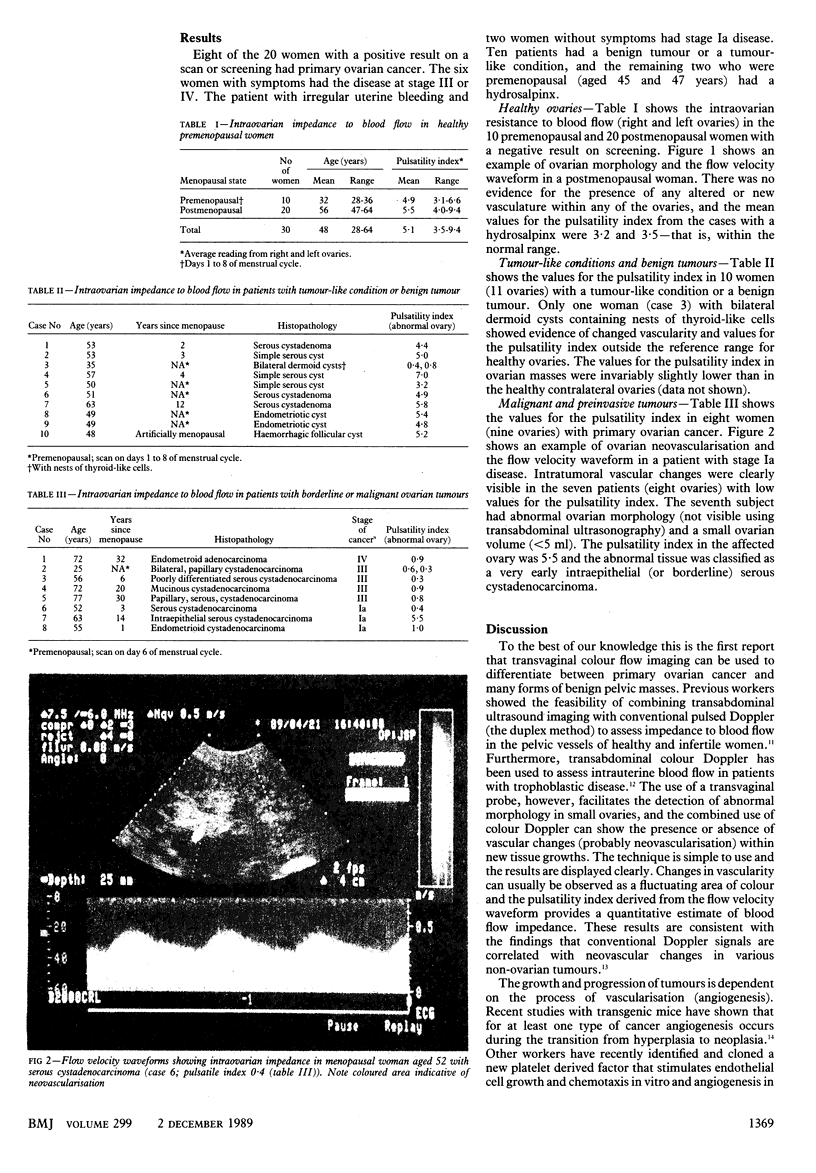
OBJECTIVE--To assess whether changes in the intraovarian vasculature or blood flow impedance can be used to identify potentially malignant masses. DESIGN--Open, non-comparative prospective study. SETTING--Ovarian screening clinics at King's College Hospital and the Hallam Medical Centre. SUBJECTS--50 Women selected on the basis of their medical history and the result of a previous transvaginal ultrasound scan. Thirty women (10 premenopausal (scan taken on days 1 to 8 of the menstrual cycle) and 20 postmenopausal) had normal ovaries, and 20 had at least one ovary with an abnormal morphology or volume, or both. INTERVENTIONS--Women with a positive result on screening were referred for laparotomy. MAIN OUTCOME MEASURES--Presence or absence of coloured areas (neovascularisation) and the pulsatility index within each ovary. The pulsatility index is a measure of the impedance to blood flow, a low value indicating decreased impedance and a high value increased impedance to blood flow. RESULTS--Two women with a positive result on screening had hydrosalpinges, 10 a benign tumour or a tumour-like condition, and eight primary ovarian cancers. No areas of neovascularisation were seen in the 30 women with morphologically normal ovaries and the two patients with hydrosalpinges; the pulsatility index ranged from 3.1 to 9.4. Similarly, nine patients (10 affected ovaries) with a non-malignant mass had no signs of neovascularisation and the pulsatility index varied from 3.2 to 7.0. One patient with bilateral dermoid cysts containing nests of thyroid-like cells had vascular changes and pulsatility index values of 0.4 and 0.8. Seven patients (eight ovaries) with primary ovarian cancer (one stage IV, four stage II, and two stage Ia) showed clear evidence of neovascularisation and pulsatility index values were from 0.3 to 1.0. One patient with an intraepithelial serous cystadenocarcinoma in a small ovary (less than 5 ml volume) had no signs of any vascular change and the pulsatility index was 5.5. CONCLUSION--Transvaginal colour flow imaging may be used to identify potentially malignant ovarian masses and help elucidate the early stages of tumorigenesis. The routine application of this technique may reduce the rate of false positive results of an ultrasonography based screening procedure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baber R. J., McSweeney M. B., Gill R. W., Porter R. N., Picker R. H., Warren P. S., Kossoff G., Saunders D. M. Transvaginal pulsed Doppler ultrasound assessment of blood flow to the corpus luteum in IVF patients following embryo transfer. Br J Obstet Gynaecol. 1988 Dec;95(12):1226–1230. doi: 10.1111/j.1471-0528.1988.tb06810.x. [DOI] [PubMed] [Google Scholar]
- Campbell S., Bhan V., Royston P., Whitehead M. I., Collins W. P. Transabdominal ultrasound screening for early ovarian cancer. BMJ. 1989 Dec 2;299(6712):1363–1367. doi: 10.1136/bmj.299.6712.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S., Goessens L., Goswamy R., Whitehead M. Real-time ultrasonography for determination of ovarian morphology and volume. A possible early screening test for ovarian cancer? Lancet. 1982 Feb 20;1(8269):425–426. doi: 10.1016/s0140-6736(82)91622-1. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Shepherd J. H. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989 Aug;96(8):889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto K., Sakuma S., Ishigaki T., Makino N. Intratumoral blood flow: evaluation with color Doppler echography. Radiology. 1987 Dec;165(3):683–685. doi: 10.1148/radiology.165.3.2825234. [DOI] [PubMed] [Google Scholar]
- Taylor K. J., Burns P. N., Wells P. N., Conway D. I., Hull M. G. Ultrasound Doppler flow studies of the ovarian and uterine arteries. Br J Obstet Gynaecol. 1985 Mar;92(3):240–246. doi: 10.1111/j.1471-0528.1985.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Taylor K. J., Ramos I., Carter D., Morse S. S., Snower D., Fortune K. Correlation of Doppler US tumor signals with neovascular morphologic features. Radiology. 1988 Jan;166(1 Pt 1):57–62. doi: 10.1148/radiology.166.1.2447604. [DOI] [PubMed] [Google Scholar]
- Thompson R. S., Trudinger B. J., Cook C. M. Doppler ultrasound waveform indices: A/B ratio, pulsatility index and Pourcelot ratio. Br J Obstet Gynaecol. 1988 Jun;95(6):581–588. doi: 10.1111/j.1471-0528.1988.tb09487.x. [DOI] [PubMed] [Google Scholar]
- Timor-Tritsch I. E., Bar-Yam Y., Elgali S., Rottem S. The technique of transvaginal sonography with the use of a 6.5 MHz probe. Am J Obstet Gynecol. 1988 May;158(5):1019–1024. doi: 10.1016/0002-9378(88)90210-4. [DOI] [PubMed] [Google Scholar]