Abstract
Background
The relative ease of targeted gene disruption in the social amoeba Dictyostelium has stimulated its widespread use as an experimental organism for cell and developmental biology. However, the field has been hamstrung by the lack of techniques to recombine disrupted genes.
Results
We describe new techniques for parasexual fusion of strains in liquid medium, selection and maintenance of the resulting stable diploid strains, and segregation to make recombined haploids. We have used these techniques to isolate rasS/gefB double nulls. The phenotypes of these mutants are no more severe than either parent, with movement, phagocytosis and fluid-phase endocytosis affected to the same degree as in rasS or gefB single nulls. In addition, we have produced diploids from one AX2- and one AX3-derived parent, providing an axenic strain with fewer secondary phenotypes than has been previously available.
Conclusions
The phenotype of the rasS/gefB double mutant suggests that the RasS and GefB proteins lie on the same linear pathway. In addition, axenic diploids and the techniques to generate, maintain and segregate them will be productive tools for future work on Dictyostelium. They will particularly facilitate generation of multiple mutants and manuipulation of essential genes.
Background
A large body of work in the 1970s and 1980s showed that parasexual recombination of haploid Dictyostelium discoideum strains was a potent tool for generating multiple mutants and constructing relatively complex genetic experiments [1-3]. During normal starvation, pairs of haploid cells can occasionally fuse, apparently at random, to give diploid progeny. These are stable enough to grow, develop and form spores while remaining diploid. If cells of two different strains, each carrying a different selectable marker, are starved together, diploids will be formed from one cell of each parental strain. These can be separated from the haploid background by applying both selections simultaneously, so each haploid parent is killed but diploids survive. As long as selection is maintained, the diploids may remain reasonably stable, but there is a continual process of haploidization in which individual lines lose one chromosome of each diploid pair. This segregation is apparently random, which means that diploids can be used to reassort chromosomes from different haploid strains, in much the same way as sexual recombination. The process has therefore been called parasexual genetics, because the two parents are usually of the same mating type, and because crossovers between the paired chromosomes of diploids very rarely occur. Loomis (1987) gives a thorough discussion of the mechanics of parasexual genetics [4].
More recently, Dictyostelium has gained popularity as an experimental organism because of the relative ease with which gene disruption mutants may be generated. However, genetics has almost never been used in conjunction with gene disruption. The main reason for this lies in the way cells are grown. Earlier work on parasexual genetics chiefly used cells which had been grown on bacterial lawns [5-7]. Such cells are healthy and grow rapidly, but are unsuitable for most molecular genetic manipulations. The bacteria cause several difficulties. They provide a large reservoir of exogenous DNA, which complicates experiments, and they frequently sequester or break down the drugs used to select transformed cells. Most molecular genetic experiments are therefore performed on cells grown axenically in liquid medium. However, the techniques most frequently used to select for diploids in bacterially-grown Dictyostelium have proved to be unworkable under axenic conditions. The bsg selectable markers require growth on Bacillus subtilis [8], and while selections using temperature sensitive mutants have been successful, the resulting diploid strains have been highly unstable and unsuitable for genetic manipulations, apparently because diploid growth is extremely inefficient at the high temperatures used for selection [9].
A set of techniques for generating and handling diploid strains would be invaluable for Dictyostelium workers. One major problem for the field has been the relative lack of selectable markers. Only three markers have been widely used for gene disruption – pyr56 [10], thyA [11] and blasticidin resistance (Bsr) [12]. Selections with pyr56 and thyA have never been performed together, and other drugs such as G418 and hygromycin have proved inefficient for gene disruption. These experimental limitations have made the generation of double disruptants difficult, and more complex mutants seriously problematical. An experimentally usable parasexual cycle would enable existing mutants to be crossed, even if they were made using the same selectable marker, and thus greatly diminish this problem.
In this paper we describe techniques for generating, handling and segregating diploid Dictyostelium in axenic medium. We have used these techniques to recombine rasS and gefB mutants, generating a rasS-/gefB- double null. RasS is one of at least seven Dictyostelium ras proteins [13], and GefB one of a large family of Ras guanine nucleotide exchange factors (RasGEFs), which activate Ras proteins [13]. The exact numbers in each family will not be known until the genome is completely sequenced, but at least 20 have already been identified, making redundancy nearly certain. A thorough analysis of Ras pathways in Dictyostelium will therefore depend on an effective method of recombining gene disruptants. RasS and GefB are particularly suitable for this study because of conflicting data about their genetic relationship [14]. Mutants in both genes move unusually rapidly and have serious defects in fluid-phase endocytosis [15,16], but recent work has shown that the two mutants move rapidly using different mechanisms [14]. Two explanations are possible. Either RasS and GefB lie in parallel pathways which are needed for related biological functions, or the pathway is linear and GefB is the principal GEF for RasS but the differences in motility are incidental. In the first case, double mutants should display a compound phenotype, while a linear pathway should yield double mutants with no additional defects. In this paper we describe a genetic approach to this question.
Results
Selection and Maintenance of Diploids in Axenic Culture
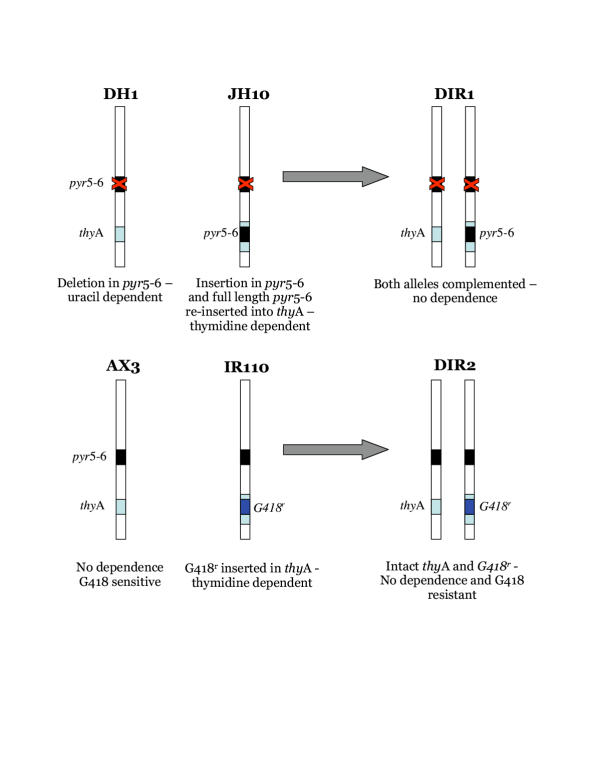
Previous attempts at growing diploid Dictyostelium in axenic culture have not been productive. This is mainly caused by incompatibility between axenic growth and the selectable markers used to maintain the strains. Diploids are formed at low efficiency during normal Dictyostelium starvation [17]. Experimental isolation of diploids therefore requires genetic selections which allow diploids, but not haploids, to grow. Typical selections involve two parents, each of which carries a different recessive mutation, so neither parent can grow under appropriate selective conditions [7]. In diploids both mutants are complemented, so growth is possible. Some selections, for example growth on Bacillus subtilis, cannot be used without bacteria, while temperature sensitivity selections are apparently too harsh for efficient axenic growth of diploids [9]. We therefore designed schemes for selecting diploids which work optimally under axenic conditions. Fig. 1 shows diagrams of two such schemes. In fig. 1a one parent, DH1, carries a deletion in the pyr56 gene and thus requires exogenous uracil for growth [18]. The other parent is JH10, which requires added thymidine because of an insertion of pyr56 in the thyA gene [19]. Thus neither parent is able to grow in FM minimal medium. Diploids formed by the fusion of JH10 and DH1 cells carry a single copy of both pyr56 and thyA, however, and are thus able to grow in FM.
Figure 1.
Selection of diploid strains. The figure shows a schematic representation of the genotype on chromosome 3.
A second scheme is shown in fig. 1b. Instead of combining two mutant strains, a wild type parent can be fused with a mutant in which the thyA gene has been disrupted using the G418r marker. Neither haploid can grow in axenic medium containing G418 but no added thymidine, but diploids grow normally. Again, diploids are stable because both markers are situated at the same locus. This scheme has several advantages and disadvantages compared with the pyr56/thyA method. In particular, previously existing mutants may be recombined without further genetic modification, but the progeny will be G418 resistant and therefore cannot be transformed by most common shuttle vectors.
Diploids are generated experimentally by shaking 5 × 106 cells of JH10 and DH1 overnight in 10 ml FM medium, then transferring the whole mixture to a Petri plate and incubating for 10 days. As negative controls, 5 × 106 cells of each parent are used alone. Under these conditions we generally see from 10 to 500 diploid colonies per plate. Growth in the control plates is never seen, though many DH1 cells survive without growth. Following one such fusion, we isolated one diploid clone and named it DIR1. These cells were used for most of the tests described below.
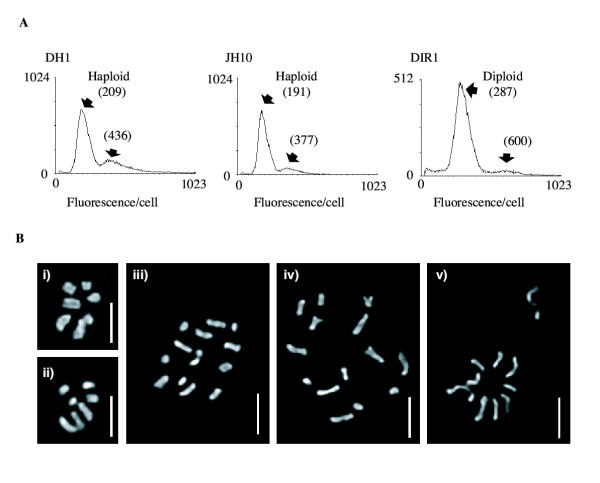
We verified that DIR1 cells were true diploids using fluorescence activated cell sorting (FACS) and chromosome staining. Fig. 2a shows a typical FACS result. After propidium iodide staining, both haploid parents exhibit a peak intensity at a DNA content of about 181 ± 13, with a smaller additional peak at 380 ± 29. DIR1 diploids, on the other hand, contained a large peak at 307 ± 14 with a smaller peak at 650 ± 42, an increase of ~70% for both peaks. This is as expected as the mitochondria contribute almost 30% of the total cellular DNA [20].
Figure 2.
The ploidy of DH1, JH10 and DIR1 cells was verified by (A) FACS analysis of DNA content, and (B) cytological staining of mitotic nuclei to visualise the chromomsomes. Strains are: (i) DH1, (ii) JH10, (iii) & (iv) DIR1. Panel (v) shows a possible mitotic non-disjunction event after exposure to thiabendazole. Bars indicate 2 μm.
Figure 2b shows mitotic cells from DIR1 and both parents stained with the DNA-specific dye DAPI. The larger complement of chromosomes of the diploids is clearly visible. It is interesting to note that there are plainly seven chromosomes in many of the images from haploid parents, and fourteen chromosomes in the diploids. We have examined a large number of mitotics spreads, and while it is often ambiguous whether apparently overlapping blobs are separate chromosomes, about half of all haploid nuclei examined clearly contain seven separate entities. We also checked cells from other strains, including AX2 and the nonaxenic parents NC4 and V12 (data not shown). In each case, seven mitotic chromosomes were clearly visible. Although older data had concluded that Dictyostelium contains seven chromosomes [2,21-23], it has been difficult to find markers in more than six linkage groups, leading to a consensus that only six bona fide chromosomes exist [24-26]. This argument has recently been addressed by Sucgang et al. (2003), who conclude that one of the seven chromosomes seen by DAPI staining derives from multiple copies of the ribosomal DNA aggregated into a single, chromosome-sized body which disintegrates during FISH [27].
Growth and Stability of Diploids
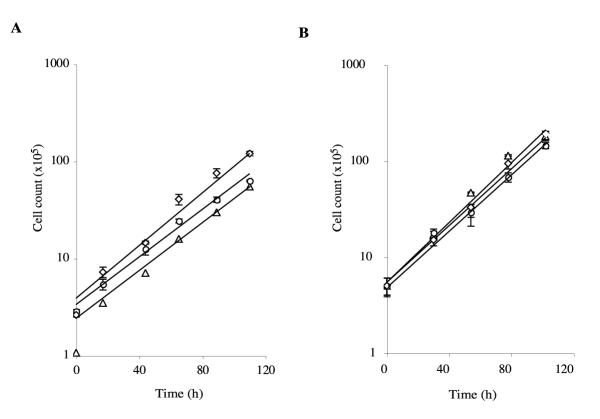
Diploid cells grow well in axenic medium under a variety of conditions (Fig. 3). In petri dishes, DIR1 cells grow as rapidly under selective conditions (without additions) as either parent (with thymidine or uracil added as appropriate). The doubling time under these conditions is just over 20 hrs for DIR1 diploids and both parents. When cells are grown in shaken culture, the growth rate of diploids is also similar to that of haploids. Shaken DIR1 double every 21 hours, compared with approximately 19 hours for JH10 and DH1. The long-term stability and ploidy of DIR1 diploids is ensured by the way in which the JH10 parental strain was generated. JH8, the strain from which JH10 was made, contains an deletion in the pyr56 gene and JH10 was then generated from this by re-insertion of the intact pyr56 gene into thyA. As indicated in fig. 1a, this means that the intact pyr56 gene in JH10 is situated at the same genetic locus as the thyA gene in DH1. This means that no normal segregation can generate a haploid that is able to grow in FM minimal medium. Only an unequal crossover or similar, extremely rare, event can generate haploid cells containing both thyA and pyr56. DIR1 diploids are thus exceedingly stable when grown under selective conditions. The growth of diploids is strongly favoured even in milder conditions – thyA- haploids die and pyr56- cells grow extremely poorly in normal, unsupplemented axenic medium (data not shown). Even after 30 generations of growth under these conditions, >60% cells are diploid. It is important to note that, due to the difficulty in getting perfectly separate chromosomes, the proportion of diploid cells estimated by cytological staining is likely to be underestimated – many of the cells scored as having 12 or 13 chromosomes are actually diploid.
Figure 3.
Growth of DIR1 diploids in FM medium in (A) dishes and (B) shaken flasks. Circles – DH1 in FM + uracil; diamonds – JH10 in FM + thymidine; triangles – DIR1 in FM.
Previous work using axenically grown diploids has been thwarted by instability of diploid cells when selection cannot be maintained. We were therefore surprised to find that DIR1 diploids were remarkably stable even in the absence of selection. As shown in table 2, even after 20 generations grown in medium supplemented with both uracil and thymidine, 42% of the cells were still diploid. Similarly, DIR1 diploids could be grown for prolonged periods on bacterial plates without reverting to the haploid state, despite the lack of selection under these conditions. 96% of bacterially grown cells were still diploid after 2 weeks growth, making growth on bacterial lawns feasible for cloning or assessing development.
Table 2.
Stability of DIR1 diploid cells under different growth conditions. Diploid cells were grown as shown and ploidy measured by cytological analysis.
| Growth conditions | No. Generations | % Haploid | % Diploid |
| FM | 30 | 3 | 73 |
| AM | 20 | 5 | 61 |
| FM + thymidine & uracil | 20 | 8 | 42 |
Table 1.
Strains used in this work.
| Strain | Parent(s) | Genotype | Ref. |
| AX2 | NC4 | Wild type (axenic) | [34] |
| AX3 | NC4 | Wild type (axenic) | [35] |
| DH1 | AX3 | pyr56- | [18] |
| JH10 | AX3 | thyA- | [19] |
| IR110 | AX3 | thyA::G418r | this work |
| IR150 | AX2 | thyA::G418r | this work |
| DIR1 | DH1/JH10 | pyr56+/-, thyA+/- | this work |
| DIR2 | AX3/IR150 | thyA+/thyA::G418r | this work |
| JK41 | AX3 | rasS-, gefB- | this work |
| JK42 | AX3 | rasS-, gefB- | this work |
Resegregation from Diploid to Haploid Using Mitotic Inhibitors
In previous work, bacterially grown diploids were segregated to give haploids by growing them on bacterial plates containing microtubule inhibitors such as benomyl (also known as benlate) or thiabendazole [28,29]. As shown in table 3, DIR1 diploids plated clonally on lawns of Klebsiella on agar supplemented with benomyl or thiabendazole segregated rapidly to give true haploids.
Table 3.
Segregation of DIR1 diploids on bacterial plates supplemented with microtubule inhibitors. Cells were plated clonally on bacterial lawns and, after two weeks, samples from the edge of the colonies were picked and screened for nutritionally dependent growth.
| Growth conditions | No. Generations | Proportion Diploid |
| - | 2 weeks | 28/29 |
| 20 μg/ml benomyl | 2 weeks | 0/12 |
| 2 μg/ml thiabendazole | 2 weeks | 0/22 |
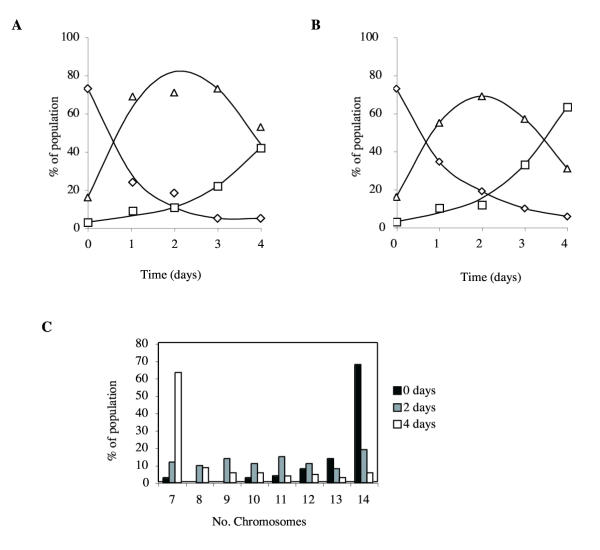
Since the aim of this work was to avoid the need for bacterial growth, we also tested the effects of benomyl and thiabendazole during axenic growth. DIR1 cells were grown in the presence of 10 μg/ml benomyl or 5 μg/ml thiabendazole, and samples were taken every day and cytologically stained to count chromosomes. Fig. 4 shows the process of segregation of diploid cells. After two days growing in benomyl or thiabendazole, as many as 69% of the cells were aneuploid, with the rest split between diploid and haploid. Four days of treatment resulted in a population in which nearly all cells were haploid. It is therefore clear that segregation under axenic conditions is an efficient way of generating haploid progeny from diploid parents.
Figure 4.
Segregation of diploids. Diploid cells were grown in axenic medium in the presence of (a) 10 μg/ml benomyl and (b & c) 5 μg/ml thiabendazole. DIR1 cells were grown for the times indicated in axenic medium supplemented with thymidine and uracil. Ploidy was measured by cytological staining and scoring 100 cells for chromosome number. Diamonds indicate diploid cells, squares indicate haploid cells, and triangles indicate aneuploids. Note that the true level of aneuploidy is overstated because of overlaps between chromosomes in the diploid images.
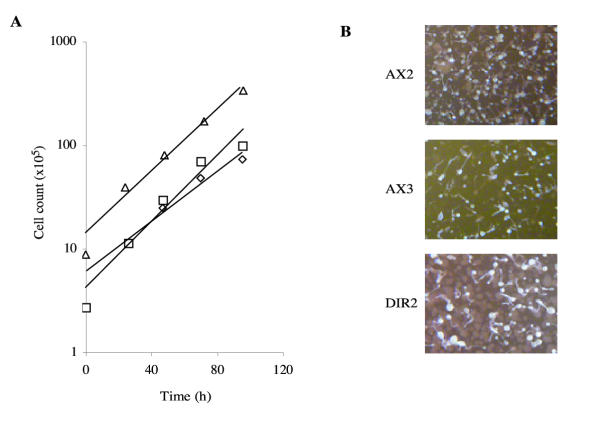
Generation of Axenic AX2/AX3 Diploids
We generated diploids with one AX2 and one AX3 parent in order to confirm that the mechanisms of axenic growth were compatible between the two strains (figure 5). Several researchers have observed major differences in behaviour between AX2 and AX3, including cell size, macropinosome formation, and the phenotypes of mutations which affect proteins such as IQGAPs [30,31] and RasS (R.H.I., unpublished data). These differences led us to wonder whether additional mutations were needed for optimal growth in addition to the three known axenic loci, and these were different in AX2 and AX3. In this case, diploids with one AX2 and one AX3-derived parent would grow more slowly than either parent, or even not at all. To our initial surprise, AX2/AX3 diploids (here named DIR2) are as healthy or healthier than either parent, giving robust growth in axenic medium, and producing large colonies which sometimes produce larger fruiting bodies than either parent (fig. 5). In retrospect this observation is less surprising. AX2 and AX3 are known to carry the same three axenic mutations. Each was generated independently by heavy mutagenesis of NC4. They therefore presumably both carry a large number of secondary mutations, but in different loci. In AX2/AX3 diploids like DIR2, most of the secondary mutations from each parent are likely to be complemented by intact genes in the other.
Figure 5.
Diploid hybrids between AX2 and AX3. (A) Growth of DIR2 (AX2/AX3) diploids in shaken flasks (squares) and dishes (diamonds) and AX2 cells in dishes (triangles) in HL-5 medium. (B) Fruiting body morphology of cells after development on clearing plates.
Generation and Characterisation of a rasS/gefB Double Knockout
One of the earlier uses of parasexual genetics was to recombine mutations from different parents, in particular to generate multiple mutants. We used the diploid system to address an interesting question from our previous research. Null mutants in rasS and gefB show a number of similar phenotypes, including diminished endoctyosis and faster cell translocation [15,16]. However, to our surprise, we recently found that the rapid movement was driven by different mechanisms in rasS- and gefB- cells [14]. We wished to understand whether RasS and GefB act together in the same pathway – in which case the differences in mechanism are incidental, and double mutants will resemble both single mutants – or in parallel pathways, controlling different aspects of cell movement, in which case double mutants should show a much more severe phenotype than either parent.
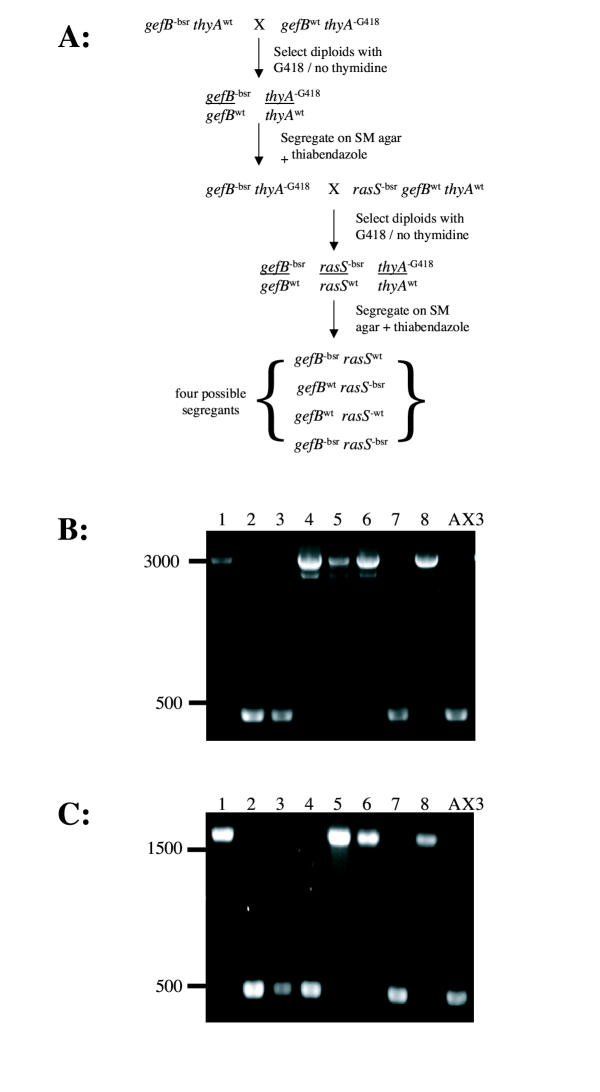
We used the Thy/G418 system to generate rasS/gefB double nulls (the scheme is shown in fig. 6a), because as described earlier it allowed us to cross pre-existing strains. First, a gefB null strain was crossed with the Thy/G418 strain IR110 to give a gefB-/gefBwt thyAG418/thyAwt diploid. This was segregated to give haploids, and a gefB- thyAG418 clone was selected. This in turn was crossed with a rasS null to give a rasS-/rasSwt gefB-/gefBwt thyAG418/thyAwt diploid. When this diploid was segregated, all possible combinations of mutant and wild type in rasS and gefB were found, as shown by PCR screens (fig. 6b &6c). We selected two rasS- gefB- double mutant clones and named them JK41 and JK42. The whole procedure took a little over one month, with relatively few hours of hands-on work during that period.
Figure 6.
Production of a rasS/gefB double mutant by parasexual recombination. (A) Schematic diagram showing the recombination process (B) PCR screen for rasS disruption. Wild-type alleles give a 450 bp product, disrupted alleles give a product of 3000 bp.(C) PCR screen for gefB disruption. Wild-type alleles give a 480 bp product, disrupted alleles give a product of 1550 bp.
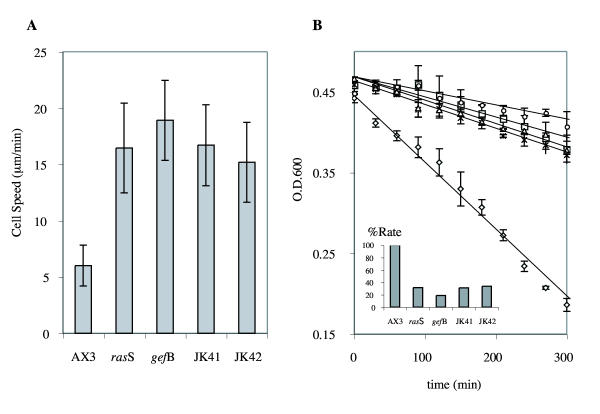
Examination of the double mutants revealed phenotypes very similar to either parent. In particular, there was no sign of a synthetic phenotype – in each case, the double mutants were no worse affected than their parents. Colonies resembled both parents in the morphology of their feeding fronts, producing fruiting bodies which resembled those produced by the gefB- parent (data not shown). In two assays which have been used to define the phenotypes of rasS- and gefB- mutants – unstimulated cell speed and phagocytosis – the double mutants were as severely affected as each parent, but no worse. Figure 7 shows two such assays. In fig. 7a, the cell speed of unstimulated, bacterially-grown double mutants is around three times the speed of wild type cells, comparable with both rasS- and gefB- parents. Fig. 7b shows the rate at which cells clear a suspension of bacteria. Wild type cells diminished the OD600 from 0.45 to below 0.2 in 5 hours. In the same interval, rasS- and gefB- mutants only cleared the OD600 to around 0.4. Both double mutants behaved in the same way, revealing a serious defect in phagocytosis in all mutant strains, but one that is not worsened by the double disruption.
Figure 7.
Relative rates of (a) motility and (b) phagocytosis of rasS-, gefB- and double mutant strains. Rates of phagocytosis were determined by the decrease in optical density of a bacterial suspension as bacteria are removed. The rate of phagocytosis relative to wild-type cells is plotted in the inset. Motility values are the means ± SD of 10 cells. OD values are the means ± SD of triplicate experiments.
Since the double mutants are consistently similar to both singly mutated parents, we conclude that the RasS and GefB proteins make up successive stages of a single pathway.
Discussion
In this work, we have adapted the techniques of Dictyostelium parasexual genetics to make them a versatile and important tool for future studies. Parasexual genetics and diploid cells have been rarely used in the past 20 years, principally because the selection schemes which were previously used are not appropriate for axenic culture, and are therefore impractical for most of the techniques and transformed cell lines used in molecular genetics. We have used a selection scheme which is ideal in axenic culture, and has the additional advantage of making highly stable diploids, because the selectable markers are present at the same genetic locus of each parent. We anticipate that our protocols will be widely used in coming years.
The ability to handle diploids allows several possibilities. Firstly, as described for rasS and gefB in this work, pre-existing mutants may be recombined to give double and multiple knockouts. Currently this technique does not permit intrachromosomal rearrangements, so only one locus per chromosome can be manipulated. Mitotic recombination and sexual crossover are both known to occur in Dictyostelium, though [32,33], so we look forward to addressing this limitation in the near future. Secondly, it will now be possible to make gene disruptions in genes thought to be required for survival. This will allow a test for genetic lethality, because heterozygote mutants will be unable to segregate into haploids containing the gene disruption. Thirdly, we will be able to address a number of issues where the parental background of mutants affects their phenotype. In many cases, inconsistent results have been obtained by comparing AX2- and AX3-based cells. For cells to grow axenically, at least three genes must be mutated. In the two most widely used axenic strains, AX2 and AX3, these mutations were generated by heavy mutagenesis followed by a long period of selection for growth in liquid medium [34,35]. As a result, both AX2 and AX3 (which were generated independently) are less robust in growth on bacteria and development than their parent, NC4. Classical Dictyostelium mutagenesis using DNA-damaging agents causes multiple mutations, so we presume that both AX2 and AX3 strains have several secondary mutations which are responsible for this multifaceted lack of robustness. By making AX2/AX3 hybrids, we have generated a strain missing the secondary mutations which presumably cause inconsistency, and which should therefore be a much closer relative of wild type cells.
The lack of a synthetic phenotype in rasS and gefB double mutants strongly suggests that these genes are a part of the same genetic pathway. When the gefB gene was first disrupted [15], the mutant phenotype's similarities to those of rasS nulls [16] led to the conclusion that both genes acted in the same pathway. However, a detailed analysis of the motility of rasS and gefB mutants showed that, while both moved rapidly, the mechanisms underlying the rapid movement were completely different [14]. rasS null cells move by extending a number of short-lived, rapidly-extending pseudopods, while gefB nulls use a single, dominant pseudopod which is much larger than usual. These differences raised the possibility that rasS and gefB might be involved in different aspects of motility. This work strongly suggests that only a single pathway is involved, which leads to an opposite conclusion. The movement phenotypes of rasS and gefB mutants, different as they are on the surface, must be caused by the same underlying defect. Presumably this defect leads to rapid actin turnover, but the manner in which the excess actin is organised can vary from cell type to cell type. This is a surprising conclusion for the cell motility field, and an elegant demonstration of the usefulness of parasexual genetics. We also look forward to more detailed studies on the motility of the double mutants, which would show which pseudopod behaviour is dominant, or whether a third mechanism of rapid motility is seen.
Conclusions
The diploid systems described in this work are an important technical advance and will facilitate a number of experiments that are currently impractical. We have used parasexual recombination to show that rasS and gefB work through the same genetic pathway, presumably because the RasGEFB protein directly activates RasS.
Methods
Cell strains and culture
For selection of diploids 5 × 106 cells of each parental strain were co-cultured in shaking flasks overnight in FM medium [36]. For selections with G418 the medium was subsequently changed to normal HL-5 medium and selected at 10 μg/ml. All medium was supplemented with a cocktail of vitamins (20 μg/l biotin, 5 μg/l cyanocobalamin, 0.2 mg/l folic acid, 0.4 mg/l lipoic acid, 0.5 mg/l riboflavin, 0.6 mg/l thiamine). Cells were cloned on SM agar plates in association with Klebsiella aerogenes [37]. When screening for nutritionally dependant growth, either 20 μg/ml uracil or 100 μg/ml thymidine were added to normal medium as indicated.
Cytological staining
For cytological examination of cell ploidy, 5 × 106 cells were seeded in a 5 cm petri dish containing acid-washed glass coverslips and allowed to adhere for 2 hours. In normal growth the mitotic index of axenic cells is <1%, therefore to facilitate observation of multiple mitotic nuclei the cells were treated with 33 μM nocodazole for a further 2 hours. This raised the mitotic index to 10–20% [38].
Prior to fixation the medium was aspirated off and replaced with ice-cold distilled water for 10 minutes. Cells were then fixed in ice-cold Carnoy's solution (3:1 ethanol:acetic acid) for 1 hour and then replaced with fresh fix for a further 10 minutes. The fix was then aspirated off and the coverslips left to air-dry. The coverslips were then stained by mounting in 3 μl Vectashield mounting medium (Vector laboratories) containing 10 μg/ml DAPI.
Coverslips were then examined with a Zeiss 100× oil immersion lens (N. A. 1.4) on a Zeiss fluorescence microscope with a further 2.5× magnification set on the optovar. Images were recorded with a digital camera and slices taken through a cell. Images were processed by deconvolution software supplied with Openlab (Improvision).
When determining ploidy, approximately 100 cells with clearly condensed chromosomes were scored in each case.
FACS analysis
24 hours prior to analysis, axenically grown cells were seeded at 20% confluency in 9.5 cm petri dishes. On the day of analysis cells were harvested and pelleted before resuspension and fixation in 10 ml methanol at -15°C. The cells were then washed twice in PBS before being pelleted and treated with 100 μl of 200 μg/ml RNase A (in PBS) for 20 minutes at 22°C. DNA was then stained with 400 μl of 50 μg/ml propidium iodide (in PBS) and analysed by flow cytometry in a Coulter FACS machine using an excitation wavelength of 488 nm. At least 10,000 cells were counted for each sample.
Disruption of thyA
The cloned thyA vector pGEM25 (a kind gift from Dr. Rick Firtel) was used to make a gene disruption construct. The 2 Kb XbaI fragment from pRIT14 [39] containing the neomycin resistance gene under control of the actin 15 promoter was blunted and ligated into the SwaI site of pGEM25 in the same direction as the thyA gene. The disruption construct was then generated by digestion with HindIII.
kAX3 cells were then transformed by electroporation using a modified version of the protocol of Tuxworth et al. [39]. Briefly, 1.6 × 107 cells growing in log phase were resuspended in 0. 4 ml sucrose buffer and mixed with 20 μg of linearised DNA. They were then electroporated at 1.1 kV, 3 μF with a 5 Ω resistor in series. After 10 minutes incubation on ice, cells were healed for 15 minutes by mixing with 2 μl of a solution of 100 mM CaCl2, 100 mM MgCl2 before addition of HL-5 medium. After 1 day of recovery selection was started at 10 μg/ml G418. After 10 days numerous G418 resistant colonies were seen and cloned onto SM agar plates in association with Klebsiella aerogenes. These clones were then picked and screened for growth dependant on exogenous thymidine.
Endocytosis assay
For phagocytosis assays using bacterial cells, the decrease in OD600 of a suspension of E. coli B/r inoculated with Dictyostelium was used as an indicator of phagocytic rate as described [40]. Briefly, clearing plates of amoebae were harvested and 2 × 107 cells were added to a suspension of bacteria in KK2 at an O.D. of approximately 0.6. This was then shaken at 180 rpm. Samples were removed every 30 min and their OD600 measured using a spectrophotometer.
Analysis of cell speed
Cell speed of bacterially grown cells was measured by removing an inoculation of cells from the very edge of the feeding front of a colony, resuspending in KK2 buffer and allowing the cells to adhere to a 5 cm Petri dish. After 30 minutes, washing once, the cells were then filmed using time-lapse recordings with a 32x phase-contrast objective on a Zeiss Axiovert inverted microscope. 15 frames at 1 minute intervals were recorded and analysed using NIH Image 1.62 software to gain an estimate of mean cell speed in terms of mean centroid displacement per frame. Centroids were determined by eye and at least 10 cells were measured for each strain.
Authors' Contributions
RHI designed and verified the selections, and supervised the work. JK performed all the remainder of the work and generated the figures. The writing of the paper was split equally.
Acknowledgments
Acknowledgements
We are grateful to Dr. Jeff Hadwiger for JH10 cells, and Dr. Gerry Weeks for comments on the manuscript.
This work was supported by an MRC Senior Fellowship to R.H.I. and BBSRC project grant 6/G17939. We are also grateful for the assistance of the Birmingham Functional Genomics laboratory, supported by JIF grant 6/JIF13209, and the Biosciences Life Sciences Imaging facility.
Contributor Information
Jason King, Email: jasonking50@yahoo.co.uk.
Robert H Insall, Email: r.h.insall@bham.ac.uk.
References
- Sussman M, Schindler J, Kim H. "Sluggers", a new class of morphogenetic mutants of D. discoideum. Exp Cell Res. 1978;116:217–227. doi: 10.1016/0014-4827(78)90078-2. [DOI] [PubMed] [Google Scholar]
- Brody T, Williams KL. Cytological analysis of the parasexual cycle in Dictyostelium discoideum. J Gen Microbiol. 1974;82:371–383. [Google Scholar]
- Coukell MB. Parasexual genetic analysis of aggregation-deficient mutants of Dictyostelium discoideum. Mol Gen Genet. 1975;142:119–135. doi: 10.1007/BF00266094. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Genetic tools for Dictyostelium discoideum. In: Spudich J A, editor. In Methods in Cell Biology. Vol. 28. Orlando, FL, Ac. Press; 1987. pp. 31–65. [DOI] [PubMed] [Google Scholar]
- Sussman RR, Sussman M. Ploidal inheritance in the slime mould Dictyostelium discoideum: Haploidization and genetic segregation of diploid strains. J Gen Microbiol. 1963;30:349–355. doi: 10.1099/00221287-30-3-349. [DOI] [PubMed] [Google Scholar]
- Sinha U, Ashworth JM. Evidence for the existence of elements of a para-sexual cycle in the cellular slime mould, Dictyostelium discoideum. Proc R Soc Lond B. 1969;173:531–540. [Google Scholar]
- Katz ER, Sussman M. Parasexual recombination in Dictyostelium discoideum: selection of stable diploid heterozygotes and stable haploid segregants. Proc Natl Acad Sci USA. 1972;69:495–498. doi: 10.1073/pnas.69.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell PC, Henderson RF, Mosses D, Ratner DI. Sensitivity to Bacillus subtilis: A novel system for selection of heterozygous diploids of Dictyostelium discoideum. J Gen Microbiol. 1977;100:207–211. [Google Scholar]
- Williams KL, Kessin RH, Newell PC. Genetics of growth in axenic medium of the cellular slime mould Dictyostelium discoideum. Nature. 1974;247:142–143. doi: 10.1038/247142a0. [DOI] [PubMed] [Google Scholar]
- Faure M, Camonis JH, Jacquet M. Molecular characterization of a Dictyostelium discoideum gene encoding a multifunctional enzyme of the pyrimidine pathway. Eur J Biochem. 1989;179:345–358. doi: 10.1111/j.1432-1033.1989.tb14560.x. [DOI] [PubMed] [Google Scholar]
- Dynes JL, Firtel RA. Molecular complementation of a genetic marker in Dictyostelium using a genomic DNA library. Proc Natl Acad Sci USA. 1989;86:7966–7970. doi: 10.1073/pnas.86.20.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. A transformation vector for Dictyostelium discoideum with a new selectable marker bsr. Plasmid. 1993;30:150–154. doi: 10.1006/plas.1993.1042. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Insall RH. Dictyostelium: an ideal organism for genetic dissection of Ras signalling networks. Biochim Biophys Acta. 2001;1525:262–271. doi: 10.1016/S0304-4165(01)00111-8. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Wilkins A, Wessels DJ, Soll DR, Insall RH. Pseudopodium dynamics and rapid cell movement in Dictyostelium Ras pathway mutants. Cell Motil Cytoskel. 2002;53:150–162. doi: 10.1002/cm.10064. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Chubb J, Insall RH. A novel Dictyostelium RasGEF is required for normal endocytosis, cell motility and multicellular development. Curr Biol. 2000;10:1427–1437. doi: 10.1016/S0960-9822(00)00797-1. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Wilkins A, Thomas GM, Insall RH. The Dictyostelium RasS protein is required for macropinocytosis, phagocytosis and the control of cell movement. J Cell Sci. 2000;113:709–719. doi: 10.1242/jcs.113.4.709. [DOI] [PubMed] [Google Scholar]
- Sussman M. Isolation of diploid strains of Dictyostelium discoideum from haploid population. Nature. 1964;201:216. doi: 10.1038/201216a0. [DOI] [PubMed] [Google Scholar]
- Insall RH, Borleis J, Devreotes PN. The aimless RasGEF is required for processing of chemotactic signals through G-protein-coupled receptors in Dictyostelium. Curr Biol. 1996;6:719–729. doi: 10.1016/s0960-9822(09)00453-9. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Firtel RA. Analysis of Galpha4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Devel. 1992;6:38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- Weijer CJ, Duschl G, David CN. A revision of the Dictyostelium discoideum cell cycle. J Cell Sci. 1984;70:111–131. doi: 10.1242/jcs.70.1.111. [DOI] [PubMed] [Google Scholar]
- Darcy PK, Wilczynska Z, Fisher PR. Phototaxis genes on linkage group-V in Dictyostelium discoideum. FEMS Microbiol Lett. 1993;111:123–127. doi: 10.1016/0378-1097(93)90192-5. [DOI] [PubMed] [Google Scholar]
- Robson GE, Williams KL. The mitotic chromosomes of the cellular slime mould Dictyostelium discoideum: a karyotype based on Giemsa banding. J Gen Microbiol. 1977;99:191–200. [Google Scholar]
- Zada-Hames Analysis of karyotype and ploidy of Dictyostelium discoideum using colchicine induced metaphase arrest. J Gen Microbiol. 1977;99:201–208. [Google Scholar]
- Cox EC, Vocke CD, Walter S, Gregg KY, Bain ES. Electrophoretic karyotype for Dictyostelium discoideum. Proc Natl Acad Sci USA. 1990;87:8247–8251. doi: 10.1073/pnas.87.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A, Maghakian D, Bergesch P, Loomis WF. Physical mapping of genes to specific chromosomes in Dictyostelium discoideum. Genomics. 1992;13:49–61. doi: 10.1016/0888-7543(92)90201-3. [DOI] [PubMed] [Google Scholar]
- Loomis WF, Welker D, Hughes J, Maghakian D, Kuspa A. Integrated maps of the chromosomes in Dictyostelium discoideum. Genetics. 1995;141:147–157. doi: 10.1093/genetics/141.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucgang R, Chen G, Liu W, Lindsay R, Lu J, Muzny D, Shaulsky G, Loomis W, Gibbs R, Kuspa A. Sequence and structure of the extrachromosomal palindrome encoding the ribosomal RNA genes in Dictyostelium. Nucleic Acids Res. 2003;31:2361–2368. doi: 10.1093/nar/gkg348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Barrand P. Parasexual genetics in the cellular slime mould Dictyostelium discoideum: haploidisation of diploid strains using ben late. FEMS Microbiol Lett. 1978;4:155–159. doi: 10.1016/0378-1097(78)90049-6. [DOI] [Google Scholar]
- Welker DL, Williams KL. Mitotic arrest and chromosome doubling using thiabendazole, cambendazole, nocodazole, and ben late in the slime mould Dictyostelium discoideum. J Gen Microbiol. 1980;116:397–407. [Google Scholar]
- Adachi H, Takahashi Y, Hasebe T, Shirouzu M, Yokoyama S, Sutoh K. Dictyostelium IQGAP-related protein specifically involved in the completion of cytokinesis. J Cell Biol. 1997;137:891–898. doi: 10.1083/jcb.137.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Escalante R, Firtel RA. A Ras GAP is essential for cytokinesis and spatial patterning in Dictyostelium. Development. 1997;124:983–996. doi: 10.1242/dev.124.5.983. [DOI] [PubMed] [Google Scholar]
- Katz ER, Kao V. Evidence for mitotic recombination in the cellular slime mold Dictyostelium discoideum. Proc Natl Acad Sci USA. 1974;71:4025–4026. doi: 10.1073/pnas.71.10.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker DL, Williams KL. A genetic map of Dictyostelium discoideum based on mitotic recombination. Genetics. 1982;102:691–710. doi: 10.1093/genetics/102.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Ashworth JM. Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970;119:171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman R, Sussman M. Cultivation of Dictyostelium discoideum in axenic culture. Biochem Biophys Res Commun. 1967;29:53–55. doi: 10.1016/0006-291x(67)90539-6. [DOI] [PubMed] [Google Scholar]
- Franke J, Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci USA. 1977;74:2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. In: Spudich J A, editor. Methods in Cell Biology. Vol. 28. Orlando, FL, Ac. Press; 1987. pp. 9–29. [DOI] [PubMed] [Google Scholar]
- Roos UP. Probing the mechanisms of mitosis with Dictyostelium discoideum. In: Spudich J A, editor. Methods in Cell Biology. Vol. 28. Orlando, FL, Ac. Press; 1987. pp. 261–279. [DOI] [PubMed] [Google Scholar]
- Tuxworth RI, Cheetham JL, Machesky LM, Spiegelmann GB, Weeks G, Insall RH. Dictyostelium RasG is required for normal motility and cytokinesis, but not growth. J Cell Biol. 1997;138:605–614. doi: 10.1083/jcb.138.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W, Schleicher M, Noegel AA. Redundancy in the microfilament system - abnormal development of Dictyostelium cells lacking two F-actin cross-linking proteins. Cell. 1992;68:53–62. doi: 10.1016/0092-8674(92)90205-q. [DOI] [PubMed] [Google Scholar]