Abstract
Background
Predicting intrinsically disordered proteins is important in structural biology because they are thought to carry out various cellular functions even though they have no stable three-dimensional structure. We know the structures of far more ordered proteins than disordered proteins. The structural distribution of proteins in nature can therefore be inferred to differ from that of proteins whose structures have been determined experimentally. We know many more protein sequences than we do protein structures, and many of the known sequences can be expected to be those of disordered proteins. Thus it would be efficient to use the information of structure-unknown proteins in order to avoid training data sparseness. We propose a novel method for predicting which proteins are mostly disordered by using spectral graph transducer and training with a huge amount of structure-unknown sequences as well as structure-known sequences.
Results
When the proposed method was evaluated on data that included 82 disordered proteins and 526 ordered proteins, its sensitivity was 0.723 and its specificity was 0.977. It resulted in a Matthews correlation coefficient 0.202 points higher than that obtained using FoldIndex, 0.221 points higher than that obtained using the method based on plotting hydrophobicity against the number of contacts and 0.07 points higher than that obtained using support vector machines (SVMs). To examine robustness against training data sparseness, we investigated the correlation between two results obtained when the method was trained on different datasets and tested on the same dataset. The correlation coefficient for the proposed method is 0.14 higher than that for the method using SVMs. When the proposed SGT-based method was compared with four per-residue predictors (VL3, GlobPlot, DISOPRED2 and IUPred (long)), its sensitivity was 0.834 for disordered proteins, which is 0.052–0.523 higher than that of the per-residue predictors, and its specificity was 0.991 for ordered proteins, which is 0.036–0.153 higher than that of the per-residue predictors. The proposed method was also evaluated on data that included 417 partially disordered proteins. It predicted the frequency of disordered proteins to be 1.95% for the proteins with 5%–10% disordered sequences, 1.46% for the proteins with 10%–20% disordered sequences and 16.57% for proteins with 20%–40% disordered sequences.
Conclusion
The proposed method, which utilizes the information of structure-unknown data, predicts disordered proteins more accurately than other methods and is less affected by training data sparseness.
Background
Various kingdoms of life appear to have proteins or protein segments that lack a folded structure [1-3]. These proteins and segments are thought to be intrinsically disordered structures providing essential biological functions [4-8], so predicting such disorder should help us understand protein functions. Disorder have been found in proteins involved in regulatory and signaling events [4,9-11] and may provide conformational flexibility that allows proteins to interact with several structurally different targets [5,12,13].
Many studies have shown that the primary structure of disordered regions is distinct from that of structured regions [14], and this has encouraged the development of many prediction methods based on the amino acid sequence. PONDR [15], GlobPlot [16], DISOPRED [17,18], VL3 [19], DISEMBL [20], IUPred [21], and RONN [22] predict the probability of any given residue being in a disordered region by using information about the amino acid sequences near that residue. A different approach predicts disorder by binary classification of amino acid sequences into mostly disordered sequences and mostly ordered sequences [23-25]. The former approach is based on the view that features of the local sequence are a more important than features of the whole structure.
Two methods have been used for binary classification. Uversky et al. suggested that a mostly disordered protein sequence could be discriminated from an ordered one by plotting the average hydrophobicity of the residues in the sequence against the net charge of the sequence [23,24], and that method has been implemented as the web-based FoldIndex application [26]. Garbuzynskiy et al., on the other hand, classified proteins as ordered or disordered by estimating the number of contacts of the whole protein [25]. Both methods classify a target protein by using a linear discriminant function.
Linear discriminant analysis, like other classification methods, infers a discriminant function that minimizes the misclassification of training data. The parameter optimization of the linear equation is therefore strongly influenced by the distribution of training data. In the prediction of protein disorder, the analysis depends on the protein sequences that are already known to be folded or unfolded. The training will be successful if the amount of training data is large enough to approximate the distribution of all protein sequences. If the quantity of training data is too small, however, the classification boundary overfits to a local cluster of protein structures.
It is hard to find disordered proteins not only because protein structures are often determined by X-ray diffraction analysis and information about proteins that could not be crystallized for X-ray analysis is seldom reported but also because not every failure to crystallize is due to disorder. Previous studies have estimated the proportion of disordered proteins in various genomes. PONDR estimated that 60% of eukaryotic proteins and 28% of bacterial proteins include disordered regions more than 40 residues long [1,27]. DISOPRED2, on the other hand, estimated that 33.0% of eukaryotic proteins and 4.2% of bacterial proteins include disordered regions longer than 30 residues and estimated that no more than 0.5% of the sequences in the Protein Data Bank (PDB) include disorder regions longer than 30 residues [2]. Despite the appreciable frequency of disordered proteins, the sequences of few mostly disordered proteins are publicly available. Although the current version (release 3.3) of DisProt [28], which is a public database providing information about disordered proteins, provides the sequences of 458 proteins, only 82 of those proteins are more than 70% disordered.
We therefore think that protein databases might be biased against disordered proteins. If there are a lot of unknown disordered proteins, the structural distribution of proteins in nature will differ from that of proteins whose structure has been determined experimentally. Since training classifiers on data biased in this respect neglects of the actual distribution of natural proteins, the discriminative boundary should be adjusted to compensate the sparseness of the training data. Semi-supervised learning has been gaining increasing attention for dealing with problems due to data sparseness. Conventional supervised-learning methods, including support vector machines (SVMs) and neural networks, use only labeled data when optimizing the parameters of the discriminant function. In binary classification, the labeled data is a set of samples each of which is known to be positive or negative. When semi-supervised learning builds a model to improve predictions, it takes into account not only the labeled data but also the unlabeled data by adapting to the distribution of unlabeled data.
A huge amount of known-sequence data is available. UniProt (UniProt50 release 48.9), for example, which is a widely used database of protein sequences, contains 974,638 nonredundant proteins [29], many of which can be expected to include a lot of disorder. We therefore, think it efficient to utilize the information of structure-unknown proteins by using semi-supervised learning to avoid training data sparseness. And prediction that considers a robust model will provide a new indicator for protein disorder.
In this study we developed a novel method for predicting disordered proteins by using Joachims' spectral graph transducer (SGT) [30], which is a binary classification algorithm based on semi-supervised learning. It constructs a k-nearest neighbor (kNN) graph with both labeled and unlabeled examples as vertices, and the edge weight between two vertices represents their similarity. If the graph is separated into two subgraphs, both labeled and unlabeled vertices are classified into two categories. The SGT takes into account both the prediction accuracy of labeled training data and the distribution of unlabeled data, because it cuts the kNN graph so as to minimize both the misclassification of labeled vertices and the sum of edges weights across the cut. We apply the SGT to the disorder prediction problem with structure-known sequences as labeled data and structure-unknown sequences, including query sequences, as unlabeled data. The proposed method can therefore be used for training both structure-known sequences and a huge amount of structure-unknown sequences, and it creates a model that incorporates a larger protein structural space. We examined how data with no structural information improves the prediction of disordered proteins and we compared the accuracy of the proposed SGT-based method with the accuracy of an SVMs-based method and the accuracies of two other previous methods. We compared this SGT-based binary-classification method with per-residue methods by comparing their predictions for both mostly disordered proteins and mostly ordered proteins. We also estimated the false positive rate when the proposed method was used for partially disordered proteins.
Results and Discussion
Effect of structure-unknown proteins on disorder prediction
Since the SGT constructs a model on both labeled data and unlabeled data, the accuracy of its predictions is influenced structure-unknown sequences as well as structure-known sequences. Here we examine how structure-unknown sequences affect prediction accuracy.
Does structure-unknown data increase prediction accuracy?
We tested different quantities of unlabeled samples in order to find out whether structure-unknown sequences have a positive or negative effect.
The SGT classifies unlabeled data as either a disordered protein or an ordered protein, so query sequences are also treated as unlabeled data. We tried to increase prediction accuracy by using as unlabeled data not only query sequences but also large numbers of structure-unknown protein sequences.
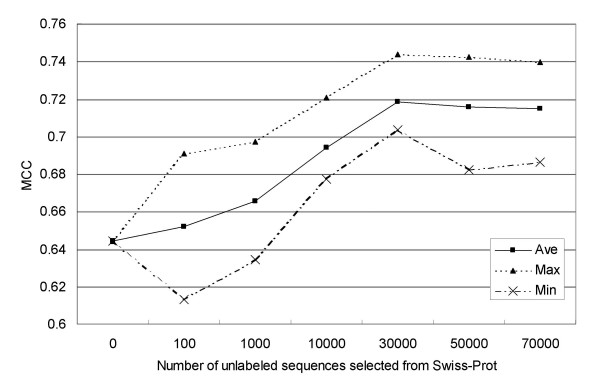
To investigate the effect of structure-unknown sequences, we prepared different quantities of unlabeled samples that were added to query sequences. Each set of unlabeled samples (structure-unknown sequences) was chosen randomly from the Swiss-Prot database. We prepared 10 different datasets for each experiment in order to avoid sampling bias, and the results we obtained are shown in Figure 1. Note that the x-axis in Figure 1 does not include the number of query sequences, and the total number of unlabeled samples in these experiments was the sum of the number of query sequences and the number of proteins selected from the Swiss-Prot database.
Figure 1.
Average (square), maximum (triangle) and minimum (X) values of Matthews correlation coefficient (MCC) for 10 different datasets in each of the seven experiments with different quantities of unlabeled data.
As shown in Figure 1, the maximum, minimum and average Matthews correlation coefficient (MCC) for the 10 datasets were highest in the experiment with 30,000 structure-unknown samples selected from the Swiss-Prot database. The computational cost of building and decomposing a kNN graph increases with the number of examples, so smaller numbers of examples are more practical with respect to computation time. Since the average MCC is almost the same for 30,000, 50,000 and 70,000 examples, 30,000 is the most practical number of examples as well as the one yielding the highest average MCC.
The recent research reported that regions of predicted disorder were found to be conserved within a large number of protein families and domains [31]. The proposed method considers information about conserved regions through similarities among sequences. The results shown in Figure 1 indicate that the proposed method effetely utilized information about conservation of protein disorder.
Curated data or uncurated data?
To investigate whether classification accuracy is affected by the quality of unlabeled samples, we used unlabeled samples from three different databases: Swiss-Prot, UniProt50 and TrEMBL. Each set of 30,000 or 70,000 unlabeled samples was chosen randomly, and we used 10 datasets from each database in order to avoid sampling bias. The results are listed in Table 1. Swiss-Prot outperformed UniProt and TrEMBL, which indicates the quality of unlabeled data is an important factor for prediction accuracy.
Table 1.
Average sensitivity (SEN), specificity (SPC), two-state accuracy (Q2) and Matthews correlation coefficient (MCC) for 10 different data sets in each experiment with different databases.
| SEN | SPC | Q2 | MCC | |
| 30000 unlabeled sequences | ||||
| Swiss-Prot | 0.684 | 0.978 | 0.938 | 0.718 |
| Uniprot | 0.581 | 0.976 | 0.922 | 0.636 |
| TrEMBLE | 0.567 | 0.980 | 0.925 | 0.643 |
| 70000 unlabeled sequences | ||||
| Swiss-Prot | 0.701 | 0.973 | 0.936 | 0.715 |
| Uniprot | 0.570 | 0.979 | 0.923 | 0.638 |
| TrEMBLE | 0.504 | 0.978 | 0.914 | 0.585 |
Swiss-Prot is a reliable database, which is carefully organized by human curators. TrEMBL is a computer-annotated supplement to Swiss-Prot, which contains amino acid translations of all the EMBL nucleotide sequence entries, including sequences automatically predicted by gene-finding programs. UniProt consists of Swiss-Prot and TrEMBL. This means that TrEMBL and UniProt might include a lot of artificial translation of pseudogenes, which are not translated into proteins in vivo. Such databases containing noise sequences are inferred to have a background distribution distinct from that of native protein sequences, and this distinction would have a negative effect on prediction.
Which similarity measurement is best?
SGT divides a kNN graph into two subgraphs for binary classification. Since the edge weight of the graph represents the similarity of two vertices, a similarity measurement for two protein sequences has to be defined. We based predictions on three measurements (amino acid composition, composition of physicochemical properties and BLAST score) and examined which is best. The results obtained using 30,000 structure-unknown sequences are listed in Table 2. The amino acid composition yielded the best results (most discriminative predictions), and the BLAST score yielded the worst results (least discriminative predictions). Compositionally based similarity measurements were thus better for predicting dissimilar proteins than was motif or sequence similarity measurement.
Table 2.
Average sensitivity (SEN), specificity (SPC), two-state accuracy (Q2) and Matthews correlation coefficient (MCC) for predictions using different similarity measurements: amino acid composition (AA comp), physicochemical property composition (PP comp) and BLAST score.
| SEN | SPC | Q2 | MCC | |
| AA Comp | 0.684 | 0.978 | 0.938 | 0.718 |
| PP Comp | 0.569 | 0.982 | 0.926 | 0.650 |
| BLAST Score | 0.663 | 0.920 | 0.885 | 0.546 |
Comparison with previous methods
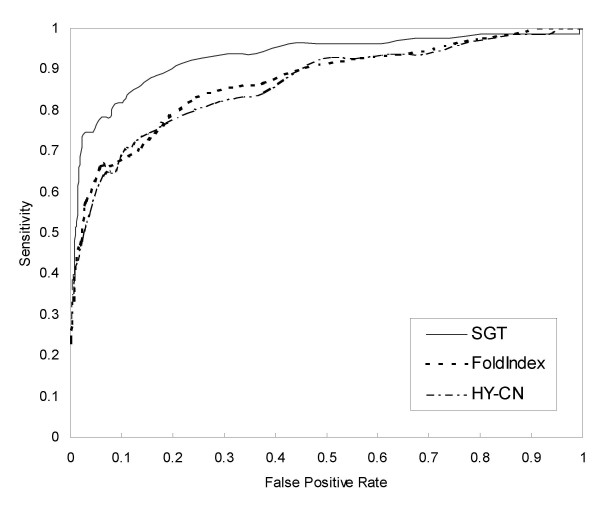
We compared the proposed method with two previous methods: FoldIndex [23] and plotting hydrophobicity against the number of contacts (hydrophobicity-contactnumber plot) [25]. FoldIndex output was obtained from the web server provided by the Israel Structural Proteomics Center, and we implemented a method for hydrophobicity-contactnumber plot because no web server or tool was available. We optimized its parameters on the same data we used for training our SGT-based method. For the proposed method, amino acid composition similarity measurement and 30,000 structure-unknown samples were used. The results are listed in Table 3. The proposed method yielded a MCC 0.202 points greater than that obtained using FoldIndex and yielded a MCC 0.221 points greater than that obtained using the method calculating the number of hydrophobic residues in contact. We also show Receiver Operating Characteristic (ROC) curves in Figure 2.
Table 3.
Sensitivity (SEN), specificity (SPC), two-state accuracy (Q2) and Matthews correlation coefficient (MCC) for the proposed method (SGT), FoldIndex, the method plotting hydrophobicity against the number of contacts (HY-CN) and an SVMs-based method (SVMs).
| SEN | SPC | Q2 | MCC | |
| SGT | 0.723 | 0.977 | 0.943 | 0.744 |
| FoldIndex | 0.663 | 0.918 | 0.884 | 0.542 |
| HY-CN | 0.663 | 0.909 | 0.876 | 0.523 |
| SVMs | 0.614 | 0.979 | 0.930 | 0.673 |
Figure 2.
ROC curves comparing our SGT-based method and previous methods.
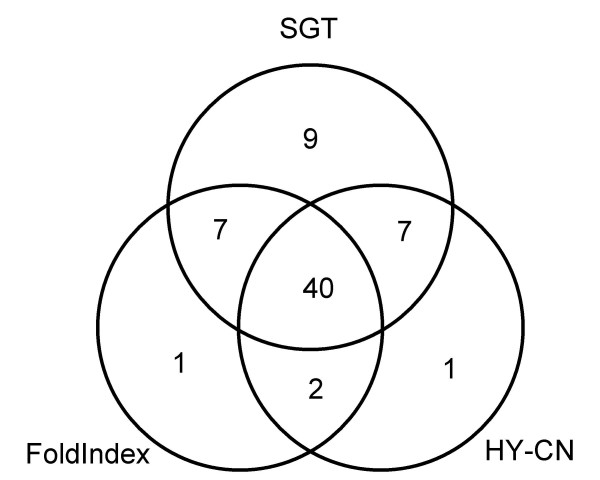
The proposed method has two advantages over previous methods. The first advantage is that it can construct a nonlinear classification boundary taking account of the background distribution of a large amount of protein sequences, which enables the classifier to avoid training data sparseness. The second advantage is that it uses more information than the previous methods do. FoldIndex and hydrophobicity-contactnumber plot postulate that a few physicochemical properties of target proteins are the main factor of disorder. Although this strategy provides a simple and clear indicator, it overlooks many disordered proteins because other complex factors are involved in protein disorder. Classification over a larger feature space should facilitate more accurate prediction. Simple indicators cannot express some features of disorder. Plotting average hydrophobicity against net charge or the number of residues in contact, for example, does not always reflect the sequence complexity, which is an important factor in the discrimination of disordered proteins [32]. If an amino acid that is used repeatedly has average hydrophobicity, net charge and contact number values, such information will remain hidden. Although the composition of the entire sequence cannot be used to distinguish the local sequence complexity, it reflects long-range complexities. We conjecture that the hydropobicity-vs-(net charge) and hydropobicity-vs-(contact number) feature spaces should be considered subsets of the amino-acid-composition feature space. The two advantages of the proposed method enable it to identify more disordered proteins. For the same false positive rate (5%), SGT found 23 disordered proteins that FoldIndex or plotting hydrophobicity against contact number did not find and found nine proteins that neither previous method found. And neither FoldIndex nor plotting hydrophobicity against contact number found four disordered proteins that SGT did not find (Figure 3).
Figure 3.
Comparing true positives among the SGT-based method, FoldIndex and the method plotting hydrophobicity against the number of contacts (HY-CN).
Comparison with support vector machines
Many forms of biological data are classified using support vector machines, neural networks, or other types of traditional machine learning. These algorithms are supervised learning procedures where the classifier is trained on labeled data. Spectral Graph Transducer, which is associated with semi-supervised learning, differs from other forms of supervised learning in that it uses unlabeled data. We also tested SVMs with the same features we use in SGT in order to investigate whether semi-supervised learning with unlabeled sequences is effective for predicting disorder. We compared SGT with SVMs, which is known to be a powerful classifier and has been widely applied to biological data analyses, using amino acid compositions as the feature vector. The SVMs package libSVM was used; Performance of major three kernels (linear, polynomial, RBF) was compared, and RBF kernel, which gave the best result, was used. All parameters were tuned by grid-search. For SGT, 30,000 structure-unknown samples were used. These results are also shown in Table 3. SGT gave a MCC 0.07 points better than the SVMs did.
Supervised learning methods, including SVMs, are especially sensitive to the training data distribution when the given data set is a small one. Therefore, if biased data are provided, the predictive tendency will differ even if predictions are made using the same data. We compared the predictive tendencies with different training data as follows: (1) evaluation data were divided into three groups (Data-A, Data-B and Data-C); (2) each datum was predicted using a classifier trained on different data; and (3) the correlation coefficient between the two results was calculated. (e.g., a classifier trained on Data-A classifies all sequences from Data-C, another classifier trained on Data-B also classifies all sequences of Data-C. and then the coefficient of the correlation between two results is calculated). The average correlation coefficient for SGT was 0.14 higher than that for the SVMs (Table 4). An SGT-based method, which uses a huge number of unlabeled samples, makes prediction robust with regard to training data sparseness. This result indicates that SGT prediction is less affected by training data bias and provides accurate predictions even with a poor data set. Experimentally determined protein structures can potentially bias the data set. Previous research has shown that discriminating disorder from order is similar to finding the classification boundary between crystal structures and solution structures [14]. This is an unavoidable problem as long as a limited dataset is used, but distribution of structure-unknown data modified the training data bias.
Table 4.
Correlation coefficients for the proposed method (SGT) and an SVMs-based method (SVMs) trained on different datasets.
| Data-A | Data-B | Data-C | Average | |
| SGT | 0.92 | 0.85 | 0.94 | 0.90 |
| SVMs | 0.83 | 0.83 | 0.63 | 0.76 |
Comparison with per-residue predictors
There are many studies in which the probability of any given residue being in a disordered region was predicted. Although the methods used in those studies are not directly comparable to our method, comparing the proposed method to per-residue predictors gives helpful information about the accuracy of the proposed method.
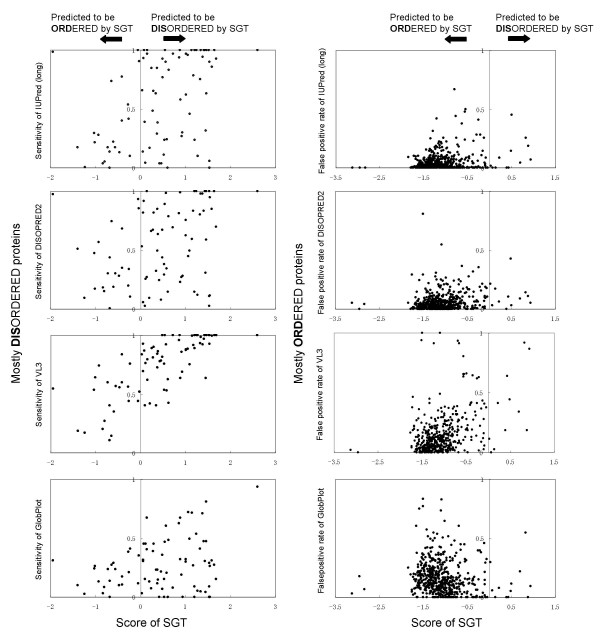
We select four successful per-residue predictors for comparison: VL3 [19], GlobPlot [16], DISOPRED2 [18] and IUPred (long) [21]. The VL3, GlobPlot and IUPred (long) results were obtained from web servers, and the DISOPRED2 results were obtained from a stand-alone program [33]. Detailed results are shown in Figure 4, which shows the results of two types of evaluation. In the graphs on the left side, showing results for mostly disordered proteins (at least 70% of their residues are disordered), the sensitivities of each per-residue predictor are plotted against the SGT scores. In the graphs on the right side, showing results for mostly ordered proteins (at least 95% of their residues are ordered), the false positive rates of each per-residue predictor are plotted against the SGT scores. The SGT gives each protein a score that shows how likely the protein is to be disordered. It assigns positive score when it predicts a query protein to be disordered. For example, if a point is plotted in the lower right portion of one of the graphs on the left, the proposed method can correctly classify the target sequence while the corresponding per-residue predictor cannot find a lot of disordered residues.
Figure 4.
Relations between the SGT score and the sensitivities and false positive rates of four per-residue predictors.
Table 5 also compares per-residue sensitivity on mostly disordered proteins, sensitivity and specificity on mostly ordered proteins of the proposed method to those of per-residue predictors. When we evaluated SGT prediction, we regarded all residues of the target protein to be predicted to be disordered if the SGT assigned positive score to the protein. And we also regarded all residues of the target protein to be predicted to be ordered if the SGT assigned a negative score to the protein. (I.e., the sensitivity of the proposed method becomes 0.7 if the SGT score is positive and the query sequence includes 70 disordered residues and 30 ordered residues).
Table 5.
Per-residue sensitivity (SEN) on mostly disordered proteins (average disorder length is 228.54) and sensitivity (SEN) and specificity (SPC) on mostly ordered proteins (average disorder length = 2.47) for the proposed method (SGT) and four per-residue predictors.
| Mostly DISORDERED proteins Ave DR length = 228.54 |
Mostly ORDERED proteins Ave DR length = 2.47 |
||
| SEN | SEN | SPC | |
| SGT | 0.834 | 0.009 | 0.991 |
| VL3 | 0.782 | 0.562 | 0.880 |
| IUPred (long) | 0.666 | 0.262 | 0.955 |
| Disopred2 | 0.645 | 0.780 | 0.954 |
| GlobPlot | 0.311 | 0.324 | 0.838 |
Specificity for mostly disordered proteins cannot be calculated because sequences selected from DisProt have no information for ordered regions. The 71 predictions by VL3 for mostly ordered sequences were excluded because the VL3 server does not return results for sequences that include ambiguous characters such as 'Z'.
DISOPRED2 and IUPred have low false positive rates on mostly ordered proteins. DISOPRED2 successfully predicts short disordered regions in mostly ordered proteins (proteins with an average disorder length of 2.47 residues per sequence), but does not detect 35.5% of the disordered regions in mostly disordered proteins (proteins with an average disorder length of 228.54 residues per sequence). VL3, on the other hand, successfully finds 78.2% of the disordered regions in mostly disordered proteins but produces a lot of false positives on mostly ordered proteins.
These per-residue predictors try to find exact position of disorder by classifying a fixed window length to be disordered or ordered. Because their prediction thus concentrates on the local trend of disorder, they miss the global trend of disorder. And because a shorter window size includes less information, the local trend of disorder is more difficult to discriminate than the global trend of disorder. When a classification scheme such as neural networks or SVMs is used to determine a classification boundary between similar examples, there is inevitably a trade-off between getting a large number of false positives and getting a large number of true positives. This trade-off is strongly influenced by ratio of positive/negative training examples.
Predicting disorder by classifying proteins as mostly disordered or mostly ordered is a rough approximation but has the advantage of detecting long disordered regions with a low false positive rate by neglecting short disordered regions. The proposed method predicts 83.4% of the disordered regions in mostly disordered proteins, and its false positive rate on mostly ordered proteins is only 0.9%. We therefore think insight is obtained by predict both disordered regions and disordered proteins, since a region-based-prediction provides information local trends of disorder while a protein-based-prediction gives information about large-area trends of disorder.
Evaluation on partially disordered proteins
Here we describe the results of prediction on partially disordered proteins. Not all proteins are mostly disordered or mostly ordered and many are partially disordered. Evaluating our method on partially disordered proteins gave us practical information we could use for estimating the false positive rates that would occur when it is used for large-scale genome analysis. As shown in Table 6, the proposed method is insensitive for the partially (5–20%) disordered proteins, although the method can predict that 16.67% of the moderately (20–40%) disordered proteins are disordered.
Table 6.
Frequency of partially disordered proteins predicted to be mostly disordered by the proposed method.
| Disorder frequency f | Number of proteins predicted to be disordered | Total number of proteins | frequency of proteins predicted to be disordered (%) |
| 5% ≤ f < 10% | 5 | 256 | 1.95 |
| 10% ≤ f < 20% | 2 | 137 | 1.46 |
| 20% ≤ f < 50% | 4 | 24 | 16.67 |
Prediction of disordered proteins in large databases
To provide illustrative examples of novel predictions made by our SGT-based method, we made predictions on several databases.
Ward et al., using DISOPRED2, estimated 18.9% of eukaryotic genomes and 5.7% of bacterial genomes to be disordered and found long (> 30 residues) disordered segments in 2.0% of archaean proteins, 4.2% of bacterial proteins and 33.0% of eukaryotic proteins [2]. Bogatyreva et al., evaluating the expected number of contacts, estimated that 12%, 3% and 2% of the proteins in eukaryotic, bacterial and archaean proteomes are totally disordered and that long (> 41 residues) disordered segments occur in 16% of archaean proteins, 20% of bacterial proteins and 43% of eukaryotic proteins [34]. The proposed method predicts that an average of 4.14% of archaean proteins, 7.0% of bacterial proteins and 28.5% of eukaryotic proteins are mostly disordered. The frequencies estimated for 5 archaean, 14 bacterial and 5 eukaryotic genomes, in addition to the overall totals for each domain, are listed in Table 7. In line with the results of previous genome-wide analysis, eukaryotic genomes are predicted to code for much more disorder than prokaryotic genomes do. This is consistent with much experimental evidence that has shown that dynamic flexibility of the protein structure is more often related to eukaryotic protein function than to bacterial and archaean protein function [12].
Table 7.
Estimated frequencies of disordered protein in 24 representative genomes.
| Kingdom | Species | Number of total sequences | Disordered protein frequency (%) |
| Archaea | Halobacterium sp. NRC-1 | 2605 | 4.57 |
| Archaea | Pyrococcus horikoshii | 1535 | 2.41 |
| Archaea | Thermoplasma volcanium | 1526 | 3.87 |
| Archaea | Sulfolobus solfataricus | 2977 | 3.76 |
| Archaea | Nanoarchaeum equitans | 536 | 9.89 |
| Bacteria | Escherichia coli K-12 | 4302 | 4.21 |
| Bacteria | Acidobacteria bacterium Ellin345 | 4777 | 4.92 |
| Bacteria | Staphylococcus aureus RF122 | 2515 | 5.81 |
| Bacteria | Mycobacterium tuberculosis H37Rv | 3991 | 4.03 |
| Bacteria | Fusobacterium nucleatum | 2067 | 5.22 |
| Bacteria | Rhodopirellula baltica | 7325 | 14.06 |
| Bacteria | Chlamydophila pneumoniae AR39 | 1110 | 9.91 |
| Bacteria | Treponema pallidum T. pallidum | 1031 | 6.89 |
| Bacteria | Synechocystis sp. PCC6803 | 3454 | 5.07 |
| Bacteria | Porphyromonas gingivalis | 1909 | 7.70 |
| Bacteria | Chlorobium tepidum C. tepidum | 2255 | 7.54 |
| Bacteria | Dehalococcoides ethenogenes | 1580 | 6.39 |
| Bacteria | Deinococcus radiodurans | 3181 | 3.99 |
| Bacteria | Thermotoga maritima | 1846 | 7.04 |
| Eukaryota | Arabidopsis thaliana | 25545 | 22.51 |
| Eukaryota | Caenorhabditis elegans | 22844 | 21.33 |
| Eukaryota | Drosophila melanogaster | 19376 | 30.21 |
| Eukaryota | Homo sapiens | 40877 | 36.85 |
| Eukaryota | Saccharomyces cerevisiae | 5869 | 18.73 |
| Archaea | 9179 | 4.14 | |
| Bacteria | 41343 | 7.00 | |
| Eukaryota | 114511 | 28.50 | |
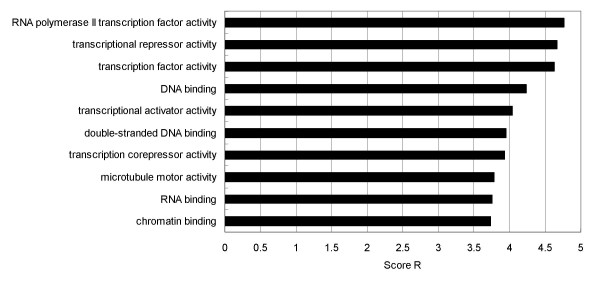
The proposed method also predicts that 15.46% of all sequences in the Swiss-Prot database are disordered. To investigate functional annotations of those sequences that were predicted to be disordered, we calculated the normalized ratio of annotated GO molecular function terms: R(T) = Rd(T)/Rs(T), where Rd(T) is the ratio of the proteins annotated by GO term T to all the proteins predicted to be disordered and Rs(T) is the ratio of the proteins annotated by GO term T to all the sequences in the Swiss-Prot database. The top 10 of the GO molecular function terms that describe more than 50 protein annotations, the 10 with the highest normalized ratios Rd(T) are listed in Figure 5. The proposed method was biased to find transcriptional-factor-related, RNA-binding-related and DNA-binding-related proteins to be disordered. Binding to nucleic acids requires interaction between the nucleic acid phosphate backbone and charged amino acids, which have a propensity for disorder (disorder propensities of amino acids and physiochemical properties are shown in Figures 6 and 7). Therefore it is not necessarily appropriate to suggest that all RNA-binding and DNA-binding proteins need dynamic flexibility, though previous papers have discussed the relation between disorder and proteins binding RNA and DNA [2,10,31,35]. The global analysis over large databases by the proposed method is an on-going study, and in the future we will use the proposed method to find new disordered proteins and will promote its use in further functional analysis of disordered proteins in collaboration with experimental laboratories.
Figure 5.
The 10 GO molecular function terms with the highest normalized ratios R.
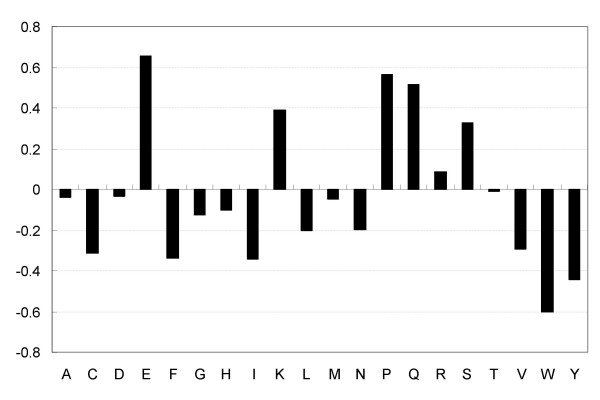
Figure 6.
Disorder propensities of 20 amino acids. Each propensity was calculated as Di/Oi, where Di is the frequency of amino acid Ai in disordered proteins and Oi is the frequency of amino acid Ai in ordered proteins.
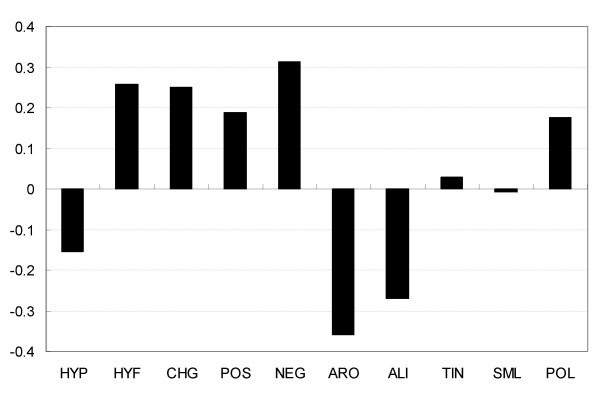
Figure 7.
Disorder propensities of 10 physicochemical properties. Each propensity was calculated as Di/Oi, where Di is the frequency of amino acids with the physicochemical feature Pi in disordered proteins and Oi is the frequency of amino acids with physicochemical feature Pi in ordered proteins. (HYP: hydrophobic, HYF: hydrophilic, CHG: charged, POS: positively charged, NEG: negatively charged, ARO: aromatic, ALI: aliphatic, TIN: tiny, SML: small, POL: polar)
Conclusion
In this study we proposed a semi-supervised learning approach for predicting disordered proteins. Disordered proteins are getting more and more attention because many of them are found to be functionally important. Few proteins however, are known to be disordered because information about a protein that could not be crystallized for X-ray analysis is seldom reported, even if the protein might be disordered. We therefore expect the distribution of disorder among proteins whose structure has been determined experimentally to differ from that of disorder among all natural proteins. Since the predictions made by previous methods are based on structure-known data, they are strongly affected by the bias for information about readily crystallized proteins. To avoid training data sparseness and to structure the hypothesis space based on the entire protein distribution, we have proposed a prediction method that uses Joachims' spectral graph transducer and is trained on both structure-known sequences and structure-unknown sequences.
This method yielded MCCs 0.202 points higher than the MCC yielded by the method plotting hydrophobicity vs. net charge (FoldIndex) and 0.221 points higher than the MCC yielded by the method plotting hydrophobicity against the number of contacts. When the false positive rate was 5%, we found 23 disordered proteins that were not found using those previous methods.
The proposed method predicts disorder by classifying proteins as either mostly disordered or mostly ordered. While such binary classification cannot detect partially disordered regions that per-residue predictors can find, it has the advantage of detecting long disordered regions by neglecting short disordered regions. When the proposed SGT-based method was compared with four per-residue predictors-VL3, GlobPlot, DISOPRED2, IUPred (long)-its sensitivity for disordered proteins was 0.834, which is 0.052–0.523 higher than that of the per-residue predictors and its specificity for ordered proteins was 0.991, which is 0.036–0.153 higher than that of the per-residue predictors.
The main contribution of this paper is that it provides a method in which structure-unknown protein sequences are used to increase the accuracy with which disordered proteins can be predicted. We compared the results obtained using the proposed method with the results obtained using a SVMs-based method that used the same features that the proposed method used (the composition of 20 amino acids). The proposed method resulted in a MCC 0.07 points higher than the MCC obtained using the SVMs-based method. When it and the method using SVMs were trained on two different datasets and both methods were tested on a third dataset, it provided an average correlation coefficient that was 0.14 higher than that provided by the method using SVMs. The SGT-based prediction was less affected by training data sparseness and provided more accurate predictions when the data set was a poor one. These results provide convincing evidence for a positive effect of structure-unknown protein sequences, and our SGT-based method is therefore able to serve as a new indicator of disordered protein that considers the overall protein distribution in nature.
Methods
Materials
Disordered proteins
We downloaded the current version of DisProt (version 3.3) and extracted proteins having more than 70% disorder. Then we clustered those sequences by sequence similarity of 30% using BASTclust, and selected representative sequences. We thereby obtained 82 sequences.
Ordered proteins
The data was prepared according to the following protocol. Complex proteins were excluded because their folded regions are possible to be unfolded on a single state. Because X-ray crystallographic analysis induces artifactually missing residues, high-quality data and well-refined data were selected in steps (2) and (3). BLASTclust was used for task (6).
1. Extract single-chain proteins from Protein Data Bank.
2. Extract data that has a resolution better than 2 Å and an observed R-factor less than 0.2.
3. Extract data determined by a newer version than Refmac5, SHELXL97 or CNS.
4. Extract proteins that are more than 95% ordered.
5. Exclude proteins that show disorder in the central area (between the 10th residue from the N-terminal end and the 10th residue from the C-terminal end).
6. Choose a representative sequence with 30% similarity to avoid redundancy.
We thereby obtained 526 sequences.
Unlabeled proteins
We used Uniprot50 (downloaded on 12 Jan 2006: 974,638 sequences), Swiss-Prot (release 48.9: 206,586 sequences), and TrEMBL (downloaded on 2 Feb 2006: 2,586,884 sequences). Short sequences tend to have a biased amino acid composition, which adversely affects prediction. We therefore excluded sequences shorter than 30 residues when it is used for semi-supervised training of SGT.
Partially disordered proteins
the data were prepared according to the following protocol. Complex proteins were excluded because their folded regions are possible to be unfolded on a single state. Because X-ray crystallographic analysis induces artifactually missing residues, high-quality data and well-refined data were selected in steps (2) and (3).
1. Extract single-chain proteins from Protein Data Bank.
2. Extract data that has a resolution better than 2 Å and an observed R-factor less than 0.2.
3. Extract data determined by a newer version than Refmac5, SHELXL97 or CNS.
4. Extract proteins that include more than 5% disorder.
We thereby obtained 417 sequences.
Protein sequences of 24 genomes
We used proteins sequences for 5 archaean, 14 bacterial and 5 eukaryotic genomes that were downloaded on 18 August 2006 from the NCBI ftp server.
Spectral Graph Transducer
The spectral graph transducer (SGT) is a powerful binary classification algorithm that was developed by Joachims [30]. It is based on semi-supervised learning, which for training makes use of not only labeled data (for which the answer is known) but also unlabeled data (for which the answer is unknown). This type of learning method often improves the prediction accuracy obtained when only a small amount of labeled data is available.
A goal of the classifier is to assign a label (either +1 or -1) to unlabeled examples. The SGT takes into account the information of unlabeled data by using a graph composed of both labeled data and unlabeled data. Given a set of labeled examples L = l0,...,lm and unlabeled examples U = u0,...,un, the SGT constructs a k-nearest-neighbor graph G with X = {U, L} as vertices. The graph G has n + m vertices, and edge weights between the vertices represent the similarity of the neighboring examples. The SGT assigns a label (either +1 or -1) to U by dividing G into two subgraphs G+ and G- (∀ui ∈ G+ are assigned +1, ∀ui ∈ G- are assigned -1) That is, G+ and G - define a cut in the graph. The SGT chooses the cut so that it provides a small training error (i.e., an li that is labeled +1 should belong to G+), has a low cut cost (i.e., it minimizes the sum of the edge weights across the cut) and makes the ratio of positive examples to negative examples in U the same as it is in L. This strategy is implemented by minimizing:
where
and yi is the prediction score of xi. If yi > 0, +1 is assigned to xi. The term γi is the penalty if xi ∈ L is misclassified. Therefore γi is positive for xi ∈ L+, negative for xi ∈ L- and 0 for xi ∈ U. The c is a parameter that trades off training error against cut cost, and C is a diagonal cost matrix that allows different misclassification costs for each example.
The spectral graph transducer outperforms other semi-supervised learning methods in many benchmark datasets [30]. We used the SGT package SGTlight in our experiments. Since classification accuracy is little affected by changing the two parameters c (trade-off of wrongly classifying training data) and d (number of eigenvectors) [30], we used c = 10,000 and d = 100. For the number of nearest neighbors, we tried k = 80, 90,...,120 and selected the one giving the best results (k = 100).
Similarity of two sequences
Because SGT is a kNN-based algorithm, the similarity of two sequences must be defined. We used three types of similarity measurement:
1. Amino acid composition
The amino acid composition is a basic property of proteins. Figure 6 shows the disorder propensities of 20 amino acids. A vector with 20 elements for the amino acid composition was used to calculate cosign.
2. Composition of physicochemical properties
Previous work has shown that composition alone is sufficient to recognize disorder accurately. Even a reduced alphabet of amino acids is useful for accurate prediction [36]. Figure 7 shows propensity for disorder of 10 physicochemical properties. We used a vector having 10 elements for physicochemical properties to calculate cosign. The binary definition of the physicochemical features is according to Zvelebil et al. [37].
3. Sequence similarity
Both of the two similarity measurements described above are based on compositional biases of amino acids. We also proposed a measurement based on sequence similarity or local motif. Top k raw score of BLAST search are used as similarity score between query sequence and database sequences for constructing kNN graph. The database of the BLAST search consists of training sequences.
Evaluation
We used five-fold cross validation for our experimental evaluation as follows.
1. We separate evaluation data (608 sequences) into five data sets and selected one (e.g., 121 or 122 sequences) for test data. The rest of the data (486 or 487 sequences) was used as training data.
2. Labels of test data are hidden.
3. Construct k-NN graph using training data, test data and proteins which are selected from Swiss-Prot.
4. Separate the k-NN graph into two (disordered or ordered) for prediction. Each sequence of unlabeld data (test data and proteins which are selected from Swiss-Prot) was classified as disordered or ordered.
5. We evaluate the precision of test data.
Steps 1–5 were repeated five times with different training data and test data.
Sensitivity (tp/(tp + fn)), specificity (tn/(tn + fp)), two-state accuracy ((tp + tn)/(tp + tn + fp + fn)), false positive rate (fp/(tn + fp)) and the Matthews correlation coefficient (MCC) were used for the evaluation. Because sensitivity and specificity are trade-off criteria, we needed a balancing criterion for the MCC. This criterion was calculated as
where tp is the number of true positives, tn the number of true negatives, fp the number of false positives and fn the number of false negatives.
Availability
Project Name: POODLE-W
Project Home Page: http://mbs.cbrc.jp/poodle/poodle-w.html
Operating Systems: POODLE-W is a web application that can be accessed from any OS.
Programming languages: C++, Perl(for CGI programming).
Restrictions to use by non-academics: none.
Authors' contributions
KS designed the methodology, developed the programs, implemented the experiments and did most of the writing under the guide of YM. SH and KT provided helpful insight in experiment and discussion. TN initiated the project. All authors contributed to the final version of the manuscript and approved it.
Acknowledgments
Acknowledgements
We thank Satoru Kanai from PharmaDesign, Inc, and Mikita Suyama from EMBL for their helpful discussions. We thank Kiyoshi Asai from our institute for his support.
Contributor Information
Kana Shimizu, Email: shimizu-kana@aist.go.jp.
Yoichi Muraoka, Email: muraoka@waseda.jp.
Shuichi Hirose, Email: shirose@pharmadesign.co.jp.
Kentaro Tomii, Email: k-tomii@aist.go.jp.
Tamotsu Noguchi, Email: noguchi-tamotsu@aist.go.jp.
References
- Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ. Intrinsic protein disorder in complete genomes. Genome Inform Ser Workshop Genome Inform. 2000;11:161–171. [PubMed] [Google Scholar]
- Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol. 2004;337:635–645. doi: 10.1016/j.jmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59. doi: 10.1016/S1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/S0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Natively unfolded proteins: a point where biology waits for physics. Protein Sci. 2002;11:739–756. doi: 10.1110/ps.4210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL. Natively unfolded proteins. Curr Opin Struct Biol. 2005;15:35–41. doi: 10.1016/j.sbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Oldfield CJ, Dunker AK. Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit. 2005;18:343–384. doi: 10.1002/jmr.747. [DOI] [PubMed] [Google Scholar]
- Receveur-Brechot V, Bourhis JM, Uversky VN, Canard B, Longhi S. Assessing protein disorder and induced folding. Proteins. 2006;62:24–45. doi: 10.1002/prot.20750. [DOI] [PubMed] [Google Scholar]
- Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z. Intrinsic disorder and protein function. Biochemistry. 2002;41:6573–6582. doi: 10.1021/bi012159+. [DOI] [PubMed] [Google Scholar]
- Iakoucheva LM, Brown CJ, Lawson JD, Obradovic Z, Dunker AK. Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol. 2002;323:573–584. doi: 10.1016/S0022-2836(02)00969-5. [DOI] [PubMed] [Google Scholar]
- Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293:321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- Tompa P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005;579:3346–3354. doi: 10.1016/j.febslet.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Garner E, Cannon P, Romero P, Obradovic Z, Dunker AK. Predicting Disordered Regions from Amino Acid Sequence: Common Themes Despite Differing Structural Characterization. Genome Inform Ser Workshop Genome Inform. 1998;9:201–213. [PubMed] [Google Scholar]
- Li X, Romero P, Rani M, Dunker AK, Obradovic Z. Predicting Protein Disorder for N-, C-, and Internal Regions. Genome Inform Ser Workshop Genome Inform. 1999;10:30–40. [PubMed] [Google Scholar]
- Linding R, Russell RB, Neduva V, Gibson TJ. GlobPlot: Exploring protein sequences for globularity and disorder. Nucleic Acids Res. 2003;31:3701–3708. doi: 10.1093/nar/gkg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Ward JJ. Prediction of disordered regions in proteins from position specific score matrices. Proteins. 2003;53:573–578. doi: 10.1002/prot.10528. [DOI] [PubMed] [Google Scholar]
- Ward JJ, McGuffin LJ, Bryson K, Buxton BF, Jones DT. The DISOPRED server for the prediction of protein disorder. Bioinformatics. 2004;20:2138–2139. doi: 10.1093/bioinformatics/bth195. [DOI] [PubMed] [Google Scholar]
- Obradovic Z, Peng K, Vucet S. Predicting intrinsic disorder from amino acid sequence. Proteins. 2003;53:566–572. doi: 10.1002/prot.10532. [DOI] [PubMed] [Google Scholar]
- Linding R, Jensen LJ, Diella F, Bork P, Gibson TJ, Russell RB. Protein disorder prediction: implications for structural proteomics. Structure. 2003;11:1453–1459. doi: 10.1016/j.str.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Dosztanyi Z, Csizmok V, Tompa P, Simon I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol. 2005;347:827–839. doi: 10.1016/j.jmb.2005.01.071. [DOI] [PubMed] [Google Scholar]
- Yang ZR, Thomson R, McNeil P, Esnouf RM. RONN: the bio-basis function neural network technique applied to the detection of natively disordered regions in proteins. Bioinformatics. 2005;21:3369–3376. doi: 10.1093/bioinformatics/bti534. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Gillespie JR, Fink AL. Why are "natively unfolded" proteins unstructured under physiologic conditions? Proteins. 2000;41:415–427. doi: 10.1002/1097-0134(20001115)41:3<415::AID-PROT130>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Oldfield CJ, Cheng Y, Cortese MS, Brown CJ, Uversky VN, Dunker AK. Comparing and combining predictors of mostly disordered proteins. Biochemistry. 2005;44:1989–2000. doi: 10.1021/bi047993o. [DOI] [PubMed] [Google Scholar]
- Garbuzynskiy SO, Lobanov MY, Galzitskaya OV. To be folded or to be unfolded? Protein Sci. 2004;13:2871–2877. doi: 10.1110/ps.04881304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilusky J, Felder CE, Zeev-Ben-Mordehai T, Rydberg EH, Man O, Beckmann JS, Silman I, Sussman JL. FoldIndex: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics. 2005;21:3435–3438. doi: 10.1093/bioinformatics/bti537. [DOI] [PubMed] [Google Scholar]
- Vucetic S, Brown CJ, Dunker AK, Obradovic Z. Flavors of protein disorder. Proteins. 2003;52:573–584. doi: 10.1002/prot.10437. [DOI] [PubMed] [Google Scholar]
- Vucetic S, Obradovic Z, Vacic V, Radivojac P, Peng K, Iakoucheva LM, Cortese MS, Lawson JD, Brown CJ, Sikes JG, Newton CD, Dunker AK. DisProt: a database of protein disorder. Bioinformatics. 2005;21:137–140. doi: 10.1093/bioinformatics/bth476. [DOI] [PubMed] [Google Scholar]
- Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Natale DA, O'Donovan C, Redaschi N, Yeh LS. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32:D115–119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachims T. Transductive Learning via Spectral Graph Partitioning. Proceedings of International Conference on Machine Learning. 2003. pp. 143–151.
- Chen JW, Romero P, Uversky VN, Dunker AK. Conservation of intrinsic disorder in protein domains and families: II. functions of conserved disorder. J Proteome Res. 2006;5:888–898. doi: 10.1021/pr060049p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. Sequence complexity of disordered protein. Proteins. 2001;42:38–48. doi: 10.1002/1097-0134(20010101)42:1<38::AID-PROT50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- The DISOPRED2 Disorder Prediction Server http://bioinf.cs.ucl.ac.uk/disopred/
- Bogatyreva NS, Finkelstein AV, Galzitskaya OV. Trend of amino acid composition of proteins of different taxa. J Bioinform Comput Biol. 2006;4:597–608. doi: 10.1142/S0219720006002016. [DOI] [PubMed] [Google Scholar]
- Minezaki Y, Homma K, Kinjo AR, Nishikawa K. Human transcription factors contain a high fraction of intrinsically disordered regions essential for transcriptional regulation. J Mol Biol. 2006;359:1137–1149. doi: 10.1016/j.jmb.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Weathers EA, Paulaitis ME, Woolf TB, Hoh JH. Reduced amino acid alphabet is sufficient to accurately recognize intrinsically disordered protein. FEBS Lett. 2004;576:348–352. doi: 10.1016/j.febslet.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Zvelebil MJ, Barton GJ, Taylor WR, Sternberg MJ. Prediction of protein secondary structure and active sites using the alignment of homologous sequences. J Mol Biol. 1987;195:957–961. doi: 10.1016/0022-2836(87)90501-8. [DOI] [PubMed] [Google Scholar]