Abstract
The seven-spanning calcium-sensing receptor (CaSR) activates multiple G proteins including Gq and Gi, and thereby activates a variety of second messengers and inhibits parathyroid hormone (PTH) secretion. However, the exact signaling mechanisms underlying the functional activity of CaSR are not yet fully understood. The heterozygous inactivation of CaSR or its inhibition by antibody blocking results in either familial hypocalciuric hypercalcemia or acquired hypocalciuric hypercalcemia (AHH), respectively. Here, we report the identification of a unique CaSR autoantibody in an AHH patient. Paradoxically, we find that this autoantibody potentiates the Ca2+/Gq-dependent accumulation of inositol phosphates by slightly shifting the dose dependence curve of the Ca2+ mediated activation of phosphatidylinositol turnover to the left, whereas it inhibits the Ca2+/Gi-dependent phosphorylation of ERK1/2 in HEK293 cells stably expressing human CaSR. Treatment of these same cells with a calcimimetic, NPS-R-568, augments the CaSR response to Ca2+, increasing phosphatidylinositol turnover and ERK1/2 phosphorylation, and overcoming the autoantibody effects. Our observations thus indicate that a calcium-stimulated CaSR primed by a specific autoantibody adopts a unique conformation that activates Gq but not Gi. Our findings also suggest that CaSR signaling may act via both Gq and Gi to inhibit PTH secretion. This is the first report of a disease-related autoantibody that functions as an allosteric modulator and maintains G protein-coupled receptors (GPCRs) in a unique active conformation with its agonist. We thus speculate that physiological modulators may exist that enable an agonist to specifically activate only one signaling pathway via a GPCR that activates multiple signaling pathways.
Keywords: allosteric modulation, disease, functional selectivity, G protein-coupled receptors, multiple active conformations
G protein-coupled receptors (GPCRs) comprise a family of receptors that mediate many of the cell-cell communication pathways in human tissues. In mammals, >1,000 types of GPCRs have been identified since the first of these receptors were identified. In addition, a variety of extracellular activators and ligands, including hormones, neurotransmitters, ions and amino acids, are now known to transmit their signals through GPCRs (1–3).
Within the past two decades, mutations in either GPCRs or their corresponding G proteins have been identified as the causes of both endocrine and common diseases (4–7). For example, gain-of-function mutations in the luteinizing hormone, thyroid-stimulating hormone, and PTH receptors cause familial male precocious puberty, sporadic hyperfunctional thyroid nodules, and Jansen metaphyseal chondrodysplasia, respectively. Conversely, loss-of-function mutations in the growth hormone-releasing hormone, luteinizing hormone, follicle-stimulating hormone, thyrotropin releasing hormone, thyroid-stimulating hormone, corticotropin, vasopressin 2, PTH, and endothelin B receptors cause familial growth hormone deficiency, male pseudohermaphroditism, hypergonadotropic ovarian dysgenesis, familial central hypothyroidism, familial hypothyroidism, familial corticotropin resistance, nephrogenic diabetes insipidus type I, Blomstrand chondrodysplasia, and Hirschsprung disease, respectively. In the case of the calcium-sensing receptor (CaSR), heterozygous loss-of-function mutations cause familial hypocalciuric hypercalcemia (8, 9), whereas gain-of-function mutations cause autosomal dominant hypocalcemia.
Autoantibodies against GPCRs, whether of the stimulating or the blocking type, have been shown to cause disease in the same fashion as activating or inactivating mutations of GPCRs. An example of this is the stimulating autoantibody against the thyroid-stimulating hormone receptor that causes Grave's disease, whereas a blocking autoantibody for this receptor leads to idiopathic hypothyroidism. In addition, a stimulating autoantibody against the β1-adrenergic receptor has been reported to contribute to the pathogenesis of cardiomyopathy (10). Furthermore, it has been reported that an activating autoantibody against angiotensin II type I receptor may trigger preeclampsia (11).
Recently, a blocking autoantibody against CaSR has been isolated and reported to cause acquired hypocalciuric hypercalcemia (AHH) (12, 13), which is phenotypically similar to familial hypocalciuric hypercalcemia but is not associated with any mutations in the CaSR gene. The autoantibody itself (IgG4) inhibits CaSR signaling pathways, inositol phosphate (IP) accumulation and ERK phosphorylation, by binding to specific CaSR sites (12). The case reported by Pallais et al., from which the autoantibody was identified, was an AHH patient whose symptoms regressed following glucocorticoid treatment and whose medical history provided evidence of autoimmune dysregulation including psoriasis, adult-onset asthma, Cooms'-positive hemagglutination, rheumatoid arthritis, ureitis and autoimmune hypophysitis (13). These findings suggested that the blocking autoantibody against CaSR was in fact responsible for the AHH in this patient.
The classic model of GPCR activation is the two-state model (1). Recently, however, several lines of evidence have strongly indicated that GPCRs may exist in multiple conformational states, comprising multiple active and inactive forms (1, 3, 14). For the type I PTH/PTHrP receptor, or Drosophila D1-like dopamine receptor, different types of agonists reportedly induce selective coupling to a distinct second messenger pathway (15). Different constitutive active mutants of the α1B-receptor are differently phosphorylated and internalized, also suggesting that several distinct active conformations exist (16). Furthermore, the human 5-HT2c receptor can be coupled to two different pathways in CHO cells, phospholipase C-mediated IP accumulation and phospholipase A2-mediated arachidonic acid release, and whereas different agonists can display the same potency for both responses, some agonists were found to preferentially activate the PLC-IP pathway, and others were found to favor the PLA2-AA pathway (17). PTH receptors are known to activate two different pathways, Gs/adenylyl cyclases and Gq/phospholipase C, upon stimulation with PTH (1–34). In contrast, PTH (3–34), harboring an N-terminal deletion, cannot activate adenylyl cyclase even though it retains the ability to activate MAPK, possibly via Gq (18).
It is particularly noteworthy that, as described above, for GPCRs that activate more than one signaling pathway, certain drugs and mutations may affect only one of these specific pathways. This is contrary to the idea that such receptors regulate multiple downstream signals to the same degree. In the context of disease, however, no autoantiboty that affects only one signaling pathway of a GPCR has been reported that would support this concept of a multistate model.
In our current study, we have identified a unique autoantibody against CaSR in a patient diagnosed with AHH. Although CaSR is known to activate multiple G proteins, including Gq and Gi, and to inhibit PTH secretion, the exact signaling mechanisms have not so far been elucidated (see also Discussion). The characteristics of this autoantibody helps to explain both the clinical conditions of the patient in question and the CaSR signaling mechanism involved and suggest that physiological as well as pathophysiological modulators may exist that facilitate a selective transition between multiple active conformations of GPCRs.
Results
Identification of an Autoantibody Against CaSR in the Serum of an AHH Patient.
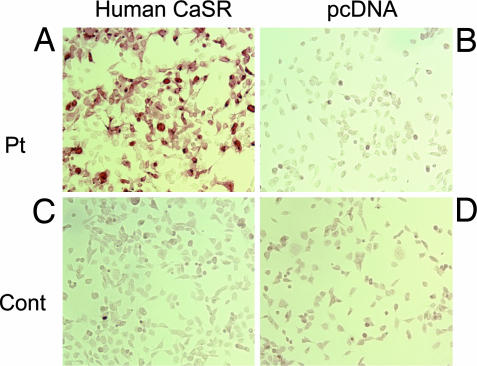
A male patient was clinically diagnosed as having AHH (Fig. 1, see Materials and Methods for a full description) but was confirmed to have no mutations in any of the 13 exons of the CaSR gene. We thus determined whether his serum reacted immunocytochemically against CaSR in a specific manner. The patient's sera (1:100) were indeed found to react against HEK293 cells either transiently or stably expressing human CaSR (293-CaSR-t and 293-CaSR-s, respectively), and not the control vector cells (293-mock-t or 293-mock-s) (Fig. 2 and data not shown). We incubated these cells with control sera (1:100), which did not react against 293-CaSR-t, 293-CaSR-s, 293-mock-t, or 293-mock-s (Fig. 2 and data not shown). We then confirmed that a monoclonal anti-CaSR antibody, LRG (raised against amino acids 374–391 of the human CaSR protein), reacted against both 293-CaSR-t and 293-CaSR-s [supporting information (SI)].
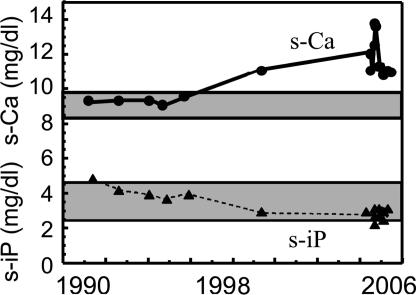
Fig. 1.
Temporal profile of the serum-corrected calcium and phosphate levels in our AHH patient subject. The corrected calcium and phosphate levels in the patient's serum over time are shown. The hatched area indicates the normal range of serum Ca, and the dotted area indicates the normal range of serum phosphate.
Fig. 2.
Immunoperoxidase staining of HEK293 cells expressing CaSR that have been reacted with both the AHH patient and control sera. (A and C) HEK293 cells transiently expressing human CaSR were incubated with AHH patient serum (1:50 dilution) (A) or with control serum (1:50 dilution) (C). A peroxidase-conjugated goat polyclonal human IgG-γ chain antibody (1:400) was used to detect autoantibodies, as described in Materials and Methods. (B and D) HEK293 cells transfected with a pcDNA3 vector were also incubated with AHH patient serum (B) or with control serum (D).
Serum from Our AHH Patient Does Not React with Three Previously Reported N-Terminal CaSR Peptides.
Synthetic peptides corresponding to amino acids 214–238, 344–358, and 374–391 of CaSR (all located within its extracellular N-terminal domain) were previously reported to react against sera from AHH patients (13), and we therefore used ELISA to examine whether these peptides also reacted with serum from our current AHH patient. However, we found that this was not the case for any of these three synthetic peptides (data not shown). We thus speculated that the serum in our AHH patient contained a type of CaSR antibody that recognized sites within the receptor protein that were different from those previously reported.
The CaSR Autoantibody Paradoxically Potentiates the Accumulation of IPs.
We next attempted to confirm our prediction that the serum from our AHH patient blocks the signaling pathways mediated through CaSR by first examining the effects of IgG purified from this patient against CaSR-dependent IP accumulation. Contrary to our expectation, this purified IgG preparation in fact potentiated the levels of IP accumulation (Fig. 3A), whereas in contrast, purified IgG from control sera showed no differences in this regard when compared with the control buffer. In addition, we observed that the dose-dependent curve representing the calcium stimulation of CaSR-mediated IP accumulation was shifted to the left following treatment with our AHH patient IgG preparation (Fig. 3A). Hence, the autoantibodies present in the serum of our patient appeared to augment Ca2+ signaling by increasing the sensitivity of the CaSR to Ca2+. The IP accumulation that occurred as a result of exposure to our AHH patient IgG preparation was also observed to be IgG concentration-dependent (Fig. 3B) and was also found to be abrogated by preboiling, indicating that these effects were bioactive IgG-specific and not due to a contaminating calcium ions or other small molecules (Fig. 3C).
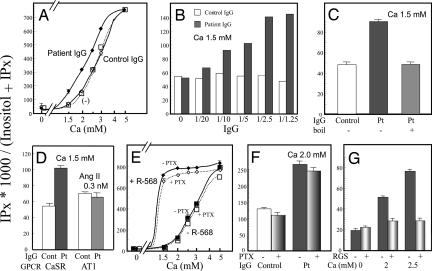
Fig. 3.
The AHH patient autoantibody potentiates Ca/CaSR-dependent IP accumulation. (A) Effects of our AHH patient autoantibody on CaSR-dependent IP accumulation stimulated by different concentrations of calcium ions. HEK293 cells stably expressing human CaSR (293-CaSR-s) were cultured in DMEM supplemented with 0.3 mM CaCl2 and [myo-3H]inositol (6 μCi/ml; Amersham Biosciences, Piscataway, NJ) for 24 h and washed with calcium-free medium containing 5 mM LiCl. The cells were then incubated with an appropriate calcium concentration after preincubation for 10 min with either patient IgG (1/2.5 volume), control IgG, or medium in the presence of 5 mM LiCl for 60 min. IP accumulation was determined as described in Materials and Methods. EC50 of calcium concentration in the presence or absence of the patient IgG (1/2.5 volume) was 2.27 ± 0.04 vs. 2.78 ± 0.05 mM (P < 0.05). (B) Effects of different concentrations of autoantibody on Ca/CaSR-dependent IP accumulation. 293-CaSR-s cells were cultured and washed as described in A and incubated with 1.5 mM calcium after preincubation for 10 min at 37°C with different volumes (1/1.25–1/20) of patient IgG or control IgG in the presence of 5 mM LiCl for 60 min. (C) Calcium-stimulated IP accumulation resulting from exposure to boiled or untreated IgG purified from the serum of our AHH patient. 293-CaSR-s cells were cultured and washed as described in A and incubated with 1.5 mM calcium after preincubation for 10 min at 37°C with a 1/5 volume of control IgG (either nonboiled or boiled patient IgG) in the presence of 5 mM LiCl for 60 min. (D) Autoantibody effects on both calcium and angiotensin II-stimulated IP accumulation. HEK293 cells stably expressing either the human CaSR or the mouse AT1a receptor were cultured and washed as described in A and then incubated with 1.5 mM calcium or with 0.3 nM AII after preincubation for 10 min with a 1/5 volume of patient IgG or control IgG in the presence of 5 mM LiCl for 60 min. (E) Effects of PTX and NPS-R-568 on calcium-stimulated IP accumulation. 293-CaSR-s were cultured and washed as described in A and incubated with an appropriate calcium concentration with or without 2 μM NPS-R-568 (+ or −) in the presence of 5 mM LiCl for 60 min. Cells were treated with or without PTX (200 ng/ml for 4 h) as indicated. (F) Effects of PTX on Ca/IgG-stimulated IP accumulation. 293-CaSR-s cells were cultured and washed as described in A and incubated with 2 mM Ca after preincubation for 10 min with a 1/2.5 volume of patient IgG. Cells were treated with or without PTX (200 ng/ml for 4 h) as indicated. (G) Effects of RGS domain of GRK2 on Ca-stimulated IP accumulation. HEK293 cells transiently coexpressing CaSR with RGS domain of GRK2 or mock were cultured and washed as described in A. Cells were then incubated with an appropriate calcium concentration. IP accumulation was estimated as described. Values represent means ± SD of triplicate determinations. Each set of results is representative of at least two additional experiments.
The purified patient IgG showed no effects on IP levels in HEK293 cells that stably express angiotensin II receptor 1 (a GPCR that couples both Gq and Gi) (19, 20) and are stimulated by angiotensin II at submaximally stimulating concentration. This result is consistent with the notion that the purified patient IgG acts specifically on CaSR and not other target proteins (Fig. 3D). In addition, the mode of action of this autoantibody resembles that reported for the calcimimetics. Calcimimetics selectively activate CaSR to inhibit PTH secretion and parathyroid cell proliferation, and have been reported to increase the sensitivity of the CaSR to activation by Ca2+ in both bovine parathyroid cells and HEK293 cells (21, 22). As previously reported (21, 22) the calcimimetic NPS-R-568 was found to potentiate Ca2+ mediated IP accumulation in our experiments (Fig. 3E), overcoming the effects by priming with the AHH patient IgG (SI Fig. 8). We next examined the effects of pertussis toxin (PTX) treatment on calcium-stimulated IP accumulation in 293-CaSR-s cells. The IP accumulation caused by Ca2+ alone, Ca2+ plus NPS R-568, or Ca2+ plus our patient IgG was minimally affected by incubation with 200 ng/ml PTX for 4 h (Fig. 3 E and F). In contrast, in HEK293 cells transiently coexpressing CaSR with RGS domain of GRK2 (23) or mock, the IP accumulation was inhibited by RGS domain of GRK2, suggesting that this increase in the IP levels occurs mostly through the activation of Gq (Fig. 3G).
The AHH Patient Autoantibody Inhibits CaSR-Dependent ERK1/2 Phosphorylation.
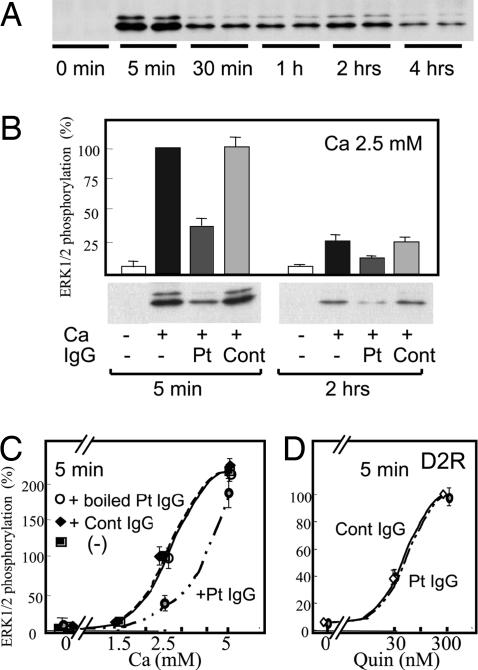
We next determined whether the purified patient IgG would affect the ERK1/2 phosphorylation that is also mediated through CaSR. In our 293-CaSR-s cells, the ERK1/2 phosphorylation levels stimulated by 2.5 mM Ca2+ were found to be maximal at the 5 min timepoint but to persist even after 4 h (Fig. 4A). Furthermore, ERK1/2 phosphorylation was detectable at a dosage of 1.0–1.5 mM Ca2+ and was saturated at ≈5 mM Ca2+. We then determined whether the IgG from our patient would affect the ERK1/2 phosphorylation levels at 5 min and 120 min in the same cells and found that, contrary to its effects on IP accumulation, the AHH patient IgG inhibited ERK1/2 phosphorylation, whereas control IgG had no effect (Fig. 4B). This inhibition of ERK1/2 phosphorylation by the patient IgG was found to be abrogated by preboiling (Fig. 4C). The purified patient IgG showed no effects on ERK1/2 phosphorylation in HEK293 cells that transiently express D2-dopamine (a typical GPCR that couples Gi) and are stimulated by quinpirole (Fig. 4D). These results indicated that the effects of the purified patient IgG were both bioactive IgG- and CaSR-specific.
Fig. 4.
The AHH patient autoantibody inhibits Ca/CaSR-dependent ERK1/2 phosphorylation. (A) Time course of Ca/CaSR-dependent ERK1/2 phosphorylation. 293-CaSR-s cells were seeded into 12-well plates and starved for 12–16 h in DMEM supplemented with 0.3 mM calcium. The cells were then stimulated with 2.5 mM calcium, and this incubation was terminated after the indicated timepoints. ERK1/2 phosphrylation was detected by Western blot analysis, using a rabbit polyclonal phospho-ERK1/2 antibody as described in Materials and Methods. (B) Effects of the AHH patient autoantibody on Ca/CaSR-dependent ERK1/2 phosphorylation. 293-CaSR-s cells were plated and starved as described in A. These cells were then stimulated with 2.5 mM calcium after preincubation for 20 min with a 1/2.5 volume of patient IgG, control IgG, or medium. These incubations were terminated after 5 min or 2 h. ERK1/2 phosphorylation was detected by Western blot analysis and was quantitated as described in Materials and Methods. Data are expressed as percentages of the values obtained in cells stimulated by 2.5 mM calcium and are representative of the mean ± SE from three independent experiments. (C) Calcium-stimulated ERK1/2 phosphorylation resulting from exposure to boiled or untreated IgG purified from the serum of our AHH patient. 293-CaSR-s cells were plated and starved as described in A. These cells were then stimulated with 2.5 mM calcium after preincubation for 20 min with a 1/2.5 volume of control IgG, either nonboiled or boiled patient IgG. These incubations were terminated after 5 min. ERK1/2 phosphorylation was quantitated as described in B. Data are expressed as percentages of the values obtained in cells stimulated by 2.5 mM calcium and are representative of the mean ± SE from three independent experiments. (D) Autoantibody effects on quinpirole-stimulated ERK1/2 phosphorylation via D2-dopamine receptor. HEK293 cells transiently expressing D2 receptor were plated and starved as described in A. These cells were then stimulated with quinpirole as indicated in the figure after preincubation for 20 min with a 1/2.5 volume of control IgG or patient IgG. ERK1/2 phosphorylation was quantitated as described in B. Data are expressed as percentages of the values obtained in cells stimulated by 300 nM quinpirole and are representative of the mean ± SE from three independent experiments.
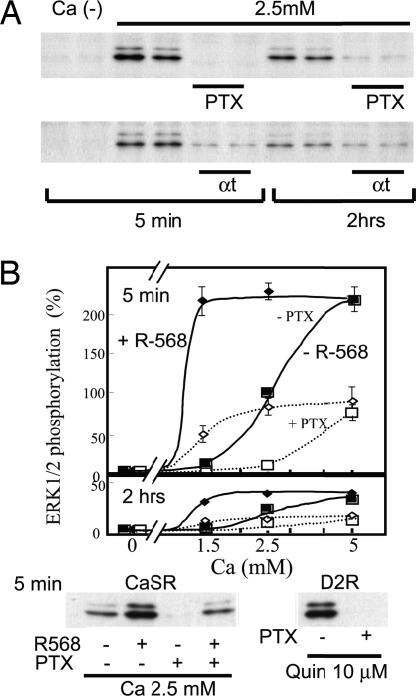
We next examined the effects of PTX treatment on calcium-stimulated ERK1/2 phosphorylation in 293-CaSR-s cells (Fig. 5A). Our results showed that ERK1/2 phosphorylation stimulated by up to 2.5 mM Ca2+ was almost completely inhibited by incubation with 200 ng/ml PTX for 4 h, suggesting that this phosphorylation occurs through the coupling of Gi to CaSR. In HEK293 cells transiently coexpressing CaSR with α-transducin (αt) or mock, ERK1/2 phosphorylation was mostly inhibited by αt, suggesting that it occurs through βγ generated by Gi upon activation (Fig. 5A).
Fig. 5.
Pertussis toxin and αt inhibit, whereas NPS-R-568 potentiates, the Ca/CaSR-dependent phosphorylation of ERK1/2. (A) Effects of pertussis toxin and αt on the Ca/CaSR-dependent activation of ERK1/2. 293-CaSR-s cells were seeded into 12-well plates and starved for 12–16 h in DMEM supplemented with 0.3 mM calcium. The cells were then stimulated with 2.5 mM calcium, and the reactions were terminated after 5 min or 2 h. Cells were treated with or without PTX. 293-CaSR-t cells were cotranfected with or without αt as indicated in the figure. (B) Effects of PTX and NPS-R-568 on the Ca/CaSR-dependent activation of ERK1/2. 293-CaSR-s cells were plated and starved as described in A and then stimulated with different concentrations of calcium with or without 2 μM NPS-R-568, as indicated in the figure. HEK293 cells transiently expressing D2 receptor were starved and then stimulated with quinpirole as indicated in the figure. The cells were treated with or without PTX, as indicated, and ERK1/2 phosphorylation was quantitatively detected by Western blot analysis as described. Data are expressed as percentage of the values obtained in cells stimulated by 2.5 mM calcium and represent mean ± SE of three independent experiments.
In contrast to the patient IgG that inhibited Ca2+ dependent ERK1/2 phosphorylation, NPS-R-568, as reported (22), was found to augment Ca2+ dependent ERK1/2 phosphorylation (Fig. 5B), overcoming the effects by priming with the purified AHH patient IgG (data not shown). Interestingly, the ERK1/2 phosphorylation stimulated by either 5 mM Ca2+ or Ca2+ plus NPS-R-568 (2 μM) was not completely inhibited by PTX (Fig. 5B), whereas ERK1/2 phosphorylation stimulated by saturating concentration (10 μM) of Quinpirole in HEK293 cells expressing D2 receptor was completetly inhibited by PTX (Fig. 5B), suggesting that under these conditions, a PTX insensitive pathway may play an additional role in ERK1/2 phosphorylation.
Discussion
We have identified a unique autoantibody to CaSR in a patient diagnosed with AHH that was found to potentiate Ca2+/Gq-dependent IP accumulation but inhibit Ca2+/Gi-dependent ERK1/2 phosphorylation in HEK293 cells expressing human CaSR. These data suggest that calcium-stimulated CaSR, primed by this autoantibody, remains in a unique active conformation that activates Gq but not Gi. Hence, this is to our knowledge the first description of a disease-related autoantibody that functions as an allosteric modulator, which will maintain a GPCR in a distinct active conformation with its agonist. These results also suggest, but do not conclusively prove, that both Gq and Gi may be important mediators of the calcium-dependent inhibition of PTH release. We first discuss the mode of action of this autoantibody based on a multistate model of GPCRs, and then further elaborate on its functional significance in CaSR signaling and the PTH secretion pathway.
Our AHH Patient Autoantibody Modulates CaSR in Multiple Active Conformations.
At present, the most widely accepted model for GPCR activation is the two-state model (1, 14), in which the receptor exists in equilibrium between an inactive (R) and active (R*) conformation. This model may explain the occurrence of spontaneous activation and inverse agonist effects in the case of GPCRs (24, 25). Recently, accumulating evidence has suggested that GPCRs can spontaneously adopt multiple conformational states, which are individually recognized and then stabilized by selective agonists (1, 14). In this multistate model, the receptor is proposed to alternate spontaneously between multiple active and inactive conformations.
With the realization that GPCRs can bind to more than one class of G protein, it was envisaged that different agonist-induced conformations of receptors could well exist with differing coupling efficacies for particular G proteins or signaling pathways (3) (see Introduction also). It is of particular interest to note also that some β2-adrenergic receptor ligands, such as ICI 118551 and propranolol, are inverse agonists of G protein-mediated signaling, which decreases cAMP and at the same time are partial agonists for the stimulation of the MAPK cascade (26). This strongly suggests that some drugs acting via a single receptor can actually exert opposing effects depending on the signaling pathway involved (15, 27).
Calcium-CaSR is known to activate two signal transduction mechanisms, the phospholipase C and ERK1/2 phosphorylation pathways. In HEK293 cells stably expressing human CaSR, we showed in our current experiments that calcium-stimulated IP accumulation depends mostly on Gq coupling but that calcium-stimulated ERK1/2 phosphorylation depends on Gi coupling. We further showed that our AHH patient IgG preparation differently modified calcium-stimulated IP accumulation and ERK1/2 phosphorylation. The autoantibody enhanced calcium-stimulated IP accumulation but inhibited calcium-stimulated ERK1/2 phosphorylation, suggesting that CaSR incubated with the patient's autoantibody adopts a distinct active conformation favoring coupling with Gq and uncoupling from Gi.
In summary, the autoantibody that we have identified in the serum from an AHH patient is unique in two respects: it supports the multistate model of GPCRs and appears mechanistically to function as an allosteric modulator of a GPCR, and thereby determining the specificity of the G protein coupling for this receptor.
Implications for CaSR Signaling and AHH.
Classically, it has been postulated that CaSR works via Gq to inhibit PTH release (28, 29), but a possible role of Gi was also suggested (29). Despite numerous studies over several decades, however, the key intracellular mechanisms by which Ca2+ and the CaSR control PTH secretion have yet to be elucidated (30).
The issue of whether Gq, Gi, or both, are responsible for inhibiting the PTH secretory response to calcium also remains to be determined, and some of the previous findings in this regard are inconsistent. Some studies have shown that the preincubation of bovine parathyroid cells with PTX partially blocks the inhibitory effects of either high Ca2+ or calcium channel agonists against both cAMP accumulation as well as on PTH release (31, 32), although these findings were not obtained in another similar study (29).
The IP-PLC signaling pathway and MAPK pathway are known to be activated in parathyroid cells by a small increase in serum Ca2+. In bovine parathyroid cells, MAPK activation depends on Gq/11-mediated activation of phosphatidylinositol-PLC and PKC and a PTX-sensitive pathway (33). One previous study has shown that in normal human parathyroid cells, ERK1/2 activation participates in the inhibition of PTH secretion induced by increasing Ca2+ (34). This report further showed that CaSR activation, through the PKC pathway and to a lesser extent Gi/βγ/PI3K, increased ERK1/2 activity in human hyperplastic parathyroid cells.
If ERK1/2 phosphorylation plays a role in regulating PTH release in human parathyroid cells, the inhibitory effects of our patient's autoantibody on ERK1/2 phosphorylation might explain the AHH phenotype in our patient. We suspect that in human parathyroid cells, Gi signaling might play a key role in inhibiting PTH secretion. We also hypothesize that Gq signaling and Gi signaling might both play important, converging roles in the inhibition of PTH secretion and that our AHH patient autoantibody might stimulate PTH secretion in parathyroid cells by the inhibition of Gi signaling, which may surmount any slight stimulation of Gq signaling.
It will be of great interest in the future to determine the region of the CaSR protein that is recognized by our AHH patient autoantibody. Currently, certain calcimimetics are becoming available to treat mainly secondary or tertiary hyperparathyroidism in end-stage renal failure. Calcimimetics are thought to react allosterically against the transmembrane domain of CaSR (21, 35) but not against the calcium binding site in its N-terminal domain. In fact, proteins, ions, and even membrane lipids, as well as many small molecules, have now been recognized to bind allosteric sites on GPCRs and thereby affect the binding and/or effect of the natural (orthostatic) ligand in either a positive or negative cooperative manner (36). Moreover, because the autoantibody detected in our patient does not react against known representative peptides in the N-terminal domain of CaSR, but does shift the concentration-response curve for Ca2+ to the left, we contend that this autoantibody reacts against CaSR allosterically. We speculate that the autoantibody against CaSR that we have uncovered reacts against a site in close proximity to the site of calcimimetic binding in CaSR as an allosteric modulator and thus exerts its effect in the presence of the orthosteric ligand, Ca2+ (37).
Materials and Methods
Subject.
A 74-year-old man, who had been receiving treatment for hypertension, presented with hypercalcemia (cCa 12.1 mg/dl, normal 8.4–9.7), mild hypophosphatemia (iP 2.8 mg/dl), and elevated PTH levels (intact PTH 120 pg/ml) without any clinical symptoms. Further analysis of this patient showed a 1,25(OH)2 vitamin D concentration of 45.8 (normal 20–60) pg/ml and PTHrP levels of <1.0 pmol/liter, suggesting primary hyperparathyroidism. However, his urinary calcium excretion was remarkably suppressed (8–10 mg/gCr) and neither ultrasound examination of the neck nor methoxyisobutyl isonitrile stress scintigraphy showed any evidence of parathyroid adenoma. Furthermore, a bone mineral density assessment showed no evidence of osteoporosis with young adult mean 96%. The clinical data obtained for this patient were therefore deemed to be compatible with a diagnosis of familial hypocalciuric hypercalcemia, which shows an autosomal dominant pattern of inheritance and is characterized by mild to moderate hypercalcemia in a relatively hypocalciuric setting, and also by normal or only slightly elevated circulating levels of PTH, although still inappropriate. Previous laboratory data for this individual, however, ruled out this genetic disease. Until the age of 68, his serum calcium and phosphate had been within normal ranges (Fig. 1). Although at the age of 69 he was found to have hypercalcemia (11.1 mg/dl) with slightly decreased IP (2.9 mg/dl), he was not further examined until the present investigation. Based on the above findings, we suspected that his hypercalcemia was caused by AHH, possibly because of a blocking autoantibody against CaSR.
Specimen Collection and Preparation.
Serum samples were collected and stored at −80° until just before use. Purified IgG were isolated by using a Montage antibody purification kit (Millipore, Billerica, MA), followed by concentration using Amicon Ultra 15 (Millipore) for immediate assay.
Cell Culture and Transfection.
HEK 293 cells, maintained in DMEM containing 10% FBS (38, 39), were transfected with pcDNA.3.1 containing DNA encoding the human CaSR, using Lipofectamine 2000. The transiently transfected cells were used for immunoperoxidase staining after 48 h in culture. Otherwise, to ensure that the HEK 293 cells stably expressed the human CaSR, clones were selected in medium containing 0.8 mg/ml G418, as described in refs. 39 and 40.
Immunoperoxidase Staining.
HEK293 cells expressing the human CaSR were fixed in 100% methanol at −20°C for 3 min. Immunoperoxidase staining was performed by using patient or control sera (1:50–100 dilution) or monoclonal anti-CaSR antibody raised against amino acids 374–391 (Affinity BioReagents, Golden, CO). Bound IgG were detected (13) by using either a peroxidase-conjugated, goat anti-human antiserum specific for the γ-chain of IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD) or a peroxidase-conjugated goat antimouse antiserum (Kirkegaard and Perry Laboratories), from the AEC+ system (Showa Kako, Osaka, Japan).
Measurement of CaSR-Stimulated IP Accumulation.
The accumulation of IPs in intact cells was assayed as described in ref. 41. Briefly, 293-CaSR-s were plated onto collagen-coated 24-well plates at 1.5 × 105 cells per well under DMEM with 0.3 mM CaCl2 and labeled with myo-[3H]inositol (6 μCi/ml, Amersham) for 24 h. After washing with a calcium-free medium containing 5 mM LiCL for 10 min, the cells were incubated with the appropriate concentrations of calcium in the presence of 5 mM LiCl for 60 min. IP and total inositol fractions were resolved on a Dowex AG1-X8 formate column (Bio-Rad, Hercules, CA), and IP accumulation was estimated by determining the ratio of IP radioactivity to the sum of IP and total inositol radioactivity.
Determination of Ca-Stimulated ERK 1/2 Activity.
Phosphorylation of ERK 1/2, which reflects one of the representative signals in the calcium-CaSR signaling pathway, was assayed as described in refs. 41 and 42. Briefly, 293-CaSR-s cells were plated onto 12 well plates and starved for 12–16 h in DMEM supplemented with 0.3 mM calcium. The cells were then stimulated with various concentrations of calcium with or without serum-purified IgG, and reactions were terminated after various times ranging from 5 min to 4 h. Proteins extracts from the 293-CaSR-s cells were then separated by SDS/PAGE and transferred to PVDF membranes. The membranes were then incubated with monoclonal anti-phospho MAPK antibody.
DNA Sequencing.
The coding exons of the CaSR genes from blood cells of our AHH patient were amplified by PCR and sequenced. The sequences were compared with the human CaSR cDNA entry (GenBank accession no. U20759).
Immunofluorescence Staining.
See SI Materials and Methods for immunofluorescence staining procedures.
Supplementary Material
Acknowledgments
We thank Kirin Pharmaceutical for providing NPS-R-568 and R. J. Lefkowitz, G. Servant, and T. Kozasa for generously donating constructs. We also thank H. R. Bourne for suggestions. This work was supported by a Grant-in-Aid for Scientific Research and Science Research on Priority Area from the Ministry of Education, Science, Sports, and Culture, Japan (to T.I.)
Abbreviations
- AHH
acquired hypocalciuric hypercalcemia
- αt
α-transducin
- GPCR
G protein-coupled receptor
- IP
inositol phosphate
- PTH
parathyroid hormone
- PTX
pertussis toxin
- CaSR
calcium-sensing receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701290104/DC1.
References
- 1.Gether U. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 2.Pierce KL, Premont RT, Lefkowitz RJ. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 3.Hill SJ. Br J Pharmacol. 2006;147(Suppl 1):S27–S37. doi: 10.1038/sj.bjp.0706455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel AM. J Clin Endocrinol Metab. 1996;81:2434–2442. doi: 10.1210/jcem.81.7.8675557. [DOI] [PubMed] [Google Scholar]
- 5.Farfel Z, Bourne HR, Iiri T. N Engl J Med. 1999;340:1012–1020. doi: 10.1056/NEJM199904013401306. [DOI] [PubMed] [Google Scholar]
- 6.Seifert R, Wenzel-Seifert K. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 7.Tao YX. Pharmacol Ther. 2006;111:949–973. doi: 10.1016/j.pharmthera.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 9.Chou YH, Pollak MR, Brandi ML, Toss G, Arnqvist H, Atkinson AB, Papapoulos SE, Marx S, Brown EM, Seidman JG, et al. Am J Hum Genet. 1995;56:1075–1079. [PMC free article] [PubMed] [Google Scholar]
- 10.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, et al. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kifor O, Moore FD, Jr, Delaney M, Garber J, Hendy GN, Butters R, Gao P, Cantor TL, Kifor I, Brown EM, Wysolmerski J. J Clin Endocrinol Metab. 2003;88:60–72. doi: 10.1210/jc.2002-020249. [DOI] [PubMed] [Google Scholar]
- 13.Pallais JC, Kifor O, Chen YB, Slovik D, Brown EM. N Engl J Med. 2004;351:362–369. doi: 10.1056/NEJMoa040008. [DOI] [PubMed] [Google Scholar]
- 14.Kenakin T. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 15.Reale V, Hannan F, Hall LM, Evans PD. J Neurosci. 1997;17:6545–6553. doi: 10.1523/JNEUROSCI.17-17-06545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mhaouty-Kodja S, Barak LS, Scheer A, Abuin L, Diviani D, Caron MG, Cotecchia S. Mol Pharmacol. 1999;55:339–347. doi: 10.1124/mol.55.2.339. [DOI] [PubMed] [Google Scholar]
- 17.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 18.Swarthout JT, Doggett TA, Lemker JL, Partridge NC. J Biol Chem. 2001;276:7586–7592. doi: 10.1074/jbc.M007400200. [DOI] [PubMed] [Google Scholar]
- 19.Kaschina E, Unger T. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 20.Makita N, Fujita T, Iiri T. AfCS Nature Molecule Pages. 2006 doi: 10.1038/mp.a000275.01. [DOI] [Google Scholar]
- 21.Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holstein DM, Berg KA, Leeb-Lundberg LM, Olson MS, Saunders C. J Biol Chem. 2004;279:10060–10069. doi: 10.1074/jbc.M312039200. [DOI] [PubMed] [Google Scholar]
- 23.Carman CV, Parent JL, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. J Biol Chem. 1999;274:34483–34492. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 24.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 25.Milligan G. Mol Pharmacol. 2003;64:1271–1276. doi: 10.1124/mol.64.6.1271. [DOI] [PubMed] [Google Scholar]
- 26.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenakin T. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 28.Conklin BR, Bourne HR. Nature. 1994;367:22. doi: 10.1038/367022a0. [DOI] [PubMed] [Google Scholar]
- 29.Brown EM, Vassilev PM, Quinn S, Hebert SC. Vitam Horm. 1999;55:1–71. doi: 10.1016/s0083-6729(08)60933-4. [DOI] [PubMed] [Google Scholar]
- 30.Kifor O, Kifor I, Brown EM. Curr Opin Nephrol Hypertens. 2002;11:397–402. doi: 10.1097/00041552-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Brown EM. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick LA, Brandi ML, Aurbach GD. Biochem Biophys Res Commun. 1986;138:960–965. doi: 10.1016/s0006-291x(86)80589-7. [DOI] [PubMed] [Google Scholar]
- 33.Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. Am J Physiol Renal Physiol. 2001;280:F291–F302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 34.Corbetta S, Lania A, Filopanti M, Vicentini L, Ballare E, Spada A. J Clin Endocrinol Metab. 2002;87:2201–2205. doi: 10.1210/jcem.87.5.8492. [DOI] [PubMed] [Google Scholar]
- 35.Petrel C, Kessler A, Dauban P, Dodd RH, Rognan D, Ruat M. J Biol Chem. 2004;279:18990–18997. doi: 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- 36.Vauquelin G, Van Liefde I. Fundam Clin Pharmacol. 2005;19:45–56. doi: 10.1111/j.1472-8206.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 37.Christopoulos A, Kenakin T. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 38.Wedegaertner PB, Bourne HR, von Zastrow M. Mol Biol Cell. 1996;7:1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iiri T, Farfel Z, Bourne HR. Proc Natl Acad Sci USA. 1997;94:5656–5661. doi: 10.1073/pnas.94.11.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iiri T, Herzmark P, Nakamoto JM, van Dop C, Bourne HR. Nature. 1994;371:164–168. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 41.Iiri T, Bell SM, Baranski TJ, Fujita T, Bourne HR. Proc Natl Acad Sci USA. 1999;96:499–504. doi: 10.1073/pnas.96.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, et al. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.