Abstract
CD1d molecules bind lipid antigens in the endocytic pathway, and access to the pathway is important for the development of CD1d-restricted natural killer T (NKT) cells. Saposins, derived from a common precursor, prosaposin, are small, heat-stable lysosomal glycoproteins required for lysosomal degradation of sphingolipids. Expression of prosaposin is required for efficient lipid binding and recognition of human CD1d molecules by NKT cells. Despite high sequence homology among the four saposins, they have different specificities for lipid substrates and different mechanisms of action. To determine the saposins involved in promoting lipid binding to CD1d, we expressed prosaposin deletion mutants lacking individual saposins in prosaposin-negative, CD1d-positive cells. No individual saposin proved to be absolutely essential, but the absence of saposin B resulted in the lowest recognition of α-galactosylceramide by NKT cells. When recombinant exogenous saposins were added to the prosaposin-negative cells, saposin B was the most efficient in restoring CD1d recognition. Saposin B was also the most efficient in mediating α-galactosylceramide binding to recombinant plate-bound CD1d and facilitating NKT cell activation. Saposin B could also mediate lipid binding to soluble CD1d molecules in a T cell-independent assay. The optimal pH for saposin B-mediated lipid binding to CD1d, pH 6, is higher than that of lysosomes, suggesting that saposin B may facilitate lipid binding to CD1d molecules throughout the endocytic pathway.
Keywords: antigen processing, lipid transfer proteins, natural killer T cells
CD1 molecules present lipid and glycolipid antigens to T cells (1). In humans, five CD1 isoforms have been identified and classified into two groups based on sequence similarity: CD1a, -b, and -c constitute group I, whereas CD1d forms group II. CD1e represents an intermediate between the two. Mice express only CD1d, but there are two homologous genes, CD1d1 and CD1d2. Diverse lipids, most derived from Mycobacteria, are presented by group 1 CD1 molecules. These include mycolic acid, glucose-monomycolate (GMM), phosphatidylinositol mannoside (2), lipoarabinomannan (LAM) and mannosyl-β-1-phosphoisoprenoid, recently redesignated mannosyl-β-1-mycoketide (3). Both endogenous and exogenous lipids can be presented by human and mouse CD1d (4, 5).
Whereas group 1 CD1 molecules present lipids to T cells with diverse T cell receptors, CD1d-restricted T cells predominantly use an invariant rearranged α chain: Vα24 in humans and Vα14 in mice. Many CD1d-restricted T cells coexpress CD161, which is expressed mostly on natural killer cells, and are referred to as natural killer T (NKT) cells (6). NKT cells rapidly secrete an array of cytokines after activation, and, although they constitute <1% of total T cells, they exert a critical influence in a variety of situations, including cancer (7) and bacterial, viral, parasitic, and fungal infections (8– 10) and autoimmune diseases (11).
Lipids must be extracted from membranes to bind to CD1d molecules, a process facilitated by lipid transfer proteins. CD1d is initially assembled in the endoplasmic reticulum (ER), and here, the protein involved is the microsomal triglyceride transfer protein (MTP). After binding self-lipids, such as phosphatidylcholine (PC) and phosphatidylinositide (PI), in the ER (12), CD1d molecules follow the secretory pathway to the cell surface and recycle between the plasma membrane and the endocytic system (13).
Access to the endocytic pathway is important for processing and presentation of lipid antigens by CD1d (14–16) and several lysosomal lipid transfer proteins, particularly the sphingolipid activator proteins (SAPs), facilitate lipid binding (14, 17, 18). SAPs are membrane-perturbing and lipid-binding proteins initially defined by their roles in sphingolipid degradation (19). Important SAPs are saposins A, B, C, and D, which are acidic, heat-stable, and protease-resistant glycoproteins of ≈8–11 kDa (19). They are proteolytically derived from a common precursor, prosaposin (PS), which is encoded by a single gene. Deficiency in saposin expression causes sphingolipid accumulation, leading to lipid-storage diseases.
The four saposins share a high degree of homology, but they have different lipid specificities. Saposin A is required for galactosylceramide degradation by galactosylceramide-β-galactosidase. Mice lacking saposin A accumulate galactosylceramide and suffer from a form of Krabbe's disease (20). Saposin B facilitates the degradation of a variety of lipids, including sulfatide by arylsulfatase A and globotriaosylceramide and digalactosylceramide by α-galactosidase A. Patients lacking saposin B accumulate these substrates in lysosomes (21). Saposin C is required for the degradation of glucosylceramide by glucosylceramide-β-glucosidase, and patients lacking this saposin develop a juvenile form of Gaucher's disease (22). Saposin D stimulates lysosomal ceramide degradation by acid ceramidase and, in vitro, sphingomyelin hydrolysis by acid sphingomyelinase. Saposin D-deficient mice accumulate ceramides in the brain and kidney (23).
The saposins apparently activate sphingolipid degradation by different mechanisms. Saposin C directly interacts with glucosylceramide-β-glucosidase and induces conformational changes in the enzyme (24). It also destabilizes membranes by supporting the interaction of the enzyme with membrane-associated substrate lipids (22, 25). Saposin B and GM2 activator protein act as physiological detergents and show broad lipid specificity. They each form a shell-like homodimer with the two subunits having different conformations and enclosing a large hydrophobic cavity (26). A lipid extraction mechanism has been proposed in which the open conformation interacts with the membrane and induces a reorganization of the lipid alkyl chains. Extraction of the lipid substrate is accompanied by a change to the closed conformation, which exposes it to the degradative enzymes as a water-soluble saposin–lipid complex (19).
Saposin C, but not other saposins, is required for lipid presentation by human CD1b (17). Saposin C can extract LAM from membranes in vitro and appears to interact directly with CD1b molecules, presumably facilitating LAM transfer. Recombinant saposins can promote lipid exchange by mouse CD1d, and mice lacking the PS gene fail to develop invariant NKT cells (27). We previously showed that mouse PS-negative cells expressing human CD1d are impaired in their ability to stimulate human NKT cells (14). Here, we examine the roles of the individual saposin(s). We find that although all four saposins can promote lipid binding to CD1d to some extent, saposin B is the most efficient.
Results and Discussion
Generation of CD1d-Positive Cells Lacking Individual Saposins.
Expression of human PS in mouse PS−/− fibroblasts [PS knockout (PSKO)1 and PSKO2] transduced with human CD1d enhanced their ability to present lipid antigens to NKT cells (14). To determine whether an individual saposin is responsible, we generated four cell lines expressing mutant PS constructs, each lacking one saposin. The retroviral constructs are shown schematically in Fig. 1A and are referred to as PSΔA, PSΔB, PSΔC, and PSΔD. Expression of the constructs in PSKO2.CD1d cells (14) was examined by Western blotting using antibodies against the individual saposins (Fig. 1B). Each cell line expressed three saposins and lacked the deleted one, whereas the cells transduced with wild-type PS expressed all four, and the cells transduced with empty vector (mock) expressed none. All the cell lines showed comparable CD1d surface expression levels (Fig. 1C). PS is proteolytically processed into mature saposins in a stepwise fashion (28), and we found that the PS deletion mutants were processed as efficiently as the wild type, shown in Fig. 1D for saposin C. Similar results were obtained for saposins A, B, and D (data not shown).
Fig. 1.
Generation of cell lines expressing PS deletion mutants. (A) Diagram of saposin deletions in the coding regions of PS mutants. In each mutant, one saposin is deleted. (B) Immunoblotting showing PS or mutant PS expression in PSKO2.CD1d cells. Cell lysates from fibroblasts expressing PS lacking individual saposins, wild-type PS or no PS (mock) were probed with rabbit antisera to saposins A, B, C, or D or with a rat anti-Grp94 antibody as a loading control. (C) Flow cytometry of PSKO2.CD1d cells transduced with wild-type or mutant PS constructs. Cells were stained with PE-conjugated mouse anti-human CD1d mAb 42.1: wild-type PS transduced cells (thick line), and PSΔA-, -ΔB-, -ΔC-, and -ΔD-transduced cells (solid, dotted, dashed and long dashed lines, respectively). Isotype control is in gray. (D) Immunoblot of PSKO2.CD1d cells expressing PS or the mutants showing processed mature saposin C. The cell lysates used in B were probed with rabbit antiserum to saposin C.
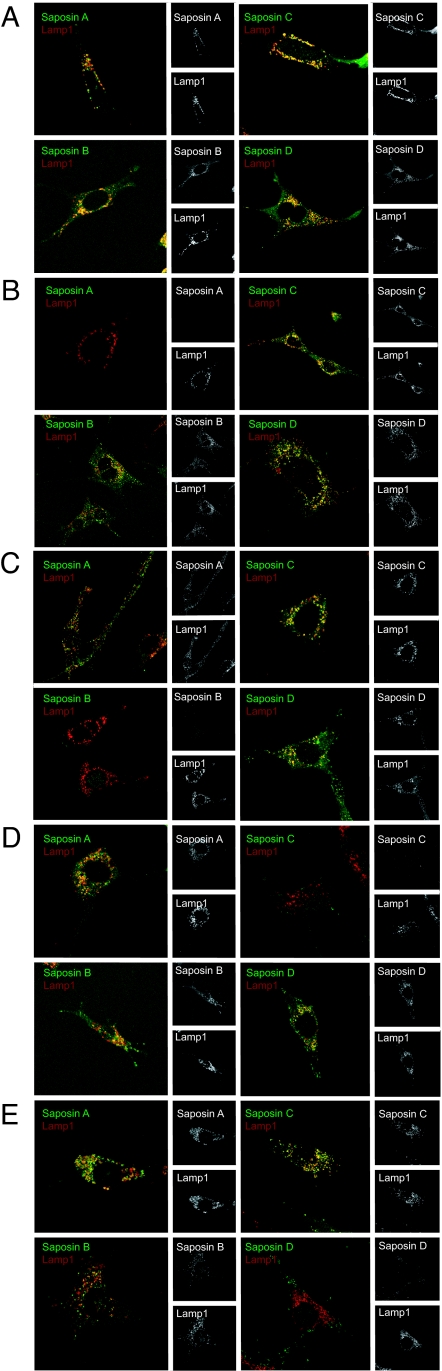
To determine whether the elimination of individual saposins affected the subcellular localization of the remaining ones, we examined the cells by confocal immunofluorescence microscopy. In cells expressing wild-type PS, all four saposins colocalized with the late endosomal/lysosomal marker, Lamp1 (Fig. 2A). In cells expressing the mutants, the deleted saposin was absent, whereas the remaining saposins still colocalized with Lamp-1. The majority of the saposins are completely or partially processed (Fig. 1D), and the intense staining in the endosomes/lysosomes probably represents the processed forms. In all the cells, we detected weak diffuse staining with the reactive saposin-specific antibodies, which probably corresponds to the ER (Fig. 2). This is likely to reflect the unprocessed forms observed by Western blotting in Fig. 1 B and D.
Fig. 2.
Immunofluorescence analysis of saposin expression in PSKO2.CD1d cells expressing wild-type PS or PS mutants lacking individual saposins. Fibroblast cell lines expressing wild-type PS (A) or PSΔA, -ΔB, -ΔC, and -ΔD (B–E, respectively) were incubated with rat anti-mouse Lamp1 and rabbit antisera to saposin A, B, C, or D, followed by Alexa Fluor 488-conjugated goat anti-rabbit Ig and Alexa Fluor 594-conjugated goat anti-rat Ig sera, respectively. Cells were examined with a confocal imaging system (Leica, Bannockburn, IL), and images were processed with Leica software.
Saposin B Most Efficiently Enhances CD1d Lipid Presentation to NKT Cells.
We examined the ability of the cell lines expressing the deletion constructs to present the lipid α-GalCer to an NKT cell clone. Although all mutant PS species were able to enhance recognition by NKT cells, the cell line lacking saposin B was the least stimulatory (Fig. 3A), suggesting that saposin B is the most efficient enhancer of CD1d lipid loading. However, the combination of saposins A, C, and D expressed by PSΔB supported NKT cell stimulation to some extent. Recognition by NKT cells in the absence of α-GalCer was similar in all cases, consistent with the previous suggestion that saposins are not necessary for recognition of endogenous lipids (14). To determine whether any of the saposins could function alone, we reconstituted saposin expression in PSKO1.CD1d cells by adding purified recombinant saposins (Fig. 3B), previously shown to be functional SAPs in vitro (29). Confocal immunofluorescence microscopy showed that the added saposins accumulated in late endosome/lysosomal compartments, shown in Fig. 3C for saposin C. Addition of any of the four saposins enhanced CD1d presentation of α-GalCer to NKT cells (Fig. 3D). However, saposin B was clearly the most efficient, consistent with the results obtained with the PS deletion mutants (Fig. 3A).
Fig. 3.
Saposin B enhances lipid loading to CD1d most efficiently. (A) PSKO2.CD1d cells expressing different PS mutants (PSΔA, -ΔB, -ΔC, and ΔD) or no prosaposin (mock) were used as antigen-presenting cells and incubated with either vehicle control (v.c.) or α-GalCer (GalC, 100 ng/ml). After fixation, 6F5 NKT cells were added, and IFN-γ secretion was measured by ELISA after 24 h. (B) Purified recombinant saposins (A–D) were separated by SDS/PAGE (15%) and detected by staining with Coomassie Brilliant blue. (C) Recombinant saposins are endocytosed and delivered to late endosomal/lysosomal compartments. PSKO1.CD1d.mock cells incubated with recombinant saposin C were stained with rabbit anti-saposin C and rat anti-mouse Lamp1 antibodies, followed by Alexa Fluor 488-conjugated goat anti-rabbit and Alexa Fluor 594-conjugated goat anti-rat Ig antibodies. (D) PSKO1.CD1d.mock cells were preincubated with saposins (A–D or A+B+C+D) overnight before incubation with α-GalCer. Control cells were left untreated and incubated with vehicle control (v.c.) or α-GalCer (GalC). After fixation, the cells were cocultured with 6F5 NKT cells for 24 h, and IFN-γ secretion was determined by ELISA.
Saposins Directly Activate Lipids for CD1d Presentation.
Saposin C appears to interact directly with CD1b to facilitate lipid binding (17). However, we were unable to detect a direct interaction between saposins and CD1d (data not shown). To investigate whether saposins directly mediate lipid binding to CD1d, we used a plate-bound CD1d-based lipid presentation assay (30). Human CD1d, expressed as an Fc-fusion protein in CHO cells and purified as described (30), was coated onto the wells of a 96-well plate, and α-GalCer was added in the presence or absence of recombinant saposins at pH 7.0. NKT cells were then added, and the response was determined by measuring the release of IFN-γ. Although there was some response in the absence of saposins, the addition of any saposin enhanced presentation of the lipid to the NKT cells (Fig. 4A). Saposin B was much more efficient than saposins A, C, or D. Titration of the molar ratio of saposin versus α-GalCer indicated that they facilitate lipid binding to CD1d specifically but that the reaction is not enzymatic (Fig. 4B). Stimulation reached a plateau when saposin B was present in a molar excess over α-GalCer, consistent with previous observations (18). This is different from the catalysis of peptide loading onto MHC class II molecules by the HLA-DM glycoprotein, where HLA-DM functions substoichiometrically, and the binding reaction follows Michelis–Menten kinetics (31).
Fig. 4.
Saposins directly activate lipids for CD1d presentation. (A) Recombinant human CD1d-Fc fusion protein was coated in triplicate in 96-well microtiter plates and incubated with vehicle control (v.c.), α-GalCer (GC, 100 ng/ml) or α-GalCer in the presence of saposins A, B, C, or D at pH 7.0. After 48 h, 6F5 NKT cells were added, and IFN-γ secretion was assayed by ELISA after a further 24 h. (B) Titration of molar ratio of saposin B: α-GalCer in the T cell stimulation assay using plate-bound CD1d. CD1d-Fc protein coated onto the wells of a 96-well plate was incubated with indicated amount of saposin B and α-GalCer (100 ng/ml), and IFN-γ secretion by the NKT cells was measured as in A. (C) CD1d-Fc-coated plates were incubated with α-GalCer in the absence (No saposins) or presence of one saposin (A–D) at indicated pHs for 48 h. T cell culture and analysis of secreted IFN-γ in the supernatant were as described in A and B.
To determine the optimal pH of saposin-mediated CD1d lipid binding, α-GalCer loading was performed over a range of pH values, and binding was assayed by using the NKT cell clone. As shown in Fig. 4C, although maximum stimulation was seen at pH 7.0 with no saposins, saposin B and D enhanced lipid loading optimally at pH 6.0. Saposins A and C were most efficient at pH 5.0, close to the physiological pH of the lysosome, and in fact, at pH 5.0, the activities of saposins B and C were equivalent. The low optimal pH for saposin-mediated lipid loading is consistent with their normal cellular distribution; both endogenous saposins (Fig. 2), (32), and the added recombinant saposins (Fig. 3D) accumulate in acidic lysosomes.
Saposins Directly Mediate Lipid Binding to CD1d.
To further examine whether saposins directly deliver lipids to CD1d, we developed a T cell-independent in vitro lipid-loading assay. Biotinylated-DHPE or -α-GalCer was incubated with a soluble, secreted version of CD1d (33) at pH 5.5, chosen to be between the optima for saposins B and C in the T cell-dependent loading assay. The lipid–CD1d complex was then isolated by using neutravidin-agarose beads, and the efficiency of lipid binding was evaluated by SDS/PAGE and immunoblotting with a mAb to the CD1d heavy chain. As shown in Fig. 5 A and B, in the absence of biotinylated lipid, there was no background binding of CD1d to the beads (lanes 2). In the absence of detergent or saposin, low spontaneous lipid binding was observed (lanes 3). In CHAPS at 0.5% or in the presence of saposin B, but not saposin C, both α-GalCer and DHPE binding to CD1d was substantially enhanced (lanes 4 and 6–11). This is consistent with the finding that saposins can transfer both glycolipid and phospholipids between membranes (34).
Fig. 5.
Saposin B activates and delivers both glycolipid and phospholipids to CD1d in vitro. (A and B) In vitro loading of biotin-α-GalCer (A) and biotin-DHPE (B) on to purified CD1d protein. Soluble CD1d was incubated with biotinylated lipids, and the resulting lipid-CD1d complex was isolated by using neutravidin-agarose beads. Bound CD1d heavy chains were detected by immunoblotting with the D5 mAb. Lanes 1: soluble CD1d used in the loading reaction; lanes 3–11: biotinylated lipids and CHAPS, saposin B, or saposin C added as indicated. (C and D) Saposin B does not remove CD1d-associated lipids. The samples shown in lanes 2 and 3 were prepared as in A and B. In lanes 4–9, CD1d was preloaded with biotinylated α-GalCer (C) or DHPE (D) in the presence of 0.5% CHAPS as in A and B. The neutravidin-agarose beads with associated CD1d–lipid complexes were then incubated under the conditions indicated before analysis of the residual biotinylated lipid-associated CD1d molecules by immunoblotting.
In 2% CHAPS, lipid binding to CD1d was inhibited (Fig. 5 A and B, lanes 5). The high concentration of detergent could compete with lipid for binding and/or remove it from the CD1d-binding groove. To test this, and to determine whether saposin B could remove lipids already associated with CD1d, CD1d was first loaded with biotinylated α-GalCer, captured using neutravidin beads, and the beads were then incubated with either detergent or saposin B. After washing, residual lipid-associated, bead-bound CD1d molecules were identified by immunoblotting. CHAPS at 0.5% and 2% induced partial and complete lipid dissociation, respectively, from CD1d, but saposin B had no effect (Fig. 5C). This suggests that saposin B is involved in the delivery of lipid substrates to CD1d but not in their removal. Saposin B was also incapable of inducing dissociation of the phospholipid DHPE from CD1d molecules (Fig. 5D). This is consistent with previous results suggesting that saposins may not induce dissociation of self-lipids from mouse CD1d; another lysosomal lipid-transfer protein, the GM2 activator protein, may be involved (27).
Our results suggest that, although all four saposins can deliver lipids to CD1d, saposin B is the most efficient. Saposins A, C, and D have high homology to each other, with pairwise sequence identities ranging from 34% to 39%. Saposin B is more distantly related, with sequence identities of 22%, 15%, and 21% with saposins A, C, and D, respectively (35). The more important difference is in the lipid activation mechanism. There are two different proposed mechanisms of saposin action, called the “solubilizer” and “liftase” models. Extensive in vitro studies suggest that saposin B uses the “solubilizer” mode, in which target lipids are extracted from bilayers to form a saposin B/lipid complex that is then presented to soluble hydrolases specific for the particular lipid head group. After the head group is hydrolyzed, the modified lipid is presumably returned to the membrane and exchanged for another (26, 34, 36). In the “liftase” mode, exemplified by saposin C, the saposin molecule facilitates binding of a lipid hydrolase to its substrate by remodeling the membrane bilayer. Saposin C reduces the bilayer thickness, possibly by partially removing (“lifting”) the lipids from one leaflet, making them readily accessible to the hydrolases, while the amphiphilic saposin molecules shield the acyl chains from the aqueous environment (36). Although both modes of saposin action could plausibly expose lipid substrates from the membrane bilayer to soluble hydrolysases, a “solubilizer” saposin might more readily deliver lipids to the CD1d-binding site. Saposin C is specifically involved in the presentation of lipid substrates by CD1b and may interact directly with CD1b to bring it in close proximity to lipid ligands (17). However, for CD1d, where no evidence for a direct interaction exists, saposin B may be the most efficient because it can directly mobilize lipid molecules.
The pH at which lipids bind to CD1d in vivo is difficult to determine. The optimal pH for biotinylated-DHPE binding in vitro in the absence of saposins is between pH 5.5 and 6 [see supporting information (SI) Fig. 6], whereas, for α-GalCer, it is pH 7.0 as measured by the lipid presentation assay (Fig. 4C). Clearly, this pH is higher than that conventionally accepted for lysosomes. Low pH is optimal for saposin-mediated α-GalCer loading, although for saposin B the functional pH range is quite broad, being optimal at pH 6.0 but still effective at pH 7.0 (Fig. 4C). Given that saposin B is superior in enhancing NKT cell stimulation when introduced into PS-negative, CD1d-positive cells (Fig. 3 A and D), it may promote lipid loading throughout the endocytic pathway, rather than exclusively in lysosomes. Substantial amounts of PS are secreted before endocytosis (37), and lipids, CD1d, and saposin B could meet at many points after endocytosis depending on where mature saposins are generated.
Exposure of the hydrophobic domains of saposins A, C, and D, and hence their affinity for phospholipid membranes, depends on low pH, but the hydrophobicity of saposin B apparently does not (38). The affinity of saposin B for lipid bilayers is low at both neutral and acidic pH, consistent with its function as an extractor/solubilizer that has only transient interactions with the membrane. Furthermore, low pH affects the structure of saposin B differently from the way it affects saposin A or C (35). Saposins A and C, as well as most other saposin-like proteins, have a compact, monomeric fold that buries a small hydrophobic core, whereas saposin B, like GM2 activator protein, has a major conformational variant that forms a homodimer that has a large hydrophobic cavity (26). For saposins A and C, low pH and the presence of detergent is required for dimer formation, whereas saposin B is dimeric at both neutral and low pH and in the presence or absence of detergent (35). These data again support the argument that the two categories of saposins interact differently with lipids in a manner relevant to the superior capacity of saposin B to facilitate lipid binding to CD1d.
Saposins are required for development of mouse invariant Vα14 NKT cells, indicating that they are involved in presenting self-lipids from thymocytes to developing NKT cells (27). The sphingolipid isogloboside b3 (iGb3) has been reported to be a self-ligand, and saposin B mediated the binding of iGb3 to mouse CD1d in a cell-free system (18). Another self-lipid identified bound by both human CD1d and CD1b is PI (33). There have been many reports that CD1d can be loaded with phospholipids and present them to T cells (30, 33, 39, 40). Saposin B, despite the fact it was initially identified as a sphingolipid activator protein, also binds and transfers phospholipids (41). It binds to some phospholipids with comparable or even higher affinities than it binds glycosphingolipids (34). Recombinant saposin B copurified with PE (26), and saposin B can mediate DHPE binding to CD1d (Fig. 5). Although the size and diversity of the self-lipid repertoire involved in NKT development and immune stimulation remains unclear, saposin B is likely to be involved in their presentation to NKT cells by CD1d during development.
Materials and Methods
Cell Lines and Antibodies.
PSKO1 and PSKO2 cells stably expressing CD1d (PSKO1.CD1d, PSKO2.CD1d) and derivatives expressing human prosaposin were described (14). Four new cell lines were produced from PSKO2.CD1d by transduction with retroviruses encoding prosaposin with the regions encoding saposin A, B, C, or D deleted. For each construct, two internal primers were used to delete the appropriate coding region by PCR. The mutant genes were cloned into the pLPCX vector, and retrovirus was generated and cell lines selected as described (42). The human CD4+Vα24+ NKT cell line, 6F5, was a gift from Steven Porcelli (Albert Einstein College of Medicine, Bronx, NY) (43). Rabbit antisera to saposins A, B, C, and D were described (32). Phycoerytherin-conjugated mouse anti-human mAb 42.1 and rat anti-mouse Lamp1 mAb were from BD–Pharmingen (San Jose, CA). Alexa Fluor 594-conjugated goat anti-rat Ig antibody was from Molecular Probes (Eugene, OR).
Reconstitution of Prosaposin-Deficient Fibroblasts.
Recombinant human saposins, expressed in Escherichia coli as His-tagged proteins, were purified as described (29). PSKO1.CD1d cells were preincubated with the saposins at 20 μg/ml for 18 h before addition of α-GalCer (100 ng/ml) or vehicle control, and NKT cell stimulation assays were performed as described (14, 42). IFN-γ secretion was assayed after 24 h by sandwich ELISA (BD–Pharmingen). All experimental samples were in the linear range of the standard curve, and results are presented as mean ± standard deviation of triplicate samples.
T Cell Stimulation Assay Using Plate-Bound CD1d.
Recombinant human CD1d-Fc (10 μg/ml) (44), was coated on Immulon plates overnight at 4°C. After washing, α-GalCer (100 ng/ml) or vehicle control was added with or without saposins for 48 h at 37°C, followed by washes with PBS and T cell medium. One hundred thousand 6F5 T cells were added to each well, and IFN-γ secretion was measured by ELISA after 24 h.
Lipid-Binding Assays.
Biotinylated DHPE (Molecular Probes) or biotinylated α-GalCer, (2S, 3S, 4R)-1-O-(α-d-galactopyranosyl)-2-[N-2-{2-[2-(2-biotinylamino-ethoxy)-ethoxy]-ethoxymethoxy}-tetracosanoylamino]-1,3,4-octadecantriol, was sonicated in TBS (pH 5.5) in the presence or absence of detergent or saposins at the indicated concentrations, and soluble CD1d, purified as previously described (33), was added. After 1 h at 37°C, the samples were neutralized, diluted in TBS (pH 7.4), and neutravidin-agarose beads added (Pierce, Rockford, IL). After 1 h at 4°C and washing, the bound proteins were separated by SDS/PAGE, and CD1d binding was analyzed by immunoblotting with the anti-CD1d antibody, D5. To examine the effect of saposins on preloaded lipids, CD1d was incubated with biotinylated lipid in the presence of 0.5% CHAPS and isolated by using neutravidin-agarose beads as described above. The beads were then incubated with indicated reagents at 37°C for 1 h before washing and analysis by immunoblotting.
Flow Cytometry, SDS/PAGE, and Immunoblotting.
These procedures were performed as described (42).
Confocal Immunofluorescence.
Confocal immunofluorescence was performed as described (15, 42). Briefly, cells were seeded on sterile coverslips and allowed to adhere overnight. They were then fixed with 3.7% formaldehyde and permeabilized in PBS containing 10% FBS, 0.1% saponin, and 10 mM glycine. For costaining, the cells were incubated with rabbit anti-saposin and rat anti-mouse Lamp1 sera, followed by Alexa Fluor 488-conjugated goat anti-rabbit Ig and Alexa Fluor 594-conjugated goat anti-rat Ig antibodies. Confocal images were acquired by using a Leica TCS SP2 confocal system and processed with Leica software.
Supplementary Material
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (P.C.), National Institutes of Health Grants AI059167 (to P.C.) and AI060777 and HL71590 (to J.G.), and a fellowship from the Cancer Research Institute (to W.Y.). G.S.B. acknowledges support in the form of a Personal Research Chair from Mr. James Bardrick and, as a former Lister Institute–Jenner Research Fellow, the Medical Research Council (U.K.) and The Wellcome Trust.
Abbreviations
- CD1
cluster of differentiation 1
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DHPE
dihexadecanoyl-sn-glycero-3-phosphoethanol-amine
- α-GalCer
α-galactosylceramide
- NKT
natural killer T
- PS
prosaposin
- PSKO
prosaposin knockout.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700617104/DC1.
References
- 1.Brigl M, Brenner MB. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 2.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Am J Pathol. 2004;165:1853–1863. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogt AB, Kropshofer H, Moldenhauer G, Hammerling GJ. Proc Natl Acad Sci USA. 1996;93:9724–9729. doi: 10.1073/pnas.93.18.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 5.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg M. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 7.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, Corazza N, Colgan SP, Onderdonk AB, Blumberg RS. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 9.Hansen DS, Siomos MA, Buckingham L, Scalzo AA, Schofield L. Immunity. 2003;18:391–402. doi: 10.1016/s1074-7613(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, Nakayama T, Taniguchi M, Saito A. J Immunol. 2001;167:6525–6532. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 11.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, et al. Nat Med. 2001;7:1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 12.Hava DL, Brigl M, van den Elzen P, Zajonc DM, Wilson IA, Brenner MB. Curr Opin Immunol. 2005;17:88–94. doi: 10.1016/j.coi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Sugita M, Grant EP, van Donselaar E, Hsu VW, Rogers RA, Peters PJ, Brenner MB. Immunity. 1999;11:743–752. doi: 10.1016/s1074-7613(00)80148-x. [DOI] [PubMed] [Google Scholar]
- 14.Kang SJ, Cresswell P. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 15.Kang SJ, Cresswell P. EMBO J. 2002;21:1650–1660. doi: 10.1093/emboj/21.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu YH, Park SH, Benlagha K, Forestier C, Jayawardena-Wolf J, Savage PB, Teyton L, Bendelac A. Nat Immunol. 2002;3:55–60. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- 17.Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, Porcelli SA, Brinkmann V, Sugita M, Sandhoff K, et al. Nat Immunol. 2004;5:169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- 18.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, et al. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 19.Kolter T, Sandhoff K. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda J, Vanier MT, Saito Y, Tohyama J, Suzuki K, Suzuki K. Hum Mol Genet. 2001;10:1191–1199. doi: 10.1093/hmg/10.11.1191. [DOI] [PubMed] [Google Scholar]
- 21.Li SC, Kihara H, Serizawa S, Li YT, Fluharty AL, Mayes JS, Shapiro LJ. J Biol Chem. 1985;260:1867–1871. [PubMed] [Google Scholar]
- 22.Ho MW, O'Brien JS. Proc Natl Acad Sci USA. 1971;68:2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda J, Kido M, Tadano-Aritomi K, Ishizuka I, Tominaga K, Toida K, Takeda E, Suzuki K, Kuroda Y. Hum Mol Genet. 2004;13:2709–2723. doi: 10.1093/hmg/ddh281. [DOI] [PubMed] [Google Scholar]
- 24.Qi X, Grabowski GA. Biochemistry. 1998;37:11544–11554. doi: 10.1021/bi980785+. [DOI] [PubMed] [Google Scholar]
- 25.Wilkening G, Linke T, Sandhoff K. J Biol Chem. 1998;273:30271–30278. doi: 10.1074/jbc.273.46.30271. [DOI] [PubMed] [Google Scholar]
- 26.Ahn VE, Faull KF, Whitelegge JP, Fluharty AL, Prive GG. Proc Natl Acad Sci USA. 2003;100:38–43. doi: 10.1073/pnas.0136947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Cantu C, III, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, et al. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonova T, Qi X, Bencosme A, Ponce E, Sun Y, Grabowski GA. J Biol Chem. 1996;271:17312–17320. doi: 10.1074/jbc.271.29.17312. [DOI] [PubMed] [Google Scholar]
- 29.Qi X, Leonova T, Grabowski GA. J Biol Chem. 1994;269:16746–16753. [PubMed] [Google Scholar]
- 30.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 31.Matsunaga I, Bhatt A, Young DC, Cheng TY, Eyles SJ, Besra GS, Briken V, Porcelli SA, Costello CE, Jacobs WR, Jr, Moody DB. J Exp Med. 2004;200:1559–1569. doi: 10.1084/jem.20041429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu Z, Witte DP, Qi X. Exp Cell Res. 2005;303:300–307. doi: 10.1016/j.yexcr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Park JJ, Kang SJ, De Silva AD, Stanic AK, Casorati G, Hachey DL, Cresswell P, Joyce S. Proc Natl Acad Sci USA. 2004;101:1022–1026. doi: 10.1073/pnas.0307847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fluharty CB, Johnson J, Whitelegge J, Faull KF, Fluharty AL. J Neurosci Res. 2001;63:82–89. doi: 10.1002/1097-4547(20010101)63:1<82::AID-JNR10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Ahn VE, Leyko P, Alattia JR, Chen L, Prive GG. Protein Sci. 2006;15:1849–1857. doi: 10.1110/ps.062256606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alattia JR, Shaw JE, Yip CM, Prive GG. J Mol Biol. 2006;362:943–953. doi: 10.1016/j.jmb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Hiesberger T, Huttler S, Rohlmann A, Schneider W, Sandhoff K, Herz J. EMBO J. 1998;17:4617–4625. doi: 10.1093/emboj/17.16.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaccaro AM, Ciaffoni F, Tatti M, Salvioli R, Barca A, Tognozzi D, Scerch C. J Biol Chem. 1995;270:30576–30580. doi: 10.1074/jbc.270.51.30576. [DOI] [PubMed] [Google Scholar]
- 39.Im JS, Yu KO, Illarionov PA, LeClair KP, Storey JR, Kennedy MW, Besra GS, Porcelli SA. J Biol Chem. 2004;279:299–310. doi: 10.1074/jbc.M308803200. [DOI] [PubMed] [Google Scholar]
- 40.De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, Joyce S. J Immunol. 2002;168:723–733. doi: 10.4049/jimmunol.168.2.723. [DOI] [PubMed] [Google Scholar]
- 41.Ciaffoni F, Tatti M, Boe A, Salvioli R, Fluharty A, Sonnino S, Vaccaro AM. J Lipid Res. 2006;47:1045–1053. doi: 10.1194/jlr.M500547-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Yuan W, Dasgupta A, Cresswell P. Nat Immunol. 2006;7:835–842. doi: 10.1038/ni1364. [DOI] [PubMed] [Google Scholar]
- 43.Paduraru C, Spiridon L, Yuan W, Bricard G, Valencia X, Porcelli SA, Illarionov PA, Besra GS, Petrescu SM, Petrescu AJ, Cresswell P. J Biol Chem. 2006;281:40369–40378. doi: 10.1074/jbc.M608518200. [DOI] [PubMed] [Google Scholar]
- 44.Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, Reddington F, Illarianov PA, Besra GS, Brenner MB, Gumperz JE. J Immunol. 2006;176:3625–3634. doi: 10.4049/jimmunol.176.6.3625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.