Abstract
The striatum and hippocampus are conventionally viewed as complementary learning and memory systems, with the hippocampus specialized for fact-based episodic memory and the striatum for procedural learning and memory. Here we directly tested whether these two systems exhibit independent or coordinated activity patterns during procedural learning. We trained rats on a conditional T-maze task requiring navigational and cue-based associative learning. We recorded local field potential (LFP) activity with tetrodes chronically implanted in the caudoputamen and the CA1 field of the dorsal hippocampus during 6–25 days of training. We show that simultaneously recorded striatal and hippocampal theta rhythms are modulated differently as the rats learned to perform the T-maze task but nevertheless become highly coherent during the choice period of the maze runs in rats that successfully learned the task. Moreover, in the rats that acquired the task, the phase of the striatal–hippocampal theta coherence was modified toward a consistent antiphase relationship, and these changes occurred in proportion to the levels of learning achieved. We suggest that rhythmic oscillations, including theta-band activity, could influence not only neural processing in cortico-basal ganglia circuits but also dynamic interactions between basal ganglia-based and hippocampus-based forebrain circuits during the acquisition and performance of learned behaviors. Experience-dependent changes in coordination of oscillatory activity across brain structures thus may parallel the well known plasticity of spike activity that occurs as a function of experience.
Keywords: basal ganglia, hippocampus, local field potential, oscillations, striatum
The striatum and the hippocampus are both forebrain structures implicated in the learning and memory of behavioral sequences, but behavioral sequences of different sorts. The striatum, as part of basal ganglia circuitry, is associated with learning sequences of actions that make up goal-directed procedures and habits (1–4). The hippocampus and adjoining cortical structures are recognized as critical for encoding and storing sequences on the basis of episodic, context-cued events (5–8). Lesion studies have dissociated striatum-dependent and hippocampus-dependent forms of learning and memory (9–11), supporting the view that these systems work independently or even competitively. In humans, there is evidence that one system can substitute for another (12). Other evidence, however, suggests that “hippocampal” deficits can follow damage in regions of the dorsal striatum interconnected with hippocampal/limbic circuits (13, 14). Furthermore, part of the ventral striatum receives direct projections from the hippocampus.
Rhythmic activity in the theta range (≈7–14 Hz in the rodent) has been proposed to be crucial for mnemonic coding in the hippocampus and related limbic structures. Pathways interconnecting the hippocampus and neocortex are thought to use these rhythms for transferring and coordinating neural representations in cortico-hippocampal circuits in relation to sequential spatial behavior (7, 15–24). Temporal spike precession relative to the hippocampal theta rhythms has further been suggested as a way to gain temporal resolution in sequence encoding in the hippocampus and in directly interconnected zones of the prefrontal cortex (5, 7, 19, 23).
These findings are mainly based on experiments in rats navigating tracks and mazes. We tested for and found robust theta-band oscillations in the striatum of rats engaged in similar-navigation tasks (25). Striatal theta rhythms were strongly modulated during performance of a procedural T-maze task, suggesting that such rhythmic activities could be important components of basal ganglia activity influencing the organization of the sequential behavioral performance.
These findings raised the intriguing possibility that the local field potential (LFP) oscillations in the striatum and those in the hippocampus might themselves be interrelated as animals learn and perform sequences of actions. To test this possibility, we recorded LFP and spike activity chronically both in the caudoputamen and in the dorsal hippocampus as rats were trained to perform this conditional T-maze task, and we measured the coherence between striatal and hippocampal theta-band LFP activity across successive weeks of training. Our findings suggest that changing patterns of striatal–hippocampal theta coherence are a cardinal feature of the neural activity that accompanies procedural learning.
Results
Striatal and Hippocampal LFP Oscillations Are Differentially Modulated During T-Maze Performance.
We recorded simultaneously in the medial caudoputamen and in the CA1 field of the dorsal hippocampus (Fig. 1A) in six rats as they performed the maze task illustrated in Fig. 1B. These sites were chosen because theta rhythms are prominent in the CA1 field (26) and because the medial striatum and the hippocampus are considered to be functionally related (4). We first analyzed the LFP activities recorded during the early phase of maze training, when the rats first reached asymptotic running times (Figs. 1–4). There were marked contrasts between the striatal and hippocampal LFP rhythms recorded during the maze runs (Fig. 2A). Hippocampal theta (7–11 Hz) rose gradually as the rats left the start zone and began to run, then peaked toward the tone-turn interval (the decision period in the task), and then gradually diminished as the goal was approached. By contrast, striatal theta-band oscillations reached an early peak near trial start, continued at nearly constant levels, and then declined quite abruptly. In the 11- to 14-Hz band, considered by some as high theta in the rat, the contrast between hippocampal and striatal oscillations was even clearer. Hippocampal power did not vary significantly, judged by 95% confidence limits, whereas the power of striatal 11- to 14-Hz activity had significant peaks at the start and end points of the runs. The power of the hippocampal rhythms in the 14- to 22-Hz (beta) band rose significantly toward the tone-turn decision period and then fell, but striatal 14- to 22-Hz power remained nearly constant. Finally, in the 30- to 50-Hz (low gamma) band, a sharp peak occurred in the hippocampal LFPs around the sounding of the warning click that indicated the beginning of each trial, but only a very small peak appeared then in the striatal LFPs.
Fig. 1.
Simultaneously recorded LFP oscillations in the caudoputamen and the CA1 field of the dorsal hippocampus exhibit distinguishable task-related modulation during instructed running in a T-maze task. (A) Nissl-stained transverse sections illustrating, at arrows, the tracks of tetrodes in the medial caudoputamen (Left) and the CA1 pyramidal cell layer (Right). CP, caudoputamen; CA1, hippocampal CA1 field; DG, dentate gyrus. (Scale bars: 1 mm.) (B) T-maze with task events. (C) Raw striatal LFP trace recorded during a single representative trial. (D and E) Mean power (red) with 95% confidence limits (black) of LFP activity in the striatum (Left) and hippocampus (Right) during a 0.75-s epoch after tone onset and plotted on linear (D) and log (E) scales. Data were averaged across values for three rats (S23, acquisition session 7; S31, acquisition session 5, S36, acquisition session 10) during the session in which each reached running-time asymptote. (F) Reconstructed spectrograms of LFP activity in the medial striatum (Upper) and in the dorsal hippocampus (Lower) averaged for data from the three rats at their running-time asymptotes, as in D. The task time was reconstructed by abutting individual peri-event windows (bracketed by white vertical lines) with widths reflecting median inter-event intervals. Labeled task event times are indicated by black vertical lines. Data are plotted as normalized power relative to pretrial baseline activity on pseudocolor log scales at the right.
Fig. 2.
LFP oscillations in the striatum and the hippocampus exhibit different task-dependent modulation. (A) Average spectral power in four frequency bands of LFPs recorded in the medial striatum (Left) and the hippocampus (Right) averaged across values for the three rats (S17, S31, and S36). Black lines indicate upper and lower 95% confidence limits. Alternating white and shaded zones indicate time windows around task events. W, warning click; Ga, gate opening; To, instruction tone onset; TS, turn start; TE, turn end; G, goal reaching. (B and C) Correlations of broad-band theta power (5–12 Hz) in the medial striatum (dark blue) and hippocampus (green) with movement velocity (B) and acceleration (C) of three individual rats [(Left) S17, acquisition session 8. (Center) S18, acquisition session 6. (Right) S23, acquisition session 7] sampled at 101-ms intervals during the 2.5 s before and 0.5 s after goal reaching in each trial. Each dot represents one such sample. Power is normalized to the median of all points within each recording site and session.
Fig. 3.
The coherence between striatal and hippocampal theta-band LFP oscillations is the strongest at the decision period of the maze runs. (A) Single-session average coherogram (S17, acquisition session 8), assembled by abutting six peri-event striatal–hippocampal coherograms, smoothed with two tapers (width = 3). Window widths reflect median inter-event intervals. The average coherence values are indicated in pseudocolor according to the scale at the right. (B) Plots of session-averaged coherence magnitude (black lines) and phase (green arrows) showing the dynamics of the synchrony between the striatal and hippocampal signals. The phase angle of significantly coherent signals is indicated by the direction of green arrows (up, 0°; down, 180°; left, 90° lead or 270° lag of hippocampus relative to striatum). Horizontal red lines indicate the level of significant coherence. Black arrows mark 9 Hz.
Fig. 4.
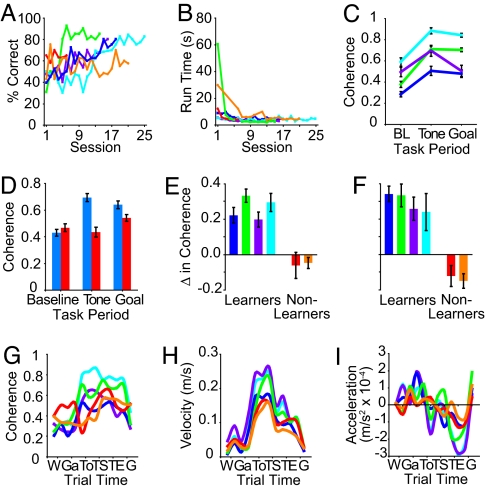
Coherence of striatal and hippocampal theta-band LFP oscillations increases in rats that successfully learn the T-maze task. (A and B) Performance accuracy (A) and running times (B) of each rat during training on the procedural T-maze task. Four rats (S17, light blue; S18, purple; S23, dark blue; S36, green) reached the acquisition criterion, but two rats (S31, red; S35, orange) did not. (C) Average magnitude of peak coherence in a 7- to 11-Hz band during 0.75-s pretrial baseline (BL), post-tone, and pre-goal periods. Each line represents coherence values for a single rat that learned the task, and averaged over all sessions for each rat (color-coded as in A). Error bars indicate standard errors of the mean. (D) Average magnitude of coherence in theta-band oscillations during 0.75-s pre-trial baseline, tone, and goal periods for the four learners (blue) and the two nonlearners (red). (E and F) Changes in coherence during the post-tone period relative to pre-trial baseline. Data from individual rats are color-coded as in A. Significant increases in coherence magnitude of striatal–hippocampal theta were found for all learners but not for non-learners in averages across all sessions (ANOVA, F = 54.42, P < 0.0001) (E) and also in averages across the first five available training sessions (ANOVA, F = 45.21, P < 0.0001) (F), during which behavioral performance of the learners and non-learners was comparable (SI Fig. 10 A and B). (G–I) Values for coherence magnitude at 9 Hz (G), running velocity (H), and acceleration (I) calculated for pre- and postevent periods for six task events (as described for Fig. 2A) averaged over all sessions for each rat (color-coded as in A).
We also tested whether the power of the striatal and hippocampal theta-band oscillations were differentially related to two measures of motor behavior that we recorded. Hippocampal theta power was highly correlated with running speed (R = 0.52–0.81, P < 0.001; Fig. 2B), but striatal theta-band power was much more weakly correlated with running speed (R = 0.03–0.48, P = 0.000–0.300; Fig. 2B; see also ref 25). Neither striatal nor hippocampal theta activity was strongly related to acceleration (R = 0.04–0.43, P = 0.000–0.200; Fig. 2C). These results demonstrate that contrasting patterns of oscillatory LFP activity in the striatum and in the hippocampal CA1 field accompanied different segments of behavior in the maze task, with the power profiles of the oscillations being different for the two structures in each of the frequency bands that we analyzed. The theta rhythms in the two regions also exhibited different relations to the rats' velocity profiles. For detailed comparisons of the striatal and hippocampal rhythms, we focused on the theta band.
Striatal and Hippocampal Theta-Band Rhythms Exhibit Highly Task-Dependent Patterns of Coherence.
Throughout the training period, there were striking modulations of coherence between the striatal and hippocampal theta rhythms as the rats ran the maze (Figs. 3 and 4). First, the magnitude of coherence was modulated during the task in the four rats that learned the task (9–13 sessions per rat, 43 total training sessions, Fig. 4 A and B). The striatal and hippocampal theta rhythms in the rats exhibited individually varying levels of coherence (0.13–0.79) during the baseline period before the runs in these rats, but, in each rat, the coherence values rose at the tone-turn period, when the rats were required to make a decision about the expected goal arm and then to execute this decision by its running direction [mean = 0.70, range = 0.27–0.96; Figs. 3 and 4C and supporting information (SI) Fig. 6]. These higher levels of coherence were largely maintained up to the period before goal reaching (mean = 0.64, range = 0.09–0.91; Figs. 3 and 4C and SI Fig. 6). The increases in coherence magnitude from the baseline period to the tone period was significant in all four rats (P = 0.0000–0.0294, t test; Fig. 4E), as were those from the baseline period to the goal period in three of four rats (P = 0.0000–0.0003). (In a seventh rat, we recorded LFPs in both the medial and lateral caudoputamen as well as the hippocampus, and we observed similarly high levels of striatal–hippocampal theta coherence for both medial and lateral sites; see SI Fig. 7). These elevated coherence magnitude values were not accounted for by correlations with either speed or acceleration during the tone-posttone period (Fig. 4 G–I and SI Fig. 8).
The patterns of coherence between the striatal and hippocampal theta rhythms were not modulated during the course of learning. We did not find a systematic change in the magnitude of coherence during the baseline, tone, and goal periods in relation to stages of learning (R = −0.0087–0.2326, P = 0.2335–0.9650; SI Fig. 9A), performance accuracy (R = 0.0801–0.1976, P = 0.2155–0.6185; SI Fig. 9B), or running speed (R = −0.1673 to −0.0031, P = 0.2959–0.9849). Nor did we find a significant correlation between the pattern of coherence, with a rise during the decision period of the task, and any of these behavioral measures (R = −0.0889–0.0883, P = 0.5853–0.6552).
Two of the rats studied did not learn the maze task. In contrast to the results for the four rats that learned the maze task, we did not find comparable increases in coherence at the tone-turn period in the rats that failed to reach the criterion for behavioral acquisition (72.5% correct for at least 2 consecutive days). The magnitude of striatal–hippocampal theta coherence was similar for the four learners and these two nonlearners during the baseline period (Fig. 4D, P = 0.3695, ANOVA). However, the levels of coherence during the tone and goal periods were significantly lower in the nonlearners than in the learners (P = 0.0000 and 0.0352, respectively, ANOVA; Fig. 4D). Accordingly, there was a significant difference in the increase of coherence from the baseline to tone periods (learners, nsession = 41, mean ± SEM = 0.264 ± 0.025; nonlearners, nsession = 19, mean = −0.053 ± 0.032; P < 0.0001, ANOVA; Fig. 4E). The coherence profiles did not parallel either the velocity or the acceleration profiles of the learners and nonlearners (see SI Fig. 8 and SI Text).
This difference held even during early training sessions, in which the four learners and the two nonlearners did not differ in performance accuracy (P = 0.43, ANOVA) or run times (P = 0.08, ANOVA; SI Fig. 10). There again was a significant increase in coherence values from baseline to tone for the learners but not for the nonlearners (learners, nsession = 18, mean = 0.299 ± 0.041; nonlearners, nsession = 9, mean = −0.134 ± 0.040; P < 0.0001, ANOVA; Fig. 4F and SI Fig. 10). Thus, even before the percent-correct values for the learners and nonlearners diverged, the four learners showed increases of coherence between the striatal and hippocampal theta rhythms during the decision period of the maze runs, whereas the two nonlearners did not exhibit such an increase. It is as though the coherence peak at the decision period did not reflect the current accuracy of performance or running speed of the rats, but whether they would learn the task.
The Phase Relations of Coherent Striatal and Hippocampal Theta Rhythms Are Modified as a Function of Learning.
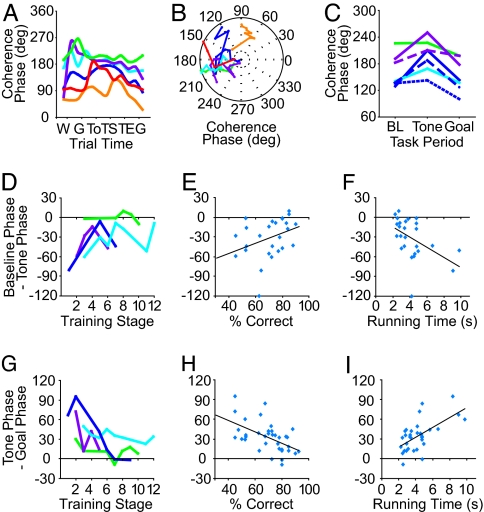
Each of the four rats that learned the maze task had a characteristic mean coherence phase profile for the striatal–hippocampal theta band oscillations recorded during the trial runs (Fig. 5 A and B). Overall, they had coherence phase angles near 180° (mean ± SEM = 171.1° ± 3.5), i.e., antiphase. We analyzed group delays between striatal and hippocampal theta rhythms. There were small but statistically significant group delays in individual sessions, but these delays did not show a consistent pattern across days or rats, failing to provide evidence that one structure consistently led the other.
Fig. 5.
The phase of striatal–hippocampal theta coherence is modulated during learning. (A) Average phase angles plotted for the six rats whose percent correct and running times are shown in the same color codes as in Fig. 3. Coherence phase was calculated by subtracting the striatal phase from the hippocampal phase and converting the angles to a 0–360° range. (B) Phase angles during the posttone period for the first to last training sessions for individual rats are shown from center to periphery of the polar plots. (C) Coherence phase angles measured at three task periods during individual sessions up to asymptote of running speed for rats S17 (1 session), S18 (2 sessions), S23 (3 sessions), and S36 (1 session). (D–I) Amounts of change in coherence phase angles from pretrial baseline period to posttone period (D–F) and from posttone period to pregoal period (G–I) during T-maze training for the four learners. Changes in coherence phase angles from pretrial baseline to posttone period were significantly correlated with learning stage (R = 0.46, P < 0.05) (D) and with running time (R = −0.48, P < 0.02) (F) but not with percent-correct response (R = 0.35, P = 0.079) (E). Tone-to-goal changes in coherence phase angles were significantly correlated with all behavioral measures: stage (R = −0.53, P < 0.01) (G), percent-correct response (R = −0.55, P < 0.001) (H), and running time (R = 0.61, P < 0.001) (I).
Significant shifts in the phase relationship between the striatal and hippocampal theta oscillations occurred during the maze runs. To examine these, we first analyzed the data recorded in the four learner rats during training sessions before and up to running time asymptote. We calculated the phase difference at ≈9 Hz between the striatal and hippocampal rhythms at baseline, tone, and goal for those sessions in which coherence values were significant (P < 0.01, 1-tailed t test). We then compared the shift in coherence phase from the baseline period to the instruction tone and from the instruction tone period to goal-reaching. For example, in the record shown in Fig. 3B (rat S17, acquisition day 8), the coherence in the theta band became significant at approximately the time of the instruction tone. From this time to the time of goal-reaching, there was a phase advance (precession) of striatal theta relative to hippocampal theta of ≈45°.
We found such phase precession during the choice-to-goal period in all rats during this early training period (Fig. 5C). The precession values varied from 12–72°, corresponding to 3.7–22.2 ms. By contrast, during the first half of the task (the baseline-to-tone period), the phase differences between the striatal and hippocampal theta rhythms changed in the opposite direction: They recessed by 1–81° degrees, corresponding to 0.3–25.0 ms (Fig. 5C). Thus, phase differences between the theta rhythms in the two regions were modulated during the course of the maze runs in such a way that they tended to increase as the rats approached the choice period and then to decrease as they ran to the goal.
The successive phase recession and precession of striatal theta relative to hippocampal theta that was so prominent early during training decreased as the rats learned the maze task. As a group, the learners showed larger changes in phase difference both for the baseline-to-tone and tone-to-goal periods early in training than late in training (for the baseline-to-tone recession, R = −0.4570, P = 0.0492, Fig. 5D; for the tone-to-goal precession, R = −0.5308, P = 0.0063, Fig. 5G). The amount of precession of the striatal theta-band oscillations was inversely related to the percent-correct performance of these rats (for the tone-to-goal precession: R = −0.5451, P < 0.001; Fig. 5H). The decreases in phase shift during training were not simply due to a shortening of the time available for phase angles to shift. The recession and precession decreased as the rats' running times decreased (Fig. 5 F and I), but correlations between the amount of phase shift and inter-event duration were not significant (SI Fig. 9 C and D) (baseline–tone, R = −0.3233, P = 0.1072; tone–goal, R = 0.3016, P = 0.0738).
The two nonlearners differed from the four learners in coherence phase angles between the striatal and hippocampal theta rhythms recorded during training. First, the nonlearners had significantly smaller phase angles (mean ± SEM = 78.1° ± 5.7, P < 0.0001, ANOVA). Second, the amount of recession and precession in the nonlearners was not correlated with the percent-correct performance (baseline-to-tone recession, R = −0.4208, P = 0.1522; tone-to-goal precession, R = 0.3232, P < 0.3055).
We observed, but did not analyze in detail, further complexity in the phase relations both within the theta band and at other frequencies. At any one time point in the maze run, the coherence phase angles between the striatal and hippocampal LFPs were clearly different at different frequencies, and there were multiple, frequency-dependent shifts in the coherence patterns between striatal and hippocampal theta as the rats ran the maze (SI Fig. 6).
Discussion
Oscillatory modulation of neuronal activity has been implicated in a wide range of functions, including sensory processing, network coordination, expectancy coding, sequence learning, episodic memory, and interval timing (7, 15, 20, 21, 23, 27–38). We demonstrate here that during goal-directed behavior, striatal theta-band oscillations have structured, task-dependent, and learning-dependent coherence relationships with the theta rhythms concurrently recorded in the CA1 field of the dorsal hippocampus. We suggest that oscillatory modulation of neuronal activity in the striatum could contribute to the interplay between basal ganglia-based circuits and concurrently active hippocampal circuits. The marked patterning of striatal–hippocampal theta coherence phase in rats that learned the task further suggests that adjustment of conjoint activity between the basal ganglia and hippocampus may be a critical part of the learning process as such goal-directed behaviors are acquired.
Striatal and Hippocampal LFP Oscillations Have Different Task-Dependent Patterns of Modulation but Can Become Coherent During the Maze Runs.
By simultaneously recording LFP activity in the striatum and the hippocampus, we directly compared the theta-band LFP rhythms in these two structures under identical behavioral conditions. Both striatal and hippocampal theta rhythms were maximal as the rats ran the maze, were reduced at rest, and fell at the end of the maze runs, but their magnitudes were modulated differently during the course of the maze runs. Remarkably, the task-modulation of the striatal and hippocampal LFP oscillations was different not only for theta rhythms but also for each frequency subrange from delta to gamma.
Despite this different task-dependent modulation of the striatal and hippocampal LFP rhythms, they exhibited periods of high coherence as the rats performed the T-maze task. For any one frequency band, the levels of coherence varied across task time, and the levels of coherence differed for different frequency bands.
The Coherence Phase Between Striatal and Hippocampal Theta-Band LFP Activity Is Modulated as a Function of Learning.
Two patterns in the coherence between striatal and hippocampal theta oscillations suggest that the relationship between these rhythms is modulated during learning. First, during the maze runs, striatal theta in the learners tended to recess and to precess relative to hippocampal theta. The coherence phase changes emphasized the decision period of the task. Striatal theta-band activity recessed (slowed) relative to hippocampal theta as the rats approached the instruction tone period, but then precessed (quickened) relative to hippocampal theta as the rats ran to the goal. The amounts of phase recession and phase precession in the learners were inversely related to success of their performance: The higher the percent correct and the shorter the run time, the smaller the adjustments of the phase angle between the theta rhythms in the striatum and hippocampus. Accordingly, the recession and the precession of striatal theta relative to hippocampal theta were largest early in training and decreased later as the animals learned.
A plausible interpretation of these findings is that early in training, when improvement in running speed and percent-correct performance had not yet been achieved, the phase relationships between the striatal and hippocampal theta-band rhythms were adjusted relative to each other during the maze runs, reflecting exploration during the maze runs to achieve an optimal relationship at the most salient event (making a cue-based decision). But as performance accuracy and running speed increased as a result of learning (the exploitation phase), these adjustments became unnecessary, because the phase relationship between the striatal and hippocampal theta rhythms was set near the start of the maze and was then maintained during the rest of the trial. In the two nonlearners, the coherence phase relation between the striatal and hippocampal theta rhythms was highly variable, possibly reflecting continuous phase adjustment. Conceivably, these rats would have gone on to learn the task; our data show only that, up to the time recording failed, neither had reached the relatively steady antiphase pattern for striatal–hippocampal coherence.
Modulation of Striatal Theta Rhythms and Their Coherence with Hippocampal Theta Rhythms Peak During the Choice Period of the Task.
For the rats that learned the task, the magnitude of coherence between the striatal and the CA1 theta rhythms rose to a peak as they reached the instruction tone part of the task, and the coherence remained high or fell only slightly as the rats made a decision about a turning direction and turned. The nonlearner sample was small (n = 2), but neither rat showed this pattern. If confirmed in a larger sample, this trend would raise the possibility that the increased coordination between the striatal and hippocampal rhythms during the decision period of the task was modulated by or was required for learning the instructional significance of the tone. Neither during the decision period nor during other time windows was the coherence well correlated with running speed or acceleration. These findings are consistent with the possibility that the coherence was modulated by cognitive processing, and that the coherence of striatal theta and hippocampal theta during the decision period of the maze may even have contributed to the learning of the task.
The magnitude and phase of coherence between the striatal and hippocampal theta oscillations varied even among the learners, and the coherence phase between two LFP signals could also fluctuate differently at different frequencies within the theta band. Other patterns of coherence held between the striatal and hippocampal LFP oscillations at different frequencies. This variability and the multiple coupling of the striatal and hippocampal theta rhythms suggest that the striatum and hippocampus are not locked in a single temporal relation; rather, their relationship is dynamic and highly task-dependent. Our findings raise the possibility that this dynamic relationship is shaped by, and may influence, the learning of goal-directed behaviors.
Network Dynamics of Striatal and Hippocampal Theta Rhythms Suggest Experience-Dependent Plasticity of Oscillatory Activity During Learning.
It is remarkable that the coherence between striatal and hippocampal theta rhythms reached levels as high as >0.9 given that the caudoputamen and dorsal hippocampus are thought not to be directly connected. The high levels of coherence that we found thus suggest that a broader network of interconnected regions shares these dynamic patterns of coherence. Because we did not find a clear relation between the levels of coherence between striatal and hippocampal theta rhythms and velocity or acceleration at the choice periods of the task, but because we did find a relation between both coherence magnitude and within-trial phase to learning and performance measures, we suggest that cognitive demands of the task influenced the relationship between the striatal and hippocampal rhythms. The fact that the choice period was the time of peak coherence in the learners and that it was also the apparent reference point for the phase adjustments during learning suggests that the coherence relationships of the striatal and hippocampal theta rhythms could be an integral part of mastering the maze task. If so, dynamic patterns of coherence across these brain structures may be a critical component of the decision and learning process of goal-directed behaviors. Task-selective, cross-structure relationships have been reported for the hippocampus and amygdala and for pairs of cortical areas (8, 39–41). Our findings suggest that cross-structure coherence patterns are built through experience and may be required for learning and that these changing coherence patterns may influence the degree of coordination with which the striatum and the hippocampus operate during goal-directed behaviors.
The phase precession of spike activity in the prefrontal cortex relative to hippocampal theta rhythms has been observed in rats running linear tracks and choice mazes as well as during foraging (18, 19), and in the choice paradigm, the prefrontal–hippocampal theta coherence is maximal in the decision period of the task (19), as we show here for striatal–hippocampal coherence. The prefrontal cortex does directly project to the striatum and could influence striatal LFP rhythms, but the prefrontal inputs do not, according to available anatomical evidence, reach the full breadth of medial and lateral sites in the caudoputamen in which we found high striatal–hippocampal theta coherence. These findings again emphasize the possibility that a distributed system of forebrain structures, ranging from striatum to the prefrontal cortex to the hippocampus, become coordinated in their rhythmic activities as goal-directed behaviors are learned and performed. We suggest that experience-dependent plasticity includes not only adjustment of firing rates but also regulation of cross-structure oscillatory activity.
Methods
Seven adult male Sprague–Dawley rats implanted with head-stages carrying 12 tetrodes targeting either the dorsomedial striatum and the dorsal hippocampus (n = 6) or the dorsolateral striatum, the dorsomedial striatum, and the dorsal hippocampus (n = 1) were trained for 6–25 days on a T-maze task that required a right or left turn at the choice point as instructed by 1- and 8-kHz tone cues indicating the location of the rewarded end goal baited with chocolate sprinkles. Approximately 40 trials were given daily until rats made correct responses in >72.5% of trials during two consecutive sessions. In each training session, single unit and LFP activities were recorded with gain and filter settings appropriate for each recording (42). Neuronal activity and movement of the rats in the maze (detected by video tracker and photobeam crossing) were monitored throughout the training session, and data were stored for later off-line analysis. Multitaper spectral analysis of LFP coherence and power was performed by using MatLab, with window durations and taper parameters adjusted as needed to characterize the features studied (SI Table 1). Standard histology was conducted following the completion of study to verify recording sites. All procedures met the approval of the Massachusetts Institute of Technology Committee on Animal Care and were in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. Detailed descriptions of methods are available in SI Text.
Supplementary Material
Acknowledgments
We thank Uri Eden for his help with the statistical analyses; Patricia Harlan for her help with the histology; and Henry F. Hall, who is responsible for the photography. This work was funded by National Institutes of Health/National Institute of Mental Health Grants MH60379 and MH071744.
Abbreviation
- LFP
local field potential.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700818104/DC1.
References
- 1.Graybiel AM. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 2.Graybiel AM. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- 4.Packard MG, Knowlton BJ. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 5.Dragoi G, Buzsaki G. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Ergorul C, Eichenbaum H. J Neurosci. 2006;26:4111–4117. doi: 10.1523/JNEUROSCI.0441-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasselmo ME. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- 8.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 9.DeCoteau WE, Kesner RP. Behav Neurosci. 2000;114:1096–1108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- 10.Packard MG, McGaugh JL. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 11.White NM, McDonald RJ. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 12.Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, Cannistraro PA, Wilhelm S. Biol Psychiatry. 2006;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Devan BD, White NM. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin HH, Knowlton BJ. Learn Mem. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzsaki G. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 16.Eichenbaum H. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 17.Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. J Neurosci. 2004;24:11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- 19.Jones MW, Wilson MA. PLoS Biol. 2005;3:2187–2199. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisman JE. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 21.Mehta MR, Lee AK, Wilson MA. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- 22.O'Keefe J, Recce ML. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 23.Siapas AG, Lubenov EV, Wilson MA. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. J Neurophysiol. 2007 doi: 10.1152/jn.00108.2007. in press. [DOI] [PubMed] [Google Scholar]
- 26.Buzsaki G. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 27.Baker SN, Kilner JM, Pinches EM, Lemon RN. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- 28.Baker SN, Chiu M, Fetz EE. J Neurophysiol. 2006;95:3904–3910. doi: 10.1152/jn.01106.2005. [DOI] [PubMed] [Google Scholar]
- 29.Buhusi CV, Meck WH. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 30.Engel AK, Fries P, Singer W. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 31.Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. Eur J Neurosci. 2003;17:1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- 32.Gray CM. J Comput Neurosci. 1994;1:11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- 33.Huxter J, Burgess N, O'Keefe J. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Annu Rev Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- 35.Mauk MD, Buonomano DV. Annu Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 36.Nerad L, Bilkey DK. J Neurophysiol. 2005;93:1246–1254. doi: 10.1152/jn.00199.2004. [DOI] [PubMed] [Google Scholar]
- 37.Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Seelig D, Aschenbrenner-Scheibe R, Kahana MJ. Proc Natl Acad Sci USA. 2003;100:7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senkowski D, Talsma D, Grigutsch M, Herrmann CS, Woldorff MG. Neuropsychologia. 2006;45:561–571. doi: 10.1016/j.neuropsychologia.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Moore RA, Gale A, Morris PH, Forrester D. Int J Psychophsyiol. 2005;60:260–273. doi: 10.1016/j.ijpsycho.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- 41.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 42.Barnes T, Kubota Y, Hu D, Jin DZ, Graybiel AM. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.