Abstract
A key factor in the use of assisted reproductive technologies (ART) for diverse species is the safety of procedures for long-term health. By using a mouse model, we have investigated the effect of in vitro culture and embryo transfer (ET) of superovulated embryos on postnatal growth and physiological activity compared with that of embryos developing in vivo. Embryo culture from two-cell to blastocyst stages in T6 medium either with or without a protein source reduced blastocyst trophectoderm and inner cell mass cell number compared with that of embryos developing in vivo. Embryo culture and ET had minimal effects on postnatal growth when compared with in vivo development with an equivalent litter size. However, embryo culture, and to a lesser extent ET, led to an enhanced systolic blood pressure at 21 weeks compared with in vivo development independent of litter size, maternal origin, or body weight. Moreover, activity of enzymatic regulators of cardiovascular and metabolic physiology, namely, serum angiotensin-converting enzyme and the gluconeogenesis controller, hepatic phosphoenolpyruvate carboxykinase, were significantly elevated in response to embryo culture and/or ET in female offspring at 27 weeks, independent of maternal factors and postnatal growth. These animal data indicate that postnatal physiological criteria important in cardiovascular and metabolic health may be more sensitive to routine ART procedures than growth.
Keywords: assisted reproductive technologies, in vitro culture
There is growing evidence that the preimplantation mammalian embryo is sensitive to environmental conditions that may influence not only immediate events of blastocyst morphogenesis but also fetal or postnatal phenotype (1). Culture conditions have been shown to slow the rate of blastocyst formation, reduce proliferation rate, and alter embryo gene expression pattern and metabolic activity compared with in vivo development (1–3). The presence of supplements, particularly ill-defined serum proteins, in mouse embryo culture medium tend to reduce fetal growth after transfer (4), whereas addition of select amino acids, growth factors, or cytokines may stimulate blastocyst development and postimplantation growth (5–7). In vitro culture conditions have also been shown to perturb mouse postnatal behavior, but effects on postnatal growth have been inconsistent (8, 9). Similarly, culture and transfer of sheep and cattle embryos, especially after exposure to serum components, has been associated with the “large offspring syndrome” condition of enhanced fetal growth, large birth weight, and a higher incidence of perinatal mortality (10). Use of animal models has further demonstrated that associated reproductive biotechnologies including ovarian hyperstimulation (11) and oocyte in vitro maturation (12) can independently contribute to abnormal fetal development. In the human, in vitro culture during assisted reproduction treatment (ART) may lead to an increase in preterm delivery, low birth weight, and perinatal mortality in singleton pregnancies compared with natural conception (13–15). Culture of human embryos to the blastocyst stage is widely practiced in ART to identify viable embryos (16). In addition, associated superovulation protocols may have adverse effects on clinical outcome (17).
The mechanistic basis of embryo sensitivity to in vitro culture likely derives from a complex of interactive processes comprising epigenetic, metabolic, and cellular components (1). Although the clinical implications of embryo culture-derived changes in fetal or postnatal phenotype are clear, embryo sensitivity is also apparent with respect to in vivo environment where links to postnatal cardiovascular and metabolic physiology have been identified (1). Thus, maternal protein undernutrition in the rat exclusively during the preimplantation period leads to altered growth and elevated systolic blood pressure (SBP) in offspring, together with altered expression of metabolic regulatory enzymes in fetal liver (18, 19). Similarly, periconceptional maternal undernutrition in the sheep has been shown to lead to perturbation in fetal cardiovascular and metabolic development (20, 21). These in vivo links between embryo environment and adult health provide support for the “developmental origins of adult disease” hypothesis (22, 23).
In the present study, we further investigate the sensitivity of routine ART procedures (embryo culture to blastocyst after ovarian hyperstimulation, embryo transfer) on mouse postnatal phenotype. Here, we include physiological parameters shown to be at risk in the in vivo dietary models referred to above. In addition to SBP, we also investigated the activity of (i) serum angiotensin-converting enzyme (ACE), which promotes cleavage of angiotensin I to angiotensin II, a potent vasoconstrictor and regulator of blood pressure elevation (24, 25), and (ii) hepatic phosphoenolpyruvate carboxykinase (PEPCK), the rate-limiting enzyme in gluconeogenesis, an enzyme recognized to be overexpressed in insulin resistance and diabetes (19, 26, 27). Both ACE and PEPCK have been shown to be useful markers of cardiovascular and metabolic health in in vivo models of embryo environmental programming (19, 24). We found that embryo culture reduced blastocyst cell number and, after transfer and independent of litter size, lead to minimal changes in postnatal growth. However, more marked changes in adult physiology including onset of hypertension were observed. Moreover, in females, physiological change in response to blastocyst culture and/or transfer was associated with increased ACE and PEPCK activity.
Results
In Vitro Culture Reduces Blastocyst Cell Number.
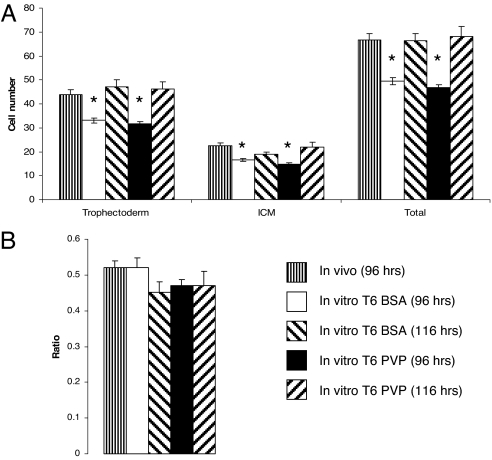
The effect of embryo culture in T6 medium from two-cell [48 h post-human chorionic gonadotrophin (hCG)] to early (96 h post-hCG) and expanded blastocyst (116 h post-hCG) stages on cell proliferation was compared with embryos developing in vivo (96 h post-hCG) (Fig. 1). Embryos that developed in vivo had significantly more blastocyst trophectoderm (TE) and inner cell mass (ICM) cells compared with cultured embryos, whether cultured in the presence (T6 BSA) or absence [T6 polyvinylpyrrolidone (PVP)] of exogenous protein, at 96 h post-hCG. If culture in T6 BSA or T6 PVP was continued for a further 20 h, to 116 h post-hCG, embryo cell numbers became comparable with those within in vivo-derived embryos at 96 h post-hCG. However, the ICM:TE cell number ratio did not differ between any treatment group (Fig. 1B).
Fig. 1.
Embryos developing in culture have fewer cells than those developing in vivo. (A) Mean (±SEM) blastocyst cell numbers at 96 and 116 h post-hCG after culture in T6 medium with or without protein (T6 BSA; T6 PVP) compared with blastocysts developing in vivo at 96 h post-hCG (n = 13–39 per treatment; ∗, P < 0.05 compared with in vivo group). (B) Mean (±SEM) ICM/TE ratio of blastocysts in A.
Embryo Environment Influences Postnatal Phenotype.
To investigate the effect of embryo environment on postnatal phenotype, we generated the following four animal treatment groups: (i) IVC-ET: in vitro-cultured embryos (T6 BSA; two-cell to early blastocyst, derived from superovulated mothers) transferred to recipients; (ii) IV-ET: in vivo-derived blastocysts (from superovulated mothers) immediately collected and transferred to recipients; (iii) NM: naturally mated mice with no embryo culture or transfer; and (iv) NM-6: NM mice with litter size reduced to six at birth (equivalent to that of IVC-ET and IV-ET mice). This design allowed us to assess postnatal phenotype and control for effects derived from embryo treatment and the ET procedure as well as maternal factors including litter size.
Litter Size and Growth Rates.
Six to 10 litters were generated for each treatment group with mean litter size, gestation length, gender ratio, and birth weight criteria as shown in Table 1. Litter sizes in the ET groups, IVC-ET and IV-ET, were not different from each other and represented 50 and 44% development to term rates per embryo transferred, respectively. Both IVC-ET and IV-ET litter sizes were significantly lower than those at birth in the two NM groups, whereas gestation length, the male:female offspring ratio, and male and female birth weights were not significantly different between treatment groups (Table 1).
Table 1.
Litter size and birth criteria (±SEM) of offspring for different treatment groups
| Group | Litter number | Gestation length, days | Mean litter size at birth | Offspring number | Male/female ratio | Mean birth weight, g |
|
|---|---|---|---|---|---|---|---|
| Males | Females | ||||||
| IVC-ET | 10 | 19 | 5.20 ± 0.42A | 52 | 0.733 | 1.648 ± 0.031 | 1.754 ± 0.048 |
| IV-ET | 8 | 19 | 6.00 ± 0.42A | 48 | 1.285 | 1.713 ± 0.032 | 1.669 ± 0.040 |
| NM6 | 8 | 19 | 11.25 ± 0.53B | 90 | 1.196 | 1.607 ± 0.021 | 1.548 ± 0.031 |
| NM | 6 | 19 | 10.67 ± 0.56B | 64 | 1.216 | 1.52 ± 0.028 | 1.522 ± 0.023 |
Different letter denotes P < 0.05.
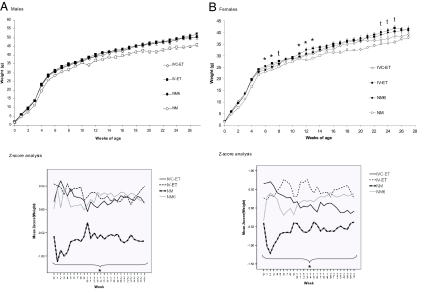
Mean weekly weights for male and female offspring for all four treatment groups from birth to 27 weeks were analyzed (Fig. 2). All mice underwent an enhanced growth spurt between 3 and 5 weeks of age when the average weekly weight increase was 2–2.5 times that occurring in previous and subsequent weeks. After 7 weeks, the weekly weight increase began to plateau so that by 11 weeks, weight increase was ≈1 g/week (Fig. 2). Assessment of overall growth rates by using Z-score analysis, where the entire data set was transformed, so giving a mean value of 0 and standard deviation of 1, revealed that the entire slopes were comparable for male and female IVC-ET, IV-ET, and NM-6 mice and significantly different from male and female NM mice, which remained smaller throughout the analysis period (Fig. 2), reflecting the difference in postnatal litter sizes between groups and identified as a significant dependent variable in the analysis. In specific weeks, however, female IVC-ET offspring were significantly reduced in weight compared with IV-ET females (weeks 6, 7, 11, 12, and 13) with this difference being at trend level (P < 0.1) at weeks 8 and 23–25 (Fig. 2B). These weight differences were independent of litter size and maternal origin, except week 6 where litter size was a significant dependent variable.
Fig. 2.
Embryo culture and transfer treatments have minimal effect on postnatal growth. Mean (±SEM) body mass and Z-score for male (A) and female (B) offspring from 1 to 27 weeks (n = 20–33 males, 20–31 females from 6–10 litters). In B, ∗ (P < 0.05) and “t” (trend; P < 0.1) denote differences between IVC-ET and IV-ET data. In Z-score plots, ∗ denotes that the entire slope is significantly different (P < 0.05) between NM and other treatment groups.
SBP.
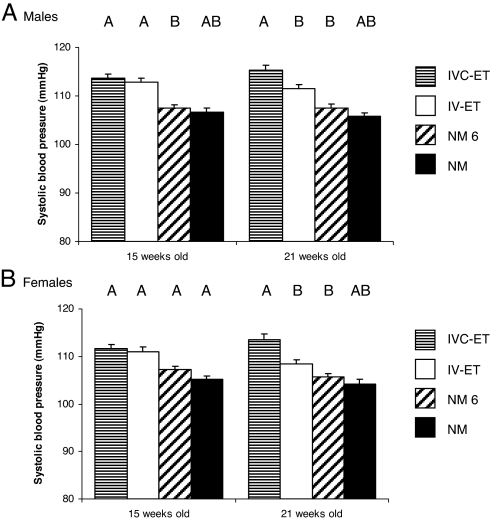
Mean SBP of offspring from the four treatment groups was determined at 15 and 21 weeks (Fig. 3). For both times, in both male and female samples, the highest mean SBP was within IVC-ET mice with descending values in the order IV-ET, NM-6, and NM mice. In both sexes, no difference in SBP was observed between IVC-ET and IV-ET offspring at 15 weeks but IVC-ET values were significantly higher than IV-ET values at 21 weeks, independent of litter size, maternal origin, and body weight. Moreover, IVC-ET offspring had significantly higher SBP than NM-6 mice at both time points (except for females at 15 weeks where difference is at trend level). Although SBP in IVC-ET offspring was also significantly higher than in NM offspring if litter size is not taken into account, this factor, when included, is a significant dependent variable, resulting in loss of independent SBP differences between these groups (Fig. 3). No differences in SBP were evident between NM and NM-6 groups.
Fig. 3.
Embryo culture and transfer treatments induce elevation in postnatal SBP. Mean (±SEM) SBP of male (n = 20–31 per treatment) (A) and female (n = 19–31) (B) offspring from 6–10 litters per treatment group. Different letter denotes P ≤ 0.05 independent of maternal origin, litter size, and weight. In addition, differences at trend level (P < 0.1) exist for IVC-ET and NM (males, 21 weeks), IV-ET and NM-6 (males, 21 weeks), and IVC-ET and NM-6 (females, 15 weeks).
Organ Allometry.
Mice were culled at 27 weeks and organ weights compared between treatments either directly or as ratio to body weight (Table 2). In general, few differences were detected between treatments, reflecting the minimal effects on growth rates (see Table 2 for statistical outcomes). However, significant differences between female IVC-ET and IV-ET left and right kidney weights and lung weight were observed, independent of maternal origin and litter size. Despite this, the differences between IVC-ET and IV-ET females were not sustained when analyzed in relation to body weight. However, the IV-ET females were significantly different from the NM-6 females for both left and right kidney/body weight ratios.
Table 2.
Mean organ weight and organ/body weight ratio of male (n = 20–30 per treatment) and female (n = 20–31 per treatment) offspring
| Liver | Left kidney | Right kidney | Heart | Lung | |
|---|---|---|---|---|---|
| Male | |||||
| Organ weight, g | |||||
| IVC-ET | 2.624 ± 0.196A | 0.384 ± 0.0137 | 0.383 ± 0.0196 | 0.291 ± 0.0078 | 0.204 ± 0.0043 |
| IV-ET | 2.482 ± 0.097a | 0.373 ± 0.0088 | 0.384 ± 0.0120 | 0.236 ± 0.0074 | 0.217 ± 0.0033 |
| NM-6 | 2.627 ± 0.130A | 0.393 ± 0.0242 | 0.357 ± 0.0188 | 0.208 ± 0.0064 | 0.208 ± 0.0057 |
| NM | 2.105 ± 0.078Bb | 0.350 ± 0.0082 | 0.331 ± 0.0072 | 0.194 ± 0.0048 | 0.197 ± 0.0041 |
| Organ/body weight ratios | |||||
| IVC-ET | 0.0514 ± 0.002a | 0.0076 ± 0.0002 | 0.0076 ± 0.0003 | 0.0044 ± 0.0001 | 0.0041 ± 0.0001 |
| IV-ET | 0.0472 ± 0.001 | 0.0071 ± 0.0001 | 0.0073 ± 0.0001 | 0.0045 ± 0.0001 | 0.0041 ± 0.0001 |
| NM-6 | 0.052 ± 0.002A | 0.0078 ± 0.0004 | 0.0071 ± 0.0003 | 0.0041 ± 0.0001 | 0.0042 ± 0.0001 |
| NM | 0.0456 ± 0.001Bb | 0.0076 ± 0.0001 | 0.0072 ± 0.0004 | 0.0042 ± 0.0001 | 0.0043 ± 0.0001 |
| Female | |||||
| Organ weight, g | |||||
| IVC-ET | 1.681 ± 0.038 | 0.231 ± 0.0067A | 0.224 ± 0.0055A | 0.0164 ± 0.0051 | 0.184 ± 0.0035A |
| IV-ET | 1.675 ± 0.065 | 0.243 ± 0.0055Bb | 0.241 ± 0.0068B | 0.1670 ± 0.0053 | 0.196 ± 0.0033B |
| NM-6 | 1.821 ± 0.061 | 0.219 ± 0.0057A | 0.209 ± 0.0084A | 0.156 ± 0.0043 | 0.184 ± 0.0047A |
| NM | 1.628 ± 0.033 | 0.222 ± 0.0046 | 0.210 ± 0.0037 | 0.146 ± 0.0021 | 0.173 ± 0.0018 |
| Organ/body weight ratios | |||||
| IVC-ET | 0.0419 ± 0.0010 | 0.0058 ± 0.0002 | 0.0056 ± 0.0002 | 0.0041 ± 0.0002 | 0.0046 ± 0.0001 |
| IV-ET | 0.0412 ± 0.0017 | 0.0060 ± 0.0001A | 0.0059 ± 0.0002A | 0.0041 ± 0.0002 | 0.0048 ± 0.0001 |
| NM-6 | 0.0436 ± 0.0010 | 0.0053 ± 0.0002B | 0.0051 ± 0.0003B | 0.0038 ± 0.0001 | 0.0044 ± 0.0001 |
| NM | 0.0428 ± 0.0009 | 0.0059 ± 0.0001A | 0.0055 ± 0.0001 | 0.0039 ± 0.0001 | 0.0046 ± 0.0001 |
Different letters denote differences either at P < 0.05 (uppercase) or as trend (P < 0.1, lowercase).
ACE Activity.
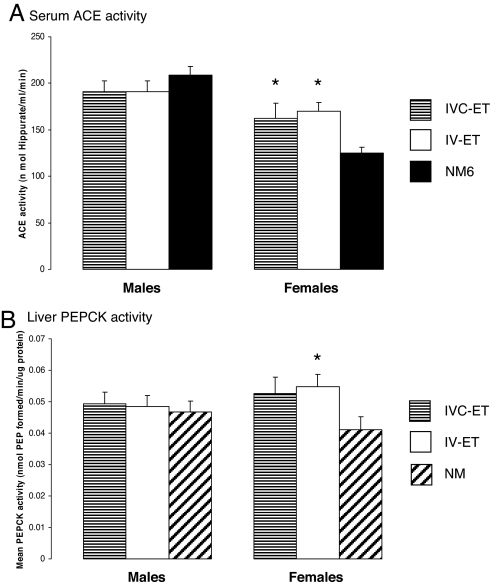
Serum ACE activity was significantly elevated at 27 weeks in female IVC-ET and IV-ET offspring compared with NM offspring, but no differences were detected in male ACE activity (Fig. 4A). The difference in female ACE activity was independent of litter size in offspring and body weight.
Fig. 4.
Embryo culture and/or transfer cause elevation in female offspring serum ACE and PEPCK activities. (A) Mean (±SEM) serum ACE activity in 16–17 male and 15–17 female offspring from 6–10 litters per treatment. (B) Mean (±SEM) liver PEPCK activity in 16–17 male and 15–18 female offspring from 8–10 litters per group. ∗, P < 0.05 compared with NM (A) or NM-6 (B) group.
PEPCK Activity.
Liver PEPCK activity at 27 weeks was assessed in IVC-ET, IV-ET, and NM-6 treatment groups. Although levels were similar within male offspring across treatments, PEPCK activity was higher in both female IVC-ET and IV-ET groups compared with NM-6 group (Fig. 4B). However, analysis taking into account both offspring litter size and body weight showed this difference from NM-6 was only significant in IVC-ET females.
Discussion
In this study, our major finding is that the culture and transfer of mouse embryos generated by ovarian hyperstimulation, routine procedures used in ART, can have lasting effects on postnatal physiology. Although culture has long been recognized to retard early development relative to in vivo environment (1, 2), and our own data using T6 medium are no exception, the breadth of postnatal consequences of the in vitro environment has not been studied extensively (1). We show that cardiovascular and metabolic changes in the adult may follow in vitro embryo manipulations. In broad terms, our data also indicate that the postnatal consequences of ART are similar to those resulting from early gestation maternal undernutrition (1, 18). Thus, abnormal early embryo environment, whether in vitro or in vivo, may have long term health consequences.
Culture of embryos from two-cell to blastocyst stages in T6 medium, either with or without a protein source, caused a reduction in blastocyst cell numbers in both TE and ICM lineages. Although several culture conditions have been shown to alter blastocyst cell number, including amino acid composition, energy substrates, growth factors, and cytokines (1, 5–7), the long-term consequences of reduced blastocyst cell numbers have not been evaluated extensively. Manipulated embryos with reduced cells have been shown to have slower fetal development possibly because of depleted stem cells for gastrulation and organogenesis (1, 28, 29). However, in the current study, depleted blastocyst cell numbers have not affected gestation length (Table 1). Moreover, quantitative measurements of the human blastocyst ICM after ART are highly indicative of blastocyst implantation potential with those displaying larger ICMs, presumably comprising more cells, more likely to implant (30). Associations between blastocyst cell number and maternal dietary treatment in vivo have also been identified with long-term consequences (18), further indicating the importance of this parameter to the developmental program.
The effect of IVC-ET treatment on postnatal growth compared with in vivo development, independent of litter size, was minimal, and limited to reduced female growth in specific weeks. The major effect on postnatal growth was mediated by litter size and evident here in the typical inverse relationship by inclusion of NM and NM-6 groups. These data are similar to those reported following mouse embryo culture in Whittens and KSOM media where no effect on postnatal growth was evident (9). In contrast, mouse embryo culture in medium containing FCS has been shown to increase female offspring weight from week 31 onward associated with increased adipose mass (8). Although our study was for a shorter postnatal duration, the physiological changes identified mainly in female offspring and discussed below might be conducive to altered growth during later life.
A major finding of our study is that preimplantation embryo culture led to an increased SBP in offspring at 21 weeks, independent of litter size, maternal origin, and body weight. Although this effect was evident in both genders, the mechanistic basis may vary between sexes. Hypertension is a multifactorial disease and its origin may be complex. We found that female, but not male, offspring from IVC-ET and IV-ET treatments exhibited elevated serum ACE activity compared with in vivo-derived controls. ACE acts to convert angiotensin I to angiotensin II, a potent vasoconstrictor following binding to angiotensin II type 1 receptors in vascular smooth muscle and elsewhere, leading to elevation of blood pressure (25, 31). Thus, one component underlying elevation in SBP in embryo-manipulated female offspring may derive from altered renin–angiotensin system. Angiotensin II has also been associated with endothelial dysfunction and heightened superoxide anion levels, which may further exacerbate hypertension (32). Moreover, ACE knockout mice are hypotensive (33). The basis for female specificity of effects on ACE activity in the current study is unknown but may, for example, reflect the inhibitory effect of estrogen on ACE expression (34).
ACE activity increase has also been observed correlating with hypertension in other models of early embryo programming, notably in response to rat maternal protein undernutrition (35) and in the mouse Ped minus phenotype (24). However, the gender specificity identified in the current study has not been observed in these models. Alteration in the renin–angiotensin system may reflect changes in renal function derived from impaired gestational nephrogenesis and a compensatory glomerular hypertrophy, a phenotype that has been recognized in the maternal dietary protein undernutrition model (35). Offspring kidneys from rat dams fed low protein diet during gestation are usually reduced in weight presumably in response to compromised nephrogenesis (35). However, where maternal dietary restriction was limited to the preimplantation period, a modest increase in offspring kidney/body weight ratio resulted, implying a compensatory growth response (18). Similarly, in the current study, kidney weight tended to be higher in female offspring only from the embryo-manipulated groups compared with NM-6, and the kidney/body weight ratio to be significantly higher in IV-ET females compared with NM-6 controls (Table 2). Collectively, these data suggest that elevated SBP in female offspring of embryo-manipulated groups may derive from combined renal and endocrine effects.
Our analysis of postnatal effects after ART treatments also included an analysis of hepatic PEPCK activity. This enzyme is recognized as a critical regulator of gluconeogenesis, and the excess hepatic glucose production characteristic of adult metabolic disease, notably type II diabetes, coincides with increased PEPCK activity (26, 27). Moreover, mice overexpressing PEPCK develop insulin resistance and diabetes (36) and expression levels of the enzyme in offspring can be set by the pattern of maternal diet during development (37). Interestingly, a maternal low protein diet administered exclusively during the preimplantation period induced elevated fetal hepatic PEPCK expression in late gestation (19). Our current studies have shown elevated mean PEPCK activity in female offspring of embryo-manipulated groups compared with in vivo developed offspring. Although this increase was only significant for IV-ET females because of other variables (litter size, body weight), such a relationship may further indicate a susceptibility of female offspring to metabolic as well as cardiovascular dysfunction.
Studies on long-term effects of ART procedures in humans have revealed evidence of higher incidence of premature birth, low birth weight, imprinting disorders, and some childhood cancers (13–15). However, effects are inconsistent, largely reflecting variation in study design, human populations, and confounding variables (14). We have identified in a mouse model that susceptibility to adult disease factors may also be increased, notably in cardiovascular function. Our data also indicate that the embryo transfer procedure itself (where embryo culture is reduced to a minimal period) may be contributory to programming postnatal phenotype for some of our outcomes, confirming the environmental sensitivity of the blastocyst stage. In addition, the superovulated condition of embryos in IVC-ET and IV-ET groups may contribute to adverse outcomes, although this is unlikely for the induction of hypertension.
Although the direct mechanisms by which the postnatal effects identified are induced in response to ART procedures are currently unknown, embryos have been shown to respond to diverse environmental stresses by epigenetic, metabolic, cellular, and physiological changes, all of which have potential for altering the developmental program (1). To our knowledge, cardiovascular effects in response to ART treatments have not been explored previously in the human, partly reflecting the relatively young age of progeny. However, our data using an animal model cannot be extrapolated directly to human ART; although the effects identified here may be important to rodents, they could have little relevance to humans. Rather, as has been previously proposed (38), we consider animal models as useful in identifying potential outcomes from ART that merit separate investigation across species.
Materials and Methods
Animals and Embryo Culture Treatments.
Animal treatments throughout this study were conducted in compliance with protocols under an appropriate United Kingdom Home Office project license, approved by local ethics committee. Virgin female (CBA × C57/BL6)F1 mice (5–6 weeks old; Biomedical Research Facility, University of Southampton) were superovulated by i.p. injection of 5 IU of pregnant mare serum gonadotrophin (PMS; Intervet, Cambridge, U.K.) and, 46 h later, 5 IU of hCG (Intervet), and caged overnight with MF1 males. The presence of a vaginal plug the following morning was taken as a sign of mating. Mice were killed by cervical dislocation and embryos were collected by flushing dissected oviducts or uteri with prewarmed H6 medium supplemented with either 4 mg/ml BSA (Sigma, St. Louis, MO; embryo culture tested; H6 BSA) or 6 mg/mL PVP (Sigma, Poole, U.K.; embryo culture tested; H6 PVP). Embryos were cultured in T6 medium (39) supplemented with either BSA (T6 BSA) or PVP (T6 PVP) under mineral oil (Sigma; embryo culture tested) at 37°C and 5% CO2 in air for up to 116 h post-hCG. Blastocyst TE and ICM cell numbers were determined by differential nuclear staining as described (40) with modifications (18).
Embryo Transfer.
Embryos cultured as above in T6 BSA from the two-cell stage (48 h post-hCG) to the blastocyst stage (96 h post-hCG), or blastocysts immediately collected from superovulated dams at this time, were placed in H6 BSA at 37°C under mineral oil for up to 3 h before ET to uteri of pseudopregnant 5- to 6-week-old virgin female (CBA × C57/BL6)F1 mice, previously mated (without superovulation) with vasectomized males. Mating times for embryo production and pseudopregnancy were identical to ensure appropriate maternal staging at ET. Standard procedures were used for ET at day 3.5 with i.p. injection of ketamine (Fort Dodge, Fort Dodge, IA) and acepromazine (C-Vet; Veterinary Products, Leyland, U.K.) anesthetic and transfer of six blastocysts to each exposed uterine horn before suture and i.p. injection of 100 μl of analgesic (Temgesic; 300 μg/ml; Reckitt & Colman Products, Wayne, NJ).
Animal Treatment Groups.
In addition to offspring derived from ET of blastocysts either cultured in vitro (termed IVC-ET mice) or developed in vivo (termed IV-ET mice), offspring from two further animal groups were generated comprising natural mating without superovulation in which litter size was either unregulated (termed NM mice) or regulated at birth to the mean litter number within IVC-ET and IV-ET mice of six (three males and three females; termed NM6 mice). Thus, controls for effects derived from ET procedure and litter size were included in the design. Of the total offspring born and after reducing NM-6 litter size (Table 1), 9 of 214 (4.2%) mice died across groups during 27 weeks of analysis to give n values as indicated in the figure legends.
Postnatal Growth.
All offspring were weaned at 3 weeks with males and females caged separately per litter with access to standard chow and water ad libitum. Offspring were weighed on the day of birth and subsequently on the same day each week for 27 weeks.
SBP.
SBP was determined at 15 and 21 weeks by tail-cuff plethysmography by using an ITC model 229 blood pressure monitor (Linton Instruments, Norfolk, U.K.). Mice were allowed to acclimatize to a room temperature of 27–30°C for at least 90 min before readings were taken, and to the tail cuff apparatus for several minutes before use. Four SBP recordings were taken per mouse at each age studied. If after 20 min all four recordings had not been taken, the mouse was released and allowed to recover for 30 min before proceeding. Heart rate was monitored as an indicator of stress, and if >500 beats per minute, readings were stopped until below this value (24).
Organ Allometry and Blood Sample Collection.
At 27 weeks of age, after cervical dislocation, a blood sample was removed via heart puncture and allowed to clot on ice. Liver, left and right kidneys, heart, and lungs were dissected out, weighed, snap-frozen in liquid nitrogen, and stored at −80°C. Blood samples were centrifuged at 10,000 × g, at 4°C for 10 min, after which serum was aliquoted and stored at −80°C.
ACE Activity.
For serum ACE activity, the method described (41) was used with modifications (24). Briefly, the method comprises serum incubation in hippuryl-l-histidine-l-leucine acetate salt (HHLA; Sigma) solution in phosphate buffer at 37°C followed by addition of cyanuric chloride (Sigma) in 1,4-dioxane (Sigma) for yellow coloration to develop. Four replicates per sample were analyzed on a Dynatech (Chantilly, VA) MR5000 plate reader at 380 nm. Samples containing only serum and chloride buffer were used as negative controls. For each analysis, a standard curve over the range 1 nM to 50 μM was prepared from a 1 mM hippuric acid (Sigma) solution in 100 mM chloride buffer, pH 8.3, and treated exactly as the incubates. Serum ACE activity was expressed as nanomoles of hippurate formed per milliliter of serum per minute; 15–17 samples across litters were randomly selected from IVC-ET, IV-ET, and NM frozen stored sera at 27 weeks.
PEPCK Activity.
Adult liver (100 mg) was homogenized in 300 μl of 50 mM Hepes, pH 7.4, and centrifuged at 1,000 × g for 10 min, and supernatant was centrifuged at 25,000 × g for 10 min, followed by 100,000 × g for 45 min, all at 4°C before storage at −80°C. After protein determination by Bradford assay (Bio-Rad, Hercules, CA), PEPCK activity in supernatant fractions was measured in the forward direction (phosphoenolpyruvate formation) essentially as described (42) and expressed as nanomoles of phosphoenolpyruvate formed per minute per microgram of protein; 15–18 samples across litters were randomly selected from IVC-ET, IV-ET, and NM-6 frozen stored livers at 27 weeks.
Statistical Analysis.
A Bonferroni one-way analysis of variance t test was used to analyze cell numbers within blastocysts (SigmaStat statistical software, version 2.0). All postnatal data (birth weights, growth weights, SBP, organ weights and ratios, serum ACE, and PEPCK activity) were analyzed by using a multilevel random-effects regression model (SPSS, version 14; SPSS, Chicago, IL), which takes into account the hierarchical nature of the data sets with between-mother and within-mother variation and different parameters measured from individual animals (24, 43). Thus, differences identified between treatment groups throughout the study are independent of maternal origin of litter, litter size, and, for nongrowth data, also of body weight unless otherwise stated. Growth data were additionally converted to Z-scores by using SPSS before random-effects regression analysis. The Z-score transformation used the entire body weight data set to standardize the weight to the same scale, with a mean of 0 and a standard deviation of 1. It allows for the analysis of entire growth curves in relation to other treatment groups, avoiding the use of multiple comparisons from weeks 0 to 27.
Acknowledgments
This work was supported by Medical Research Council Grant G9800781 (to T.P.F.) and a studentship award by the Schools of Medicine and Biological Sciences (University of Southampton) (to A.J.W.). M.H. is supported by the British Heart Foundation.
Abbreviations
- ACE
angiotensin-converting enzyme
- ART
assisted reproductive technologies
- ET
embryo transfer
- hCG
human chorionic gonadotrophin
- ICM
inner cell mass
- IV
in vivo
- IVC
IV culture
- NM
naturally mated
- PEPCK
phosphoenolpyruvate carboxykinase
- PVP
polyvinylpyrrolidone
- SBP
systolic blood pressure
- TE
trophectoderm.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. Biol Reprod. 2004;71:1046–1054. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- 2.Harlow GM, Quinn P. Aust J Biol Sci. 1982;35:187–193. doi: 10.1071/bi9820187. [DOI] [PubMed] [Google Scholar]
- 3.Rinaudo P, Schultz RM. Reproduction. 2004;128:301–311. doi: 10.1530/rep.1.00297. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Dean W, Brown D, Reik W, Feil R. Biol Reprod. 2001;64:918–926. doi: 10.1095/biolreprod64.3.918. [DOI] [PubMed] [Google Scholar]
- 5.Lane M, Gardner DK. J Reprod Fertil. 1997;109:153–164. doi: 10.1530/jrf.0.1090153. [DOI] [PubMed] [Google Scholar]
- 6.Kaye PL, Gardner HG. Hum Reprod. 1999;14:3052–3059. doi: 10.1093/humrep/14.12.3052. [DOI] [PubMed] [Google Scholar]
- 7.Sjoblom C, Roberts CT, Wikland M, Robertson SA. Endocrinology. 2005;146:2142–2153. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez De Fonseca F, Pintado B, Gutierrez-Adan A. Proc Natl Acad Sci USA. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Proc Natl Acad Sci USA. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEvoy TG, Sinclair KD, Young LE, Wilmut I, Robinson JJ. Hum Fertil (Camb) 2000;3:238–246. doi: 10.1080/1464727002000199061. [DOI] [PubMed] [Google Scholar]
- 11.Van der Auwera I, D'Hooghe T. Hum Reprod. 2001;16:1237–1243. doi: 10.1093/humrep/16.6.1237. [DOI] [PubMed] [Google Scholar]
- 12.Bertolini M, Mason JB, Beam SW, Carneiro GF, Sween ML, Kominek DJ, Moyer AL, Famula TR, Sainz RD, Anderson GB. Theriogenology. 2002;58:973–994. doi: 10.1016/s0093-691x(02)00935-4. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M, Kurinczuk JJ, Bower C, Webb S. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 14.Olivennes F, Fanchin R, Ledee N, Righini C, Kadoch IJ, Frydman R. Hum Reprod Update. 2002;8:117–128. doi: 10.1093/humupd/8.2.117. [DOI] [PubMed] [Google Scholar]
- 15.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Fertil Steril. 2004;81:551–555. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Devroey P, Bourgain C, Macklon NS, Fauser BC. Trends Endocrinol Metab. 2004;15:84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Development (Cambridge, UK) 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 19.Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, Fleming TP. Mol Reprod Dev. 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- 20.Edwards LJ, McMillen IC. Am J Physiol Regul Integr Comp Physiol. 2002;283:R669–R679. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- 21.Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker DJP. Mothers, Babies and Health in Later Life. 2nd Ed. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- 23.Gluckman PD, Hanson MA. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 24.Watkins A, Wilkins A, Osmond C, Warner CM, Comiskey M, Hanson M, Fleming TP. J Physiol. 2006;571:211–220. doi: 10.1113/jphysiol.2005.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skeggs LT, Jr, Kahn JR, Shumway NP. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consoli A, Nurjhan N, Capani F, Gerich J. Diabetes. 1989;38:550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- 27.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam PP. Teratology. 1988;37:205–212. doi: 10.1002/tera.1420370305. [DOI] [PubMed] [Google Scholar]
- 29.Power MA, Tam PP. Anat Embryol. 1993;187:493–504. doi: 10.1007/BF00174425. [DOI] [PubMed] [Google Scholar]
- 30.Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Fertil Steril. 2001;76:1157–1167. doi: 10.1016/s0015-0282(01)02870-9. [DOI] [PubMed] [Google Scholar]
- 31.Unger T. Am J Cardiol Suppl. 2002;89(2):3–9. [Google Scholar]
- 32.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Hypertension. 1999;33:323–328. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Langley-Evans SC, Langley-Evans AJ, Marchand MC. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- 36.Valera A, Pujol A, Pelegrin M, Bosch F. Proc Natl Acad Sci USA. 1994;91:9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai M, Byrne CD, Zhang J, Petry CJ, Lucas A, Hales CN. Am J Physiol. 1997;272:G1083–G1090. doi: 10.1152/ajpgi.1997.272.5.G1083. [DOI] [PubMed] [Google Scholar]
- 38.Leese HJ, Donnay I, Thompson JG. Hum Reprod. 1998;13(Suppl 4):184–202. doi: 10.1093/humrep/13.suppl_4.184. [DOI] [PubMed] [Google Scholar]
- 39.Quinn P, Barros C, Whittingham DG. J Reprod Fertil. 1982;66:161–168. doi: 10.1530/jrf.0.0660161. [DOI] [PubMed] [Google Scholar]
- 40.Handyside AH, Hunter S. J Exp Zool. 1984;231:429–434. doi: 10.1002/jez.1402310317. [DOI] [PubMed] [Google Scholar]
- 41.Raimbach SJ, Thomas AL. J Physiol. 1990;423:441–451. doi: 10.1113/jphysiol.1990.sp018032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conjard A, Brun V, Martin M, Baverel G, Ferrier B. Biochem J. 2002;368:301–308. doi: 10.1042/BJ20020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwong WY, Osmond C, Fleming TP. Reprod Biomed Online. 2004;8:574–576. doi: 10.1016/s1472-6483(10)61105-4. [DOI] [PubMed] [Google Scholar]