Plants have never worried about compliance with the Kyoto Protocol.† For millennia, they have released large amounts of gaseous compounds, such as carbon dioxide, oxygen, water vapor, and ethylene, from their foliage into the lower atmosphere. Thanks to improvements in headspace sampling techniques and mass spectrometry in the last 20 years, the list of plant volatiles has greatly expanded and now includes methanol, acetone, formaldehyde, and other short-chain carbonyl compounds, plus a host of terpenes, phenylpropanoids, benzenoids, and fatty-acid derivatives (1, 2). Are plants just “passing gas” by emitting compounds that are by-products of essential processes, or do the released substances have any real function in their lives? A major breakthrough in answering this question occurred in the early 1990s when it was discovered that the emission of plant volatiles could be triggered by herbivore feeding. The emitted compounds were shown to act as a botanical cry for help, attracting predatory and parasitic species that attack herbivores (3, 4). Herbivore-induced plant volatiles have also been demonstrated to have other ecological roles, for example, in direct deterrence of herbivores (5, 6) or in plant-to-plant communication. However, surprisingly little attention has been paid to the possible internal roles of these substances in the life of the plant until the work of Heil and Bueno in this issue of PNAS (7).
For the last few years, Martin Heil and his coworker Christian Kost have carried out a fascinating series of investigations on the antiherbivore defenses of wild lima bean plants growing along the cost of Oaxaca, Mexico (8–11). Feeding by beetles and other insects induces lima bean to release volatiles and secrete nectar from sites on the leaves (extrafloral nectaries) that attracts ants, wasps, and flies, which are herbivore enemies. By recruiting such defenders to its cause, lima bean can reduce herbivore feeding and increase its growth rate.
Previously, Heil and Kost (11) showed that volatiles from herbivore-damaged lima bean plants trigger extrafloral nectar production in undamaged plants. In the present work, Heil and Bueno (7) studied whether volatiles released from one part of a plant have any impact on other parts of the same plant. Employing a deceptively simple experimental design, they induced volatile emission from two leaves of a lima bean shoot by mechanical damage and application of jasmonic acid and then wound a second shoot from the same plant around the induced one. After 1 day, leaves of both shoots were found to produce more extrafloral nectar on their undamaged leaves than on leaves of untreated shoots. However, when the damaged leaves were enclosed in plastic bags, the effect completely vanished. Hence, increased nectar production seemed to be triggered by an airborne signal emanating from the damaged leaves rather than by one moving through the plant.
These results were corroborated by using lima bean plants transplanted from the field to a greenhouse to exclude the effects of root signaling and possible interference from volatiles of other plants. In this experiment, two leaves on a stem were damaged by beetle feeding or by the mechanical wounding–jasmonic acid treatment. Undamaged leaves on the same stem showed an increase in extrafloral nectar secretion when they were placed near the damaged leaves or when air from the damaged leaves was blown over them but not when air from the damaged leaves was blown away from them. If an internal plant signal were involved in triggering this response, one would have expected the undamaged leaves to be consistently induced because they were immediately adjacent to the damaged leaves. The fact that no increase in extrafloral nectar was observed in undamaged leaves without contact to the air passing over the damaged leaves highlights the critical role of the volatiles in this intraplant communication network.
From a broader perspective, there should be little surprise that one part of a plant releases volatile signals that impact another part. The action of ethylene as a gaseous plant hormone controlling various aspects of physiology and development has been appreciated for >70 years (12). However, herbivore-induced volatiles have usually been assumed to serve as an external signal to other organisms rather than an internal signal meant for private consumption. Moreover, after the classic studies of Clarence Ryan and coworkers (13) showing how wounding triggers an increase in defenses in distant leaves, herbivore-induced signaling pathways in plants have usually been assumed to involve vascular transmission.
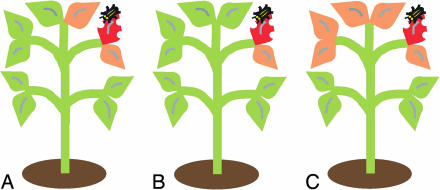
Airborne signaling may provide a much more rapid and precise way of passing information on to neighboring leaves than via the vascular system. Because of peculiarities of vascular architecture in plants, adjacent leaves are sometimes not directly connected to each other (14). In large plants, such as trees, shrubs, and vines (including the lima bean), stems from separate branches often grow together so that foliage in close proximity has only remote vascular links. Under these conditions, volatile signals should allow individual leaves to alert their neighbors about the presence of herbivores much more swiftly and accurately than might be achieved by vascular transmission (14) (Fig. 1).
Fig. 1.
Sounding the alarm. Initial herbivore damage is a good indicator of further attack. By mobilizing defensive compounds or attracting herbivore enemies, plants can increase their resistance to herbivores. However, these measures are likely to be costly, so only foliage that is likely to be attacked should be well defended. The experiments of Heil and Bueno (7) demonstrate that plants employ volatiles released from attacked leaves to induce defenses in directly adjacent leaves. Volatile-mediated signaling (A) may sound the alarm more rapidly and efficiently than signals transmitted by short-range (B) or long-range (C) vascular connections. Attacked leaves are depicted in a dark orange color and induced leaves in light orange.
The remarkable results of Heil and Bueno (7) are likely to model plant behavior faithfully under natural conditions. Unlike other experiments of this type, volatile emission was induced with insect damage rather than solely by artificial treatments, and the experiments used natural volatile blends rather than synthetic mixtures of varying composition and concentration. In addition, the lima beans studied were wild plants that had not been subjected to artificial selection.
Volatile compounds acting as intraplant signals could simultaneously function as signals to other organisms. The findings presented by Heil and Bueno (7) do not preclude such a possibility and even lend it some support. However, the internal roles probably came first in an evolutionary context. One can easily imagine that volatiles released as intraplant messages could also be perceived by other organisms in the vicinity, which could use them to their advantage. Herbivore enemies, for example, could have come to associate damage-induced volatiles with the presence of certain herbivores and to employ these compounds as attractants. Other groups of organisms that could “eavesdrop” on internal plant signals include herbivores, pathogens, and even neighboring plants. Airborne communication among neighboring plants has been a controversial topic for many years, but there are now multiple examples in the literature where plants respond to aerial cues put out by neighboring plants under herbivore attack by eliciting their own direct and indirect defenses (15–17). Lima bean can now be added to this list. In the present work, Heil and Bueno describe an experiment in which a stem of beetle-damaged leaves from one plant could stimulate increased volatile formation and extrafloral nectar production in another plant when placed in close proximity.
Green-leaf volatiles and terpenes are good candidates for testing.
These contributions aside, Heil and Bueno's major scientific achievement lies in having established the existence of intraplant signaling mediated by herbivore-induced volatiles. This milestone should stimulate various lines of further investigation. For example, insect feeding on lima bean induces the emission of whole range of substances, including green-leaf volatiles, monoterpenes, sesquiterpenes, and long-chain aldehydes. Determining which compound (or compounds) is the actual intraplant signal is a high priority. Both the green-leaf volatiles and terpenes are good candidates for testing, given their activities in laboratory and field studies on interplant communication (11, 18, 19). It would also be valuable to follow the route of intraplant volatiles from synthesis through release, reuptake, and receptor binding to determine the component proteins or genes of this signaling pathway and how they are regulated. Another important issue is to determine the costs and benefits of such intraplant communication by manipulating volatile production in field studies. How the induction of volatiles and extrafloral nectaries affect plant fitness may vary dramatically depending on the intensity of herbivore pressure. Finally, the work should inspire a search for other examples of volatile, intraplant signals that may have been overlooked previously. For example, there is some intriguing preliminary evidence that methyl salicylate, a frequently reported component of herbivore-induced volatile blends, acts in such a role (20).
Footnotes
The authors declares no conflict of interest.
See companion article on page 5467.
†The Kyoto Protocol is an international agreement on climate change that mandates countries to reduce their emission of carbon dioxide and other greenhouse gases.
References
- 1.Dudareva N, Pichersky E, Gershenzon J. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichersky E, Gershenzon J. Curr Opin Plant Biol. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 3.Dicke M, van Beek TA, Posthumus MA, Ben Dom N, Van Bockhoven H, De Groot AE. J Chem Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- 4.Turlings TCJ, Tumlinson JH, Lewis WJ. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 5.De Moraes CM, Mescher MC, Tumlinson JH. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 6.Kessler A, Baldwin IT. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 7.Heil M, Bueno JCS. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heil M. J Ecol. 2004;92:527–536. [Google Scholar]
- 9.Heil M, Kost C. Ecol Lett. 2006;9:813–817. doi: 10.1111/j.1461-0248.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 10.Kost C, Heil M. Basic Appl Ecol. 2005;6:237–248. [Google Scholar]
- 11.Kost C, Heil M. J Ecol. 2006;94:619–628. [Google Scholar]
- 12.Bleeker AB, Kende H. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Narváez-Vásquez J, Ryan CA. Planta. 2004;218:360–369. doi: 10.1007/s00425-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 14.Orians C. J Chem Ecol. 2005;31:2231–2242. doi: 10.1007/s10886-005-7099-7. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 16.Dicke M, Bruin J. Biochem Syst Ecol. 2001;29:981–994. [Google Scholar]
- 17.Farmer EE. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 18.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 19.Arimura G, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J. Biochem Syst Ecol. 2001;29:1049–1061. [Google Scholar]
- 20.Shulaev V, Silverman P, Raskin I. Nature. 1997;385:718–721. [Google Scholar]