Abstract
Disease may play a critical role in invasions by nonnative plants and animals that currently threaten global biodiversity. For example, a generalist viral pathogen has been recently implicated in one of the most extensive plant invasions worldwide, the invasion and domination of California's perennial grasslands by exotic annual grasses. To date, disease has never been quantitatively assessed as a cause of this invasion. Using a model with field-estimated parameters, we demonstrate that pathogen presence was necessary to reverse competitive outcome and to allow exotic annual grass invasion and dominance. Although pathogen-induced reversal of a competitive hierarchy has been suggested as a mechanism of species invasion, here we quantitatively demonstrate the importance of this phenomenon by using field-derived parameters in a dynamical model. Pathogen-mediated reversals in competitive balance may be critically important for understanding past, and predicting future, invasions.
Keywords: barley yellow dwarf virus, California grassland, disease ecology, integrodifference model, invasion
Invasion by exotic plant species is a critical current threat to global biodiversity, ecosystem function, and human economies (1, 2). Cascading plant compositional alterations have been documented after plant invasions (3); in the U.S., for example, 57% of imperiled plant species are threatened by invasive plant species (4). In Mediterranean climates around the world, perennial grasslands have undergone invasion by exotic annual grasses (5, 6). For example, one of the most dramatic plant invasions worldwide is the invasion and domination of the native perennial grasslands in California by exotic annual grasses. Since European settlement, this grassland has experienced one of the most broad-scale ecosystem invasions in the world (7, 8), in which >9 million hectares of native perennial grassland are now dominated by exotic annual grasses and forbs from the European Mediterranean region (8, 9).
Paradoxically, recent empirical work demonstrates that the invasive annual grasses in California are inferior competitors for resources shared with the native perennials (10, 11), supporting earlier theoretical insights into annual–perennial competition (12). Thus, mechanisms other than exploitative competition were necessary to drive the invasion of this system by competitively inferior annual grasses. Although several mechanisms have been suggested to explain the apparent enigma in which an inferior competitor has invaded and remained numerically dominant across much of California for more than a century (e.g., grazing, rainfall changes, and perennial seed limitation; see refs. 10 and 13), disease has only recently been suggested as a potential factor in this invasion (14). Although it has been shown that invasion by annual grasses can increase the prevalence of a viral pathogen (14), there have been no quantitative tests to determine whether these effects could alter the long-term competitive dominance hierarchy of native and exotic grasses in California. Here, we develop a dynamical model that uses field-based parameter estimates to examine whether the invasion by nonnative annual grasses may have altered the long-term community-wide dynamics of a globally distributed generalist virus of grasses, resulting in widespread invasion and persistent domination of the California grassland flora by a suite of otherwise competitively inferior introduced grasses from southern Europe.
Along with providing insights into the invasion of California grasslands, we elucidate the result that a disease can completely reverse the competitive hierarchy in a system, thereby mediating an exotic species invasion. To date, the species invasion literature has largely focused on how the loss or gain of natural enemies (e.g., pathogens) or the introduction of novel pathogens can alter species invasions. Much of this work falls under two general hypotheses: the escape of an invasive species from pathogens present only in its native range (enemy-release hypotheses) and the effects of native pathogens on resistance to invasion (novel-weapons hypothesis) (15). In contrast, the current study quantitatively explores the possibility that an invasive species may alter the dynamics of a host–pathogen interaction, thereby allowing invasion by a species that is competitively excluded in the absence of the pathogen.
A substantial body of work exists that examines the mediation of competition between animal hosts by generalist parasites and pathogens (16–18), and work in the last decade has shown that insect-vectored pathogens also can directly and indirectly determine the structure of plant communities (19). Pathogens have long been known to alter the competitive balance in animal communities when they favor the competitively inferior species (17, 20, 21), and recent work has illustrated that such competitive shifts also can occur in plant communities (22). Even when competitive hierarchies are not reversed, the rate of an invading competitive dominant can be dramatically increased in the presence of a pathogen (18). Thus, disease can alter the relative biomass of competitors in their environment and potentially cause extirpation of more susceptible hosts (21).
Based on experimental and observational data, it has been suggested that pathogens may allow invasion by a species previously excluded by direct competition in communities as wide-ranging as monkeys, mussels, and grasslands (14, 23, 24); however, short-term studies tell us little about the ultimate outcome of interactions among potentially long-lived species (25). To our knowledge, there have been no studies that use models fitted with field-estimated parameters to examine the long-term demographic implications of this potentially widespread phenomenon (17). Here, we develop a quantitative model to assess the role of a disease in reversing the competitive balance between native and exotic grasses in California, thus allowing exotic annual grass invasion. We focus on two primary questions: (i) Does the presence of a viral pathogen affect the susceptibility of native perennial grasslands to invasion by exotic annual grasses, and (ii) how has the conversion from perennial to annual grass domination altered pathogen dynamics?
Study System.
Barley and cereal yellow dwarf viruses (hereafter we refer to this complex of phloem-limited luteoviruses as BYDV) are one of the most economically important pathogens in grasses worldwide, causing stunting and yield losses in grain crops such as wheat, barley, and oats (26), and they are among the most prevalent of all viral pathogens (27). In California grasslands, BYDV is a persistently present disease, with occasional epidemic outbreaks (28); it infects a broad range of native and introduced grasses, leading to increased mortality and lower fecundity (29); and its origin is as yet unknown. The pathogen is persistently transmitted by at least nine aphid species in California (30, 31). However, it is not transmitted directly or intergenerationally (vertically through the seeds); annual host plants must acquire the virus each growing season, whereas perennial hosts serve as virus reservoirs.
The model used is at heart a simple Lotka–Volterra competition model combined with an implicit-vector susceptible-infected disease model; we use an integrodifference formulation to incorporate seasonality (see Methods for full description). Our model choice was driven by strategic simplicity to allow both generality and effective parameter estimation from field data.
Results
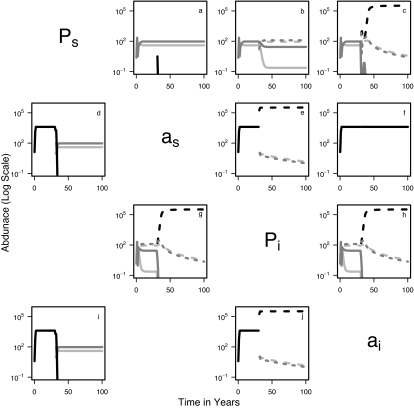
We find that disease-free perennial grasses cannot be invaded by healthy annuals (Fig. 1a), and that healthy perennials can replace annuals in disease-free systems (Fig. 1d). However, the presence of disease in the system allows invasion by annuals and long-term coexistence of a mixed annual–perennial community (Fig. 1 c, e, g, h, and j). In this case, total perennial biomass is significantly reduced, and no healthy perennials persist in the community outside of the growing season. Using experimental and field-observed data, our model predicts coexistence of annuals and perennials with significant domination by annuals; both annual and perennial grasses become infected each growing season by implicitly modeled vector movement among the groups. In contrast, annuals alone are unable to sustain BYDV infection, because BYDV infection is not transmitted intergenerationally (e.g., Fig. 1 f and i), thus a perennial reservoir is necessary for pathogen persistence through California's annual seasonal drought. In California's exotic annual grasslands, maintenance of BYDV depends on pathogen reintroduction by local movement or long-distance immigration of viruliferous aphids from perennial grasses (e.g., native grasses or perennial lawn grasses) or other reservoirs, such as irrigated grass crops (e.g., barley), where the disease can persist despite seasonality. If we include low-level immigration of viruliferous aphids in our model, the sole changes in dynamics are that the pathogen is always present in the system, annuals always dominate when present, and perennials always persist when present.
Fig. 1.
Pairwise invasion plots. These plots show the community trajectory when each species is added to an equilibrial population of its competitor in the presence or absence of disease. Within-season dynamics are not shown here; data plotted represent the end of each growing season. In each plot, the resident achieves equilibrium, and the invader arrives after 30 years. Rows represent residents, and columns represent invaders. Thus, the first row represents pathogen-free perennial system (Ps) invaded by healthy annuals (a), infected perennials (b), and infected annuals (c); the second row represents a pathogen-free annual system (as) invaded by healthy perennials (d), infected perennials (e), and infected annuals (f); the third row represents infected a perennial system (Pi) invaded by healthy annuals (g) and infected annuals (h); and the fourth row represents an initially infected annual system (ai) invaded by healthy perennials (i) and infected perennials (j). Perennial adults are dark gray, seedlings are light gray, and annuals are black. The y axis is logarithmic, and the cutoff is at 0.1.
Annual grass invasion combined with aphid preferences for annual grasses alters disease dynamics by increasing transmission rates and disease prevalence in perennial grasses. Before annual grass invasion, some perennial grasses remained uninfected each season, whereas in a mixed annual–perennial community, all perennials become infected in the first year, such that virtually no healthy perennials are seen at the end of any growing season (Fig. 1 g and h). In addition, pathogen incidence and infection prevalence in the grass community increase with invasion of annual grasses into a perennial grass–BYDV community.
Although both annuals and perennials are susceptible to BYDV, the disease favors annuals over perennials by reducing the benefits of perennial longevity; investment in future reproduction. For annuals, the disease substantially reduces biomass and fecundity (γ) but has no effect on survival, because annuals persist between seasons only as uninfected seeds. In fact, the decline in annual biomass within a season favors the surviving annuals by reducing intraspecific competition. In contrast, BYDV infection has several compounding negative effects on perennials that have survived their first year. First, infection halves the lifespan of adult perennials. Reduced survival to the subsequent year (σPI), compounded by reduced fecundity (εp), depresses the lifetime net seed production of an adult perennial (e.g., from 330 to 75 seeds per plant) to a level insufficient for an infected adult perennial to replace itself if all of its offspring were to be infected at germination. Finally, because perennial adults are superior to annuals in competition for light and nutrients, loss of perennial biomass reduces interspecific competitive pressure on annuals. Thus, the disease is more detrimental to the long-lived perennial life history than to the annual grass life history; this permits annuals to increase in relative abundance, which further amplifies viral incidence.
These model predictions are robust for a broad swath of parameter space surrounding the values estimated from available empirical data (e.g., fecundity, longevity, etc.), covering over one-third of the parameter space for all parameters examined [supporting information (SI) Appendix B]. Thus, our predictions are applicable not only to our focal species but also across the broad range of perennial and annual life histories represented in the California grass flora.
Discussion
These long-term dynamic modeling results unify seemingly contradictory results in California grassland research. The widespread invasion and subsequent domination of California perennial grasslands by annual grasses from the European Mediterranean region led to the assumption that the native perennials were poorer resource competitors (32). Recent empirical research (10, 11) upholds earlier theoretical work (12) in showing that the invasive annual grasses are poorer resource competitors than the native perennials for light, nutrients, and water. Attempts to date to explain how poorer resource exploiters can be extraordinary invaders include initial widespread alterations of herbivory, disturbance, and the abiotic environment (e.g., overgrazing, trampling by cattle, altered burning regimes, and drought), which dramatically reduced perennials. Perennial grasses persist at low densities throughout California grasslands (32), yet it is critical to note that decades of active management to favor native perennial grass recovery have, at best, achieved only greater relative abundance of perennial grasses but never exclusion of exotic annual grasses (10, 33).
Here we quantitatively demonstrate an alternate mechanism by which an inferior resource competitor could both invade and maintain dominance among competitively dominant natives. This interaction between the pathogen and the two competitor species mediates competition by two pathways: apparent competition, in which each plant species provides a source of pathogens that depresses the fecundity and survival of the superior competitor; and direct competition mediated by infection, in which the pathogen reduces the survival and fecundity of each species, thereby reducing the competitive impact of each on the other (34). In contrast to most simple two-host–one pathogen models, the pathogen causes the native perennial reservoir species, which is also the superior resource competitor, to lose in competition to the exotic annual species, because disease diminishes the duration of the adult perennial competitive refuge.
As predicted by our model, experimental studies have demonstrated that invasions by exotic annual grasses can increase BYDV prevalence (14). In addition, we do not predict complete extirpation of the perennial grasses by this mechanism as supported by observations of existing grassland community composition (10, 32), because the adult perennial size class still provides some refuge from competition. However, given that perennial grasses can live for decades to centuries, experimental studies tell us little about the long-term effects of BYDV on the ultimate community composition (25). Our modeling work allows us to extrapolate from these short-term studies to ecologically relevant time scales encompassing many generations of perennial grass hosts.
Our results imply that alterations of disturbance regimes may have hastened invasion, but the presence of a pathogen was necessary to permit and maintain invasion. In fact, the failure of active management (e.g., removal of grazing, fire suppression, and perennial seeding) to create native-only communities supports our results and suggests that the invasion by European annual grasses has shifted California grasslands into an alternate stable invaded state from which they cannot return. Of all mechanisms currently considered important in driving and maintaining this invasion, an alternative state induced by disease is the only one consistent with the observed lack of management success.
Although we parameterized our model for a relatively short-lived perennial species that is only moderately affected by BYDV, model sensitivity analyses demonstrate that our results remain qualitatively unchanged over a broad range of host and pathogen vital rates (e.g., lifespan and virulence). For example, perennial grass lifespan, a key component of their competitive advantage over annual species, ranges from 5 to >10 years for perennial grass species like Bromus carinatus and Elymus glaucus to several hundred years for Nassella pulchra (K. Rice, personal communication; refs. 35 and 36). Disease in perennials increases mortality, thereby reducing the benefits of long-term investment in reproduction. Although annuals can invade this perennial grass community only in the presence of the pathogen, longer-lived perennials reduce the extent of domination by annual grasses. In addition, the effects of the BYDV pathogen vary among the native California grass species, with 30–70% reduction in fecundity and 0–80% reduction in survival (29). In our focal species, E. glaucus, BYDV causes a moderate reduction in fecundity relative to other species (50%) and a weak reduction in survival (10%); thus, our results present a conservative estimate of the influence of this pathogen in setting in motion the invasion of exotic annual grasses into California's diverse native community.
Although the provenance of BYDV does not affect our primary conclusion, it is interesting to consider how long of a lag existed between the arrival of BYDV and the invasion and domination by exotic annual grasses. If the pathogen is native, the introduction of novel hosts altered the dynamics of this native pathogen to allow invasion. If BYDV was introduced by human transport, our results show that it must have arrived in the system before or concurrent with the exotic grasses to allow successful annual grass invasion. Work to date demonstrates that BYDV has been in California at least since the early 1900s (C. Malmstrom, personal communication; ref. 37). In addition, given that aphid vectors can disperse hundreds of miles per day on wind (38), and that symptomatic descriptions suggest that BYDV was present in North American crops over a century ago (39), evidence suggests that if it did not arise in California, BYDV has been in the system for a very long time. Although less likely, viruliferous aphids or infected plant tissue also could have been transported during an early shipment of annual grasses into the system; many intentional and accidental introductions of annual species into California have occurred throughout the past 150 years (32, 40). In this case, it might be that a wave of BYDV preceded the spread of exotic annual grasses across the landscape by only years or decades.
Although our model is essentially a local-scale model, realistic complexities, such as the effects of space and species heterogeneity on transmission and competition, may promote the coexistence of annuals and perennials by reducing interspecific interactions rather than by amplifying competitive exclusion (41, 42); however, these complexities are unlikely to change our conclusions. Although greater realism may reduce rates of pathogen transmission or slow invasion or may permit localized persistence of annuals in the absence of the pathogen, these alterations would not qualitatively change our primary result: perennials dominate in the absence of disease, whereas annuals dominate but coexist with perennials in the presence of disease. Thus, the current model result provides a robust and important initial contribution to our understanding of the long-term population-dynamic consequences of disease in the invasion of California perennial grasslands by exotic annuals.
The tight links between disease ecology and species invasions have only recently become apparent. To date, this work has focused on the direct effects of introduced pathogens on native species or on how the shift in the pathogen load of introduced species affects invasibility (15, 18, 22, 43, 44). Here we quantitatively demonstrate a striking result that links disease and invasions without requiring changes in the pathogen community. The introduction of an exotic host can alter pathogen dynamics such that a formerly uninvasible community becomes opened to invasion. The potential importance of this largely unexplored interaction is illustrated here as a plausible mechanism allowing one of the most dramatic and persistent plant invasions worldwide, the spread and domination of exotic annual grasses throughout California. Specifically, we show that BYDV must have been present in the native grassland community at least concomitantly with, if not before, the successful invasion by exotic annual grasses.
Methods
Modeling Description.
In California grasslands, the wet winter and spring growing seasons alternate with hot, dry summers, during which perennial grasses remain dormant, and annuals persist only as seeds. We use an integrodifference modeling approach to describe the punctuated seasonality of this system. During the growing season (of length τ), differential equations are the effective model, and the dormant summer season is described by difference equations [(t+τ) to (t + 1)]. We use the following susceptible-infected equation structure to summarize the multihost dynamics of the California grassland community.
Net competition terms:
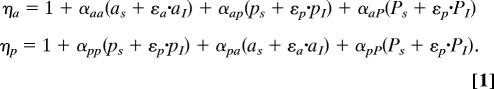 |
Vector:
Rainy season/continuous time:
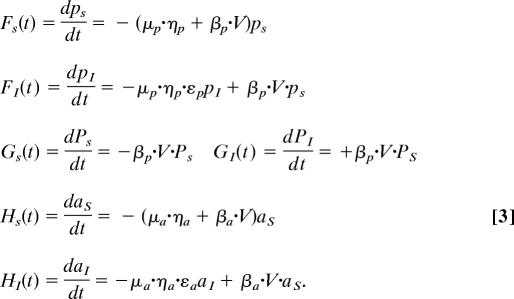 |
Dry season/discrete time:
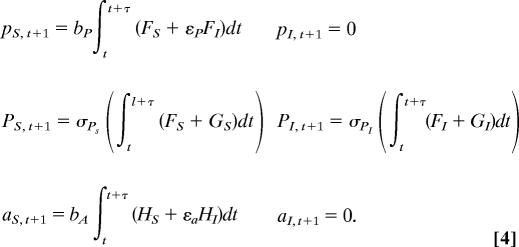 |
Perennial adults are qualitatively different from annual grasses, because they are competitively superior and less palatable to aphids; in contrast, first-year perennials are more similar to annuals in these characteristics (10, 14). Thus, to examine the role of disease in invasion, we explicitly track susceptible perennial seedlings (pS), infected perennial seedlings (pI), susceptible perennial adults (PS), infected perennial adults (PI), susceptible annuals (aS), and infected annuals (aI) (Eq. 4). Although BYDV can slow foliage and root development, prevent seed production, or even kill a host, symptoms tend to be more subtle in wild hosts (29); in the current model, we examine both reduced fecundity and disease-induced dormant season mortality. Net competition terms for perennials and annuals (Eq. 1) are described as ηp and ηa, in which αXY is the competition coefficient between host species X and Y. We use a Lotka–Volterra competition framework because of the nature of the data available for parameter estimation, as well as density making a sensible common currency for competition and disease for a systemic virus. We explicitly describe background mortality (μ), pathogen transmission (β), disease-induced fecundity and biomass reduction (ε), birth rates (e.g., bX for species X), and adult perennial summer survival (σ). Fecundity and biomass reduction are represented by the same parameter, because plant fecundity is largely a function of plant biomass and the empirical estimates were essentially identical (29). Annual and perennial rates differ for most parameters; however, rates are identical for adult and seedling perennials, except ηp and σ. F, G, and H, which represent growing season differential equations (Eq. 3), are updated annually from the difference equations.
Aphids disperse widely and reproduce rapidly, preferring and performing better on annuals than perennials (14, 30, 31). Here, we do not track aphid vectors explicitly but rather assume them to be generated instantaneously (indexed as V; Eq. 2). Although it is an extremely simplistic assumption, we believe it captures an essential effect of the aphids, given the strategic simplicity of the rest of the model and the paucity of available data on aphid dynamics in this system. The difference in aphid fecundity on annuals vs. perennials is described by λ.
Parameterization and Testing.
We examine the potential role of BYDV in the invasion of California grasslands by parameterizing this model with empirical data. Perennial bunchgrasses such as N. pulchra, E. glaucus, and B. carinatus historically ranged from Mexico to Canada but dramatically declined in abundance before the late 1800s. They remain widespread but sparse throughout California (32). These bunchgrasses fall into two functional types: short-lived (5->10 years, E. glaucus and B. carinatus) and long-lived (100+ years, N. pulchra and Nasella cernua) (K. Rice, personal communication; refs. 35 and 36). Although BYDV infection has been documented in at least 33 native and 80 exotic grasses in California (45), we focus on two common grasses, E. glaucus, a native perennial, and Bromus hordeaceus, an exotic annual, because these species have among the broadest ranges of West Coast grassland species. They have been the focus of our own multiyear BYDV monitoring and among the native perennials, the best-quality published prevalence data are available for E. glaucus (14). Although we parameterize the model for these two species, we have kept the formulation intentionally general to permit a greater breadth of predictive ability. In Discussion, we address predictions for disease and invasion in communities dominated by longer-lived perennial grasses. Table 1 provides a summary of rates and parameter values estimated from empirical data in this system.
Table 1.
Empirically derived parameter values
| Symbol | Definition | Value | Source (ref. no. or SI Appendix) |
|---|---|---|---|
| Discrete time parameters | |||
| bp | Perennial birth rate | 45.0 | SI Appendix A |
| ba | Annual birth rate | 200.0 | 10 |
| σPs | Healthy perennial (adult) survival | 0.88 | 29 |
| σPi | Infected perennial (adult) survival | 0.77 | 29 |
| εp | Fractional reduction in biomass, and thus also fecundity, of infected perennials | 0.5 | 29 |
| εa | Fractional reduction in biomass, and thus also fecundity, of infected annuals | 0.11 | 29 |
| τ | Growing season | 20 wks | SI Appendix A |
| Continuous time parameters | |||
| βP | Perennial pathogen transmission rate | 0.02 | SI Appendix A |
| βA | Annual pathogen transmission rate | 0.04 | SI Appendix A |
| λ | Vector preference and performance (aphids per annual/aphids per perennial) | 1.5 | 14 |
| μa | Annual death rate | 1 | SI Appendix A |
| μp | Perennial death rate | 0.5 | SI Appendix A |
| Competition parameters | |||
| αpp | Competition between first-year perennials | 1.3e-3 | SI Appendix A |
| αpa | The effect on first-year perennials by annuals | 6.8e-4 | SI Appendix A |
| αpP | The effect on first-year perennials by adult perennials | 0.7 | SI Appendix A |
| αaa | Competition between annuals | 1.1e-3 | SI Appendix A |
| αap | Effect on annuals by first-year perennials | 3.4e-7 | SI Appendix A |
| αaP | Effect on annuals by adult perennials | 0.7 | SI Appendix A |
Values estimated from empirical work in California grasslands and used for model parameterization. Discrete and continuous parameter units are year−1 (except ε, γ, and λ, which are unitless), and competition parameters are individual−1.
Predicting the conditions under which an introduced species will successfully invade requires knowledge of the conditions under which small populations will have positive growth rates when competing against a numerically abundant resident species. Ecological and evolutionary theory has demonstrated that two species will coexist if each has a positive growth rate when rare, but one species will displace the other if it increases when rare but the other does not (46). We used R (R Foundation for Statistical Computing, Vienna, Austria) to numerically simulate the outcome of reciprocal invasion experiments with susceptible and infected perennials and annuals as both residents and invaders. We also tested the sensitivity of our results to the estimated vital rate values (SI Appendix B).
Supplementary Material
Acknowledgments
Thanks to S. Harpole and three anonymous reviewers for comments. Support for this project was provided, in part, by National Science Foundation (NSF)/National Institutes of Health Grant EID 05-25666; U.S. Department of Agriculture Grant NRICGP 2003-35316-13767; NSF Grant DEB 02-35624; the Andrew Mellon Foundation; the National Center for Ecological Analysis and Synthesis, a Center funded by NSF Grant DEB-0072909; the University of California; and the University of California, Santa Barbara. We also thank the University of California Natural Reserve System.
Abbreviation
- BYDV
barley and cereal yellow dwarf viruses.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608573104/DC1.
References
- 1.Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Ecol Appl. 2000;10:689–710. [Google Scholar]
- 2.Pimentel D, Lach L, Zuniga R, Morrison D. BioScience. 2000;50:53–65. [Google Scholar]
- 3.Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann EH. Science. 1997;277:1300–1302. [Google Scholar]
- 4.Stein BA, Kutner LS, Adams JS. Precious Heritage: The Status of Biodiversity in the United States. New York: Oxford Univ Press; 2000. [Google Scholar]
- 5.Milton SJ. South Afr J Sci. 2004;100:69–75. [Google Scholar]
- 6.Lenz TI, Moyle-Croft JL, Facelli JM. Austral Ecol. 2003;28:23–32. [Google Scholar]
- 7.Mooney HA, Hamburg SP, Drake JA. In: Ecology of Biological Invasions of North America and Hawaii. Mooney HA, Drake JA, editors. New York: Springer; 1986. pp. 250–272. [Google Scholar]
- 8.Baker HG. In: Plant Relations in Pastures. Wilson JR, editor. East Melbourne, Australia: Commonwealth Scientific and Industrial Research Organization; 1978. pp. 368–384. [Google Scholar]
- 9.Jackson LE. J Biogeogr. 1985;12:349–361. [Google Scholar]
- 10.Seabloom EW, Harpole WS, Reichman OJ, Tilman D. Proc Natl Acad Sci USA. 2003;100:13384–13389. doi: 10.1073/pnas.1835728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbin JD, D'Antonio CM. Ecology. 2004;85:1273–1283. [Google Scholar]
- 12.Crawley MJ, May RM. J Theor Biol. 1987;125:475–489. [Google Scholar]
- 13.D'Antonio CM, Vitousek PM. Annu Rev Ecol Syst. 1992;23:63–87. [Google Scholar]
- 14.Malmstrom CM, McCullogh AJ, Johnson HA, Borer ET. Oecologia. 2005;145:153–164. doi: 10.1007/s00442-005-0099-z. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, Maron JL, Morris WF, Parker IM, Power AG, et al. Ecol Lett. 2006;9:726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 16.Dobson AP, Hudson PJ. Trends Ecol Evol. 1986;1:11–15. doi: 10.1016/0169-5347(86)90060-1. [DOI] [PubMed] [Google Scholar]
- 17.Hudson P, Greenman J. Trends Ecol Evol. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. [DOI] [PubMed] [Google Scholar]
- 18.Tompkins DM, White AR, Boots M. Ecol Lett. 2003;6:189–196. [Google Scholar]
- 19.Dobson A, Crawley M. Trends Ecol Evol. 1994;9:393–398. doi: 10.1016/0169-5347(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 20.Park T. Ecol Monogr. 1948;18:267–307. [Google Scholar]
- 21.Anderson RM, May RM. Philos Trans R Soc London B. 1986;314:533–570. doi: 10.1098/rstb.1986.0072. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell CE, Power AG. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- 23.Allison AC. In: Population Biology of Infectious Disease. Anderson RM, May RM, editors. Berlin: Springer; 1982. pp. 245–267. [Google Scholar]
- 24.Calvo-Ugarteburu G, McQuaid CD. Marine Ecol Prog Ser. 1998;169:149–163. [Google Scholar]
- 25.Briggs CJ, Borer ET. Ecol Appl. 2005;15:1111–1117. [Google Scholar]
- 26.D'Arcy C. In: Barley Yellow Dwarf: 40 Years of Progress. D'Arcy CJ, Burnett PA, editors. St. Paul, MN: Am Phytopathol Soc; 1995. pp. 9–28. [Google Scholar]
- 27.Thresh JM. Appl Biol. 1980;5:1–65. [Google Scholar]
- 28.Griesbach JA, Steffenson BJ, Brown MP, Falk BW, Webster RK. Crop Sci. 1990;30:1173–1177. [Google Scholar]
- 29.Malmstrom CM, Hughes CC, Newton LA, Stoner CJ. New Phytol. 2005;168:217–230. doi: 10.1111/j.1469-8137.2005.01479.x. [DOI] [PubMed] [Google Scholar]
- 30.Pike KS, Allison D, Boydston L, Qualset CO, Vogt HE, Summers CG. Calif Agr. 1989;43:22–24. [Google Scholar]
- 31.Halbert S, Voegtlin D. In: Barley Yellow Dwarf: 40 Years of Progress. D'Arcy CJ, Burnett PA, editors. St. Paul, MN: Am Phytopathol Soc; 1995. pp. 217–258. [Google Scholar]
- 32.Heady HF. In: Terrestrial Vegetation of California. Barbour MG, Major J, editors. New York: Wiley; 1977. pp. 491–514. [Google Scholar]
- 33.Stromberg MR, Kephart P. Restor Manage Notes. 1996;14:102–111. [Google Scholar]
- 34.Alexander HM, Holt RD. Perspect Plant Ecol Evol Syst. 1998;1:206–220. [Google Scholar]
- 35.Flowers LJ. Agronomy and Range Science. Davis: Univ of California; 1999. [Google Scholar]
- 36.Hamilton JG. Ecology, Evolution, and Marine Biology. Santa Barbara: Univ of California; 1997. p. 145. [Google Scholar]
- 37.Oswald J, Houston B. Plant Dis Rep. 1951;321:421–446. [Google Scholar]
- 38.Irwin ME, Thresh JM. Philos Trans R Soc London B. 1988;321:421–446. [Google Scholar]
- 39.Galloway BT, Southworth EA. J Mycol. 1890;6:72–74. [Google Scholar]
- 40.Mensing S, Byrne R. Fremontia. 1999;27:6–9. [Google Scholar]
- 41.Tilman D. Ecology. 1994;75:2–16. [Google Scholar]
- 42.Hastings A. Theor Popul Biol. 1980;18:363–373. doi: 10.1016/0040-5809(80)90015-5. [DOI] [PubMed] [Google Scholar]
- 43.Parker JD, Burkepile DE, Hay ME. Science. 2006;311:1459–1461. doi: 10.1126/science.1121407. [DOI] [PubMed] [Google Scholar]
- 44.Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- 45.Malmstrom CM. Grasslands. 1998;8:6–10. [Google Scholar]
- 46.Gurney WSC, Nisbet RM. Ecological Dynamics. New York: Oxford Univ Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.