Abstract
Protection of poikilothermic animals from seasonal cold is widely regarded as being causally linked to changes in the unsaturation of membrane phospholipids, yet in animals this proposition remains formally untested. We have now achieved this by the genetic manipulation of lipid biosynthesis of Caenorhabditis elegans independent of temperature. Worms transferred from 25°C to 10°C develop over several days a much-increased tolerance of lethal cold (0°C) and also an increased phospholipid unsaturation, as in higher animal models. Of the three C. elegans Δ9-desaturases, transcript levels of fat-7 only were up-regulated by cold transfer. RNAi suppression of fat-7 caused the induction of fat-5 desaturase, so to control desaturase expression we combined RNAi of fat-7 with a fat-5 knockout. These fat-5/fat-7 manipulated worms displayed the expected negative linear relationship between lipid saturation and cold tolerance at 0°C, an outcome confirmed by dietary rescue. However, this change in lipid saturation explains just 16% of the observed difference between cold tolerance of animals held at 25°C and 10°C. Thus, although the manipulated lipid saturation affects the tolerable thermal window, and altered Δ9-desaturase expression accounts for cold-induced lipid adjustments, the effect is relatively small and none of the lipid manipulations were sufficient to convert worms between fully cold-sensitive and fully cold-tolerant states. Critically, transfer of 10°C-acclimated worms back to 25°C led to them restoring the usual cold-sensitive phenotype within 24 h despite retaining a lipid profile characteristic of 10°C worms. Other nonlipid mechanisms of acquired cold protection clearly dominate inducible cold tolerance.
Keywords: Δ9-acyl desaturase, lipid composition, RNAi
Fluctuations of temperature have profound effects on biological systems, particularly poikilothermic organisms that are physiologically unable to regulate body temperature (1). Poikilotherms that inhabit environments with periodic fluctuations, such as seasons or tides, tend to display highly plastic physiologies, with prior thermal conditioning of days to weeks leading to both an altered capacity for life (i.e., adaptation of rate functions) and reversible changes in resistance to the debilitating and lethal effects of extreme high and low temperatures. Matching the thermal tolerance window to seasonal changes in temperature is a critically important component of fitness (2). Perhaps the best studied mechanism for the heat hardening response is the induction of heat-shock proteins, or chaperones (3), which mitigate the damage to protein folded structure at high temperatures and thereby protect against one of the principal causes of heat damage.
Less attention has been paid to how cold tolerance is acquired, particularly in higher animals. Perhaps the dominant hypothesis accounting for acquired protection to chill (i.e., nonfreeze) damage, originating in the early work of Heilbrunn (4), relates to membrane lipid composition. Cold conditioning generally leads to an increased proportion of unsaturated fatty acids in the major phospholipid classes (5). This maintains membrane physical structure at least partially independent of temperature and compensates for long-term temperature fluctuations (6, 7), a property that has been linked to the balance between saturates and unsaturates and thus to the cooperative lipid ordering (8). Moreover, animals inhabiting cold temperate or polar habitats tend to have much more unsaturated brain membranes than those from warmer tropical or subtropical habitats (9). If the primary lesion underpinning cold damage and death is influenced by membrane physical structure, then the lipid compositional response is likely to be the underpinning causal event in acquired cold tolerance. However, despite its widespread acceptance, this “phospholipid adaptation” hypothesis has never been explicitly tested in animals in anything other than a circumstantial manner.
The balance between saturated and unsaturated fatty acids is largely influenced by the activity of the Δ9-acyl desaturase, and this enzyme is substantially up-regulated by cold transfer in microorganisms, plants, and poikilothermic animals (10). Genetic manipulation of the desaturase in the cyanobacterium Synechocystis (11) and in higher plants (12–14) offers convincing evidence in favor of the causal role of the protein in cold adaptation. But the experimental manipulation of desaturase expression in higher animals is currently not tractable, and instead we focus on desaturase manipulation of Caenorhabditis elegans to test the role of phospholipid saturation in the acquired cold tolerance phenotype. The worm displays changes in lipid saturation upon cold acclimation (15), and we quantify the extent to which the phospholipid saturation hypothesis accounts for the acquired cold tolerance phenotype.
Results
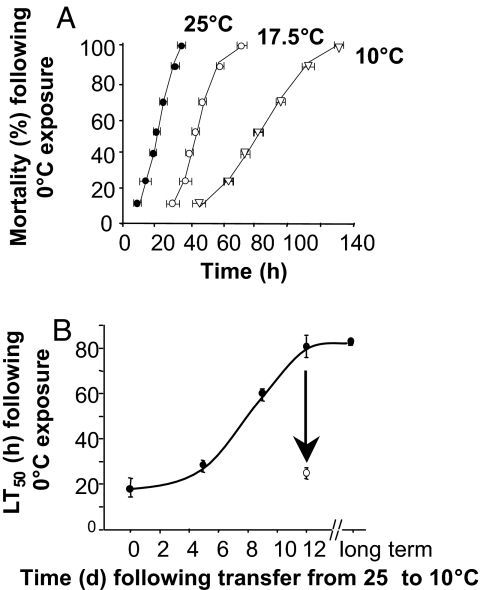
The cold tolerance limits of C. elegans and its ability to reversibly alter cold tolerance have not been previously determined. First we established cold conditions that cause measurable mortality, and whether tolerance to a lethal cold temperature was induced on reduction of culture temperature. Fig. 1A shows that 25°C-cultured worms were killed within 36 h after transfer to extreme cold (0°C), with a time to 50% mortality (LT50) of 20 ± 2 h (mean ± SD; n = 3). Worms cultured at 17.5°C and 10°C, however, showed a progressively greater tolerance of sustained cold (i.e., a right shift of the mortality curve) with maximal protection being attained after growth at 10°C, which is approaching the lowest physiological growth temperature for this animal (16). Fig. 1B shows that this cold-tolerant phenotype was induced slowly over a period of 5–12 days after transfer of cultures to 10°C. However, transfer of cold-tolerant worms back to 25°C resulted in a restoration of the normal cold-sensitive phenotype (LT50 24.3 ± 0.6 h; arrow in Fig. 1B) within 24 h.
Fig. 1.
Effect of acclimation temperature and length of acclimation period on mortality after exposure to 0°C. (A) Worms were grown at 25°C (filled circles), 17.5°C (open circles), and 10°C (open triangles). (For all groups, n = 3.) (B) Worms grown at 10°C for up to 12 days (filled circles). Worms that were transferred back to 25°C on day 12 for 24 h before 0°C-exposure are indicated by an open circle (n = 3–13). Error bars represent SD.
Lipid analysis of 25°C cold-sensitive and 10°C cold-tolerant worms (Table 1) broadly matched that demonstrated by Tanaka et al. (15) in that the 10°C worms had significantly reduced proportions of saturated fatty acids (SFA) (35.9 ± 2.3% compared with 27.3 ± 3.4%; mean ± SD; P < 0.001) and increased monounsaturated fatty acids (MUFA) (25.2 ± 3.7% compared with 43.19 ± 2.9%; mean ± SD; P < 10−6), mainly C16:1n-7 and C18:1n-7. These alterations were independent of the lipid composition of the Escherichia coli food source, which did not change significantly between the two temperatures (data not shown). We also assessed the changes in fatty acid saturation after transferring 10°C-cultured cold-tolerant worms back to 25°C for 24 h, when they lost most of their cold tolerance. The 24-h fatty acid profile was more characteristic of cold-acclimated worms [total lipids: SFA, 23.2 ± 0.5%; MUFA, 37.0 ± 1.1%; polyunsaturated fatty acids (PUFA), 39.8 ± 1.6% (mean ± SD)].
Table 1.
Fatty acid composition (%) of total lipid fraction of C. elegans grown at 25°C and 10°C
| Fatty acid | 25°C |
10°C |
||||||
|---|---|---|---|---|---|---|---|---|
| C | F5 | F7 | F5:F7 | C | F5 | F7 | F5:F7 | |
| 14:0 | 1.7 ± 0.9 | 2.5 ± 0.4 | 1.8 ± 0.1 | 1.3 ± 0.3* | 3.2 ± 3.2 | 1.0 ± 0.1 | 1.8 ± 0.6 | 1.3 ± 0.3 |
| 16:0 | 5.3 ± 2.1 | 6.1 ± 1.0 | 3.9 ± 0.3 | 4.4 ± 1.1 | 4.7 ± 1.3 | 6.3 ± 2.1 | 3.3 ± 1.2 | 7.4 ± 2.8 |
| 17:iso | 20.3 ± 5.1 | 24.4 ± 5.6 | 21.5 ± 0.6 | 17.6 ± 3.3 | 9.8 ± 2.5 | 8.2 ± 4.0 | 10.4 ± 3.3 | 8.2 ± 3.4 |
| 16:1n-7 | 3.3 ± 1.1 | 5.5 ± 0.4† | 3.6 ± 0.3 | 1.1 ± 1.0 | 9.1 ± 1.6 | 3.5 ± 0.4† | 6.0 ± 1.9* | 6.4 ± 1.7 |
| 17:0 | 0.8 ± 0.7 | 1.2 ± 0.1 | 0.8 ± 0.7 | 2.3 ± 0.8 | 1.3 ± 0.6 | 1.1 ± 0.3 | 1.2 ± 0.8 | 2.3 ± 0.7 |
| 17:1n-9 | 1.5 ± 1.4 | 1.8 ± 0.9 | 1.5 ± 0.4 | 3.4 ± 2.7 | 1.8 ± 1.5 | 1.6 ± 0.5 | 0.8 ± 0.7 | 1.6 ± 1.7 |
| 18DMA | 1.1 ± 0.6 | 2.6 ± 0.8 | 1.9 ± 0.8 | 3.9 ± 1.1* | 0.7 ± 0.7 | 2.5 ± 0.3† | 2.6 ± 0.7† | 0.8 ± 0.7 |
| 18:0 | 6.4 ± 1.4 | 4.2 ± 0.8* | 19.6 ± 3.5* | 26.9 ± 5.1* | 7.3 ± 1.6 | 8.4 ± 0.5 | 22.1 ± 3.5† | 37.5 ± 6.5* |
| 18:1n-9 | 5.0 ± 1.7 | 2.3 ± 2.1 | 1.9 ± 0.2† | 1.7 ± 0.4† | 3.3 ± 0.7 | 2.7 ± 0.3 | 1.4 ± 0.2† | 2.2 ± 0.4* |
| 18:1n-7 | 15.1 ± 0.9 | 15.2 ± 2.1 | 18.3 ± 0.4‡ | 12.5 ± 0.7* | 28.8 ± 3.3 | 22.2 ± 1.2† | 24.7 ± 2.2* | 17.2 ± 4.4* |
| 18:2n-6 | 12.3 ± 2.0 | 10.1 ± 0.9* | 4.5 ± 0.7‡ | 2.7 ± 0.6‡ | 9.6 ± 1.9‡ | 14.3 ± 0.2† | 4.4 ± 1.8† | 1.1 ± 0.9‡ |
| 18:3n-6 | 1.7 ± 1.0 | 3.0 ± 0.5 * | 0.0 ± 0,0 | 2.7 ± 2.7 | 1.4 ± 0.7 | 0.8 ± 0.5 | 1.5 ± 0.2 | 1.5 ± 0.3 |
| 18:3n-3 | 1.4 ± 1.1 | 1.5 ± 0.3 | 1.3 ± 0.3 | 1.5 ± 0.3 | 2.3 ± 1.8 | 1.9 ± 0.1 | 1.1 ± 0.4 | 0.5 ± 0.4 |
| 20:0 | 0.4 ± 0.4 | 0.2 ± 0.2 | 1.7 ± 0.4 * | 2.0 ± 1.1 | 0.3 ± 0.5 | -.4 ± 0.7 | 2.5 ± 1.0 * | 5.4 ± 2.9 |
| 20:1n-9 | 0.3 ± 0.3 | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.7 ± 1.1 | 0.2 ± 0.4 | 0.2 ± 0.1 | 0.1 ± 0.2 | 1.1 ± 1.0 |
| 20:3n-6 | 3.5 ± 0.9 | 2.5 ± 0.5 | 2.1 ± 0.4 | 1.6 ± 1.4 | 1.5 ± 0.3 | 2.0 ± 0.2 * | 1.4 ± 0.5 | 0.5 ± 0.4 * |
| 20:4n-6 | 2.7 ± 0.8 | 2.5 ± 0.8 | 1.6 ± 0.2 * | 1.5 ± 1.3 | 0.8 ± 0.2 | 0.8 ± 0.1 | 1.5 ± 0.4 * | 0.4 ± 0.4 |
| 20:4n-3 | 3.7 ± 0.8 | 3.1 ± 1.1 | 2.4 ± 0.5 * | 1.8 ± 0.2† | 2.4 ± 0.4 | 4.3 ± 0.6 † | 1.3 ± 0.9 | 0.5 ± 0.5 * |
| 20:5n-3 | 13.5 ± 2.1 | 11.0 ± 2.0 | 11.3 ± 1.6 | 10.3 ± 0.8* | 11.6 ± 1.3 | 18.1 ± 1.7† | 11.8 ± 1.4 | 4.1 ± 1.6† |
| SFA | 35.9 ± 2.3 | 41.1 ± 3.9 | 51.3 ± 3.1† | 58.4 ± 0.5‡ | 27.3 ± 3.4 | 27.8 ± 2.5 | 44.0 ± 2.7‡ | 62.9 ± 9.1* |
| MUFA | 25.2 ± 3.7 | 25.2 ± 0.7 | 25.3 ± 0.1 | 19.4 ± 1.4* | 43.19 ± 2.9 | 30.0 ± 0.7‡ | 33.0 ± 4.0* | 28.5 ± 5.1† |
| PUFA | 38.9 ± 3.9 | 33.7 ± 4.0 | 23.5 ± 3.0† | 22.1 ± 1.2‡ | 29.6 ± 4.2 | 42.1 ± 2.1† | 23.0 ± 3.6* | 8.6 ± 4.0† |
C, control; F5, fat-5 mutant; F7, fat-7 RNAi; F5:F7, combined fat-5 mutant and fat-7 RNAi. Values are weight percentage (mean ± SD). n = 7 for 25°C control, n = 6 for 10°C control, n = 4 for 10°C fat-5 mutant and 10°C combined fat-5 mutant:fat-7 RNAi, and n = 3 for all other groups. Values that are significantly different from control worms grown at either 25°C or 10°C using an unpaired t test are denoted as follows:
*, P < 0.05;
†, P < 0.005;
‡, P < 1.0 × 10−6. For E. coli, composition at 25°C was as follows: 5.3%, 14:0; 36.7%, 16:0; 11.2%, 17:iso; 7.0%, 16:1n-7; 1.2%, 18:0; 20.6%, 18:1n-7; 18.0%, others. There was no significant change in composition of these bacterial lipids after 12 days of incubation at 10°C.
In a separate experiment we confirmed that the cold-induced increase in lipid unsaturation occurred in both phosphatidylcholine (PC) and phosphatidylethanolamine (PE) fractions [supporting information (SI) Table 2], which together comprise >80% of all polar lipids. In PC the SFA decreased from 55.4% at 25°C to 41.8% after 12 days of growth at 10°C largely because of increases in the proportion of C18:1n-7. However, after just 48 h of cold exposure, PC displayed a similar decrease in SFA but with a substantial increase in the proportion of 20:5n-3 and little change in C18:1n-7. PE also showed a substantial decrease in SFA from 57.4% to 44.4% over 12 days mainly because of a reduction in C16:0 and C18:0 and a corresponding increase in C18:1n-7. However, at 48 h the proportion of C16:1n-7 was greatly reduced whereas C18:1n-7 C18:2n-6 and C20:5n-3 showed modest increases. Evidently in both phospholipid classes the short-term response was quite distinct in character from the longer-term response.
Sphingolipids have been implicated in chill resistance in plants (17, 18) although not so far in animals. Sphingomyelin constitutes 8.1% of phospholipids (19) in C. elegans and was selected to assess the effects of cold treatment upon sphingolipids more generally. We used a mass spectrometric approach to quantify the molecular species distribution of the sphingomyelin isolated from 25°C and 10°C worms (SI Fig. 6). Fatty acids consisted of a series of 2-hydroxylated C20–C26 acids (SI Table 3) consistent with the glycosylceramides of this species (20). We found no evidence of a change in sphingoid base unsaturation at 10°C or of significant differences in SFA-, MUFA-, or PUFA-containing sphingomyelin fractions (SI Table 4) (Student's t test, P > 0.05). We conclude that this lipid class does not independently confer the cold tolerance phenotype.
Three Δ9-acyl desaturases have been identified in C. elegans, namely fat-5, fat-6, and fat-7 (21). fat-6 and fat-7 are 86% homologous at the nucleotide level, and when expressed in yeast are capable of desaturating both 16:0 and 18:0 substrates to generate 16:1(n-7) and oleic 18:1(n-9) products, respectively (21). However, fat-5 is limited to 16:0 and thus can generate only 16:1(n-7), which is elongated to vaccenic acid [18:1(n-7)] (21), an abundant C. elegans fatty acid (15). 18:1(n-9), on the other hand, does not accumulate in C. elegans; rather, it appears to be rate-limiting for the production of polyunsaturates (PUFAs) (21), principally linoleic acid [18:2(n-6)] which, after elongation and further desaturation, is converted to eicosapentaenoic acid [20:5(n-3)] (15, 22, 23). This corresponds with the most significant long-term changes in fatty acid composition in 10°C-acclimated worms being in the proportions of 16:1(n-7) and 18:1(n-7) (Table 1).
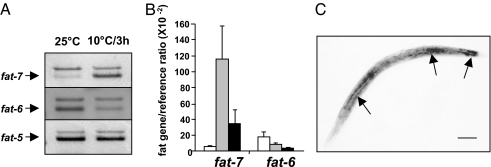
Fig. 2A and B shows that transfer of worms from 25°C to 10°C caused an ≈20-fold induction of fat-7 within 3 h, and it remained ≈6-fold higher at 24 h. By contrast, fat-6 showed a progressive reduction over the full 24-h period whereas fat-5 showed no change. fat-7 induction was blocked by the transcriptional inhibitor, α-amanitin, indicating transcriptional induction (data not shown). Using in situ hybridization in the worm we show that fat-7 was expressed in the gut cells, which matches the predominantly liver expression in fish (24) (Fig. 2C). Both fat-5 and fat-6 are also predominantly expressed in gut cells (25).
Fig. 2.
Expression profile of Δ9-desaturase homologues in C. elegans. (A) Duplex RT-PCR analysis. The top (larger) band in each duplex is the reference gene. (B) Real-time quantitative RT-PCR (n = 4). Error bars represent SD. White, gray, and black bars represent 0, 3, and 24 h, respectively, after downshift from 25°C to 10°C. The reference gene was lam-1. (C) In situ hybridization with fat-7 antisense probe. The arrows indicate intense expression in the gut and rectum. (Scale bar: 100 μm.)
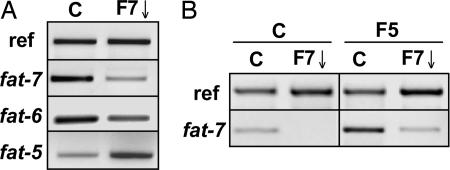
We have manipulated the transcript expression of fat-7 using the RNAi feeding technique (26), which led to a substantial (≈90%) reduction in fat-7 transcript amounts in 10°C-acclimated wild-type worms (Fig. 3A). fat-7 RNAi also reduced levels of the homologous transcript, fat-6, but resulted in increased amounts of the fat-5 transcript, suggesting a compensation of one Δ9-desaturase for ablated expression of another. Similarly, the fat-5 mutant strain displayed higher levels of fat-7 expression than wild-type (Fig. 3B). These compensatory interactions in expression of the multiple Δ9-desaturases, also identified by Brock et al. (25), made it necessary to simultaneously abrogate expression of all three by using fat-7 RNAi in a fat-5 mutant background. Lipid analysis showed that, irrespective of acclimation temperature, SFA levels were comparable between wild-type and fat-5 mutants, indicating that fat-5 expression has little influence on the status quo (Table 1). However, fat-7 RNAi resulted in a significant increase in SFA of wild-type worms (up by ≈15%), and an even greater increase when applied to the fat-5 mutant strain (up by 20–35%, depending on growth temperature). Proportions of 18:2(n-6) and 20:5(n-3) were significantly increased in 10°C fat-5 mutant worms, reflecting elevated expression of fat-7. It is worth pointing out that 18:1(n-7) is present in E. coli (15), so there is a lower limit to the reduction in MUFAs without resorting to manipulation of food source.
Fig. 3.
Expression of desaturase homologues in C. elegans using RT-PCR. (A) Effect of fat-7 RNAi on the expression of fat-5, fat-6, and fat-7. C, control; F7↓, fat-7 RNAi. The reference gene is shown as loading control. (B) Effect of fat-5 mutant on fat-7 expression. Analysis was performed on worms grown at 10°C from L1 for 12 days. C, control; F7↓, fat-7 RNAi; F5, fat-5 mutant.
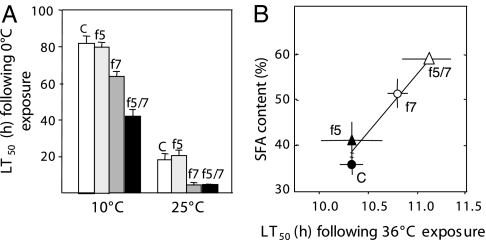
After exposure to a cold lethal temperature (0°C) we show that LT50 of wild-type and fat-5 mutant worms was comparable at each of the two growth temperatures (Fig. 4A). However, fat-7 RNAi significantly reduced LT50 at both 10°C and 25°C, indicating fat-7 activity influences both acquired cold tolerance (at 10°C) and intrinsic cold tolerance (i.e., direct transfer from 25°C to 0°C). The effect of fat-7 RNAi was further accentuated in 10°C-acclimated fat-5 mutant worms.
Fig. 4.
Relationship between thermal tolerance and fatty acid composition. (A) Effect of manipulated Δ9-desaturase expression on 0°C tolerance for worms held at 10°C and 25°C. White bars, control; light gray bars, fat-5 mutant; dark gray bars, fat-7 RNAi; black bars, fat-5 mutant/fat-7 RNAi. Values and bars represent mean ± SD (n = 3–5). (B) Correlation between SFA content and LT50 at an exposure temperature of 36°C for worms conditioned at 25°C and subjected to desaturase manipulation. C, control, unmanipulated worms; f5, those deficient in the fat-5 gene; f7, those subjected to RNAi treatment for the fat-7 gene; f5/7, worms subjected to both conditions. Values and error bars represent means ± SD (n = 3).
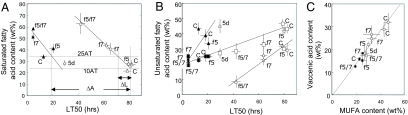
Fig. 5 shows the effects of desaturase manipulation upon fatty acid composition and relates this to changes in LT50. Fig. 5A illustrates the relationship for SFAs; the left side of the figure contains the data points for 25°C worms, which tend to be at low values for LT50. The right side contains the more cold-tolerant values for 10°C worms. For both 10°C and 25°C groups there was a strong negative correlation between LT50 and SFA content but each temperature data set occupied a different position on the x axis. Thus, worms manipulated to contain equivalent SFA contents at 10°C and 25°C did not produce equivalent cold tolerance phenotypes and evidently lipid saturation is not sufficient to explain the difference in cold tolerance. The linear SFA/LT50 relationships generated by these desaturase manipulated worms provides a calibration curve with which to quantify the predicted effects of the naturally occurring lipid response to cold upon LT50. The SFA content of 10°C and 25°C worms is indicated by the two horizontal dotted lines in Fig. 5A. The intercept of these with the SFA/LT50 curve for 10°C worms defines the LT50 values predicted for the acclimated worms (Fig. 5A, vertical dotted lines). This predicted change in LT50 (ΔL) is calculated to be just 16% of the observed difference in LT50 between 10°C and 25°C worms indicated as ΔA. Fig. 5B shows the corresponding data for MUFA and PUFAs from which it is evident that the change in SFA is largely accounted for by changes in PUFA. Although fat-5, fat-7, and the combined ablation of both genes in 10°C worms caused substantial changes in LT50, the MUFA content of these worms was not statistically different. Fig. 5C shows that over all conditions of temperature and desaturase ablation the proportion of vaccenic acid remained a constant proportion (≈75%) of all MUFAs. This relationship was unaffected by reduced desaturase expression, which suggests a sophisticated mechanism regulating MUFA composition.
Fig. 5.
Effects of desaturase manipulation on fatty acid composition related to changes in LT50. (A) Correlation between LT50 measured at 0°C, and percentage of SFAs of whole-body polar lipids for worms held at 10°C and 25°C. r2 = 0.813 for 25°C worms (filled triangles) and 0.997 for 10°C worms (open triangles). Error bars represent SD (n = 3–10). Labels are as in Fig. 4B. 5d, worms 5 days after transfer from 25°C to 10°C. All other 10°C worms were held at 10°C for 12 days. The two horizontal dotted lines signify the SFA values for control worms grown at 10°C and 25°C, as indicated. The vertical dotted lines indicate the change in LT50 due to growth at 10°C and 25°C worms (ΔA) and that are due directly to the change in SFA as estimated from the 10°C worm curve (ΔL). ΔL was only 16% of ΔA. (B) Correlation between LT50 and the unsaturated fatty acids. Filled symbols indicate worms held at 25°C, and open symbols indicate those held at 10°C; triangles indicate PUFAs, and squares indicate MUFAs. Labels are as in Fig. 4B. Lines were fitted by inspection: the MUFA with a single line but PUFAs with one line for each of the two culture temperatures. (C) Relationship between vaccenic acid [18:1(n-7)] and MUFAs across all treatment conditions. Filled symbols represent worms held at 25°C, and open symbols represent those held at 10°C. Labels are as in Fig. 4B. The illustrated regression line is y = 0.702 × x (r2 = 0.84).
We have also found a positive correlation between LT50 and SFA content after exposure to the high lethal temperature of 36°C (Fig. 4B). Thus, fat-7 RNAi increased the upper LT50, and, again, this effect was more striking in the fat-5 mutant background. This increase in resistance to high temperature exposure prompted us to investigate the effect of increased SFA content on longevity and fecundity, for there is a large body of evidence showing a correlation in C. elegans between heat resistance and both enhanced longevity (27–31) and fecundity (32). We found that worms with increased SFA levels had decreased mean lifespan and reduced fecundity (SI Fig. 7). The relationship between Δ9-desaturase expression and longevity remains enigmatic however. For example, Brock et al. (25) recorded increased lifespan in fat-5, fat-6, and fat-7 mutants compared with wild-type worms, yet a reduced lifespan in nhr-80 mutants which have greatly diminished expression of all three Δ9-desaturase genes.
To confirm that the reduction in LT50 of desaturase-manipulated worms was linked to the desaturase-mediated lipid manipulation we attempted to rescue the cold-sensitive phenotype of the desaturase-ablated worms by dietary supplementation of the product of the desaturase-mediated reaction, oleic acid. The LT50 of combined fat-5 mutant: fat-7 RNAi worms grown at 10°C and fed oleic acid was significantly higher (71.3 ± 0.6 h, mean ± SD) than that of unsupplemented fat-5 mutant:fat-7 RNAi worms (46.3 ± 4.0), but was not different from fat-5 mutant worms grown on control plates containing HT115 bacteria lacking an insert (72.7 ± 3.2 h, P > 0.05). Oleic acid supplementation produced a substantial down-regulation of fat-7 transcript levels (data not shown), indicating that this gene was strongly influenced by diet. We also noted that oleic acid supplementation improved the recovery of coordinated movement in both fat-7 RNAi and control worms after 36-h exposure to 0°C and restored normal fertility (data not shown).
Discussion
Adult C. elegans clearly possess a powerful protective response that enhances survival of lethal cold that is induced simply by transfer to the cool but nonlethal temperature of 10°C. This response takes ≈12 days to reach its maximal effect, but restoration of the cold-sensitive phenotype on transfer of cultures back to 25°C occurred within 24 h. Cold transfer leads, over time, to an increase in the fatty acid unsaturation of phosphoglycerides, including the major classes PC and PE, as with other animals (9), but we were unable to find any significant changes in sphingomyelin molecular species content, in contrast to recent observations in plants (33). In all these respects the phenotype matches that observed in other poikilothermic animals (5). For worms the change in saturation in PC appeared to have two phases, one that occurred within 48 h due largely to increased proportions of 20:5n-3, and the other over the subsequent 10 days, in which this PUFA was replaced by vaccenic acid, 18:1(n-7). The former response, which was also observed by Tanaka et al. (15), probably results from increased activity of Δ9-acyl desaturases. The reduction in proportion of SFAs, long thought to be the dominant feature of the lipid response (6), occurred within 48 h. PE also showed similar though less extreme trends.
Of the three Δ9-desaturase genes in C. elegans only fat-7 was cold-inducible. We found a powerful ≈20-fold induction within 3 h after transfer to 10°C which corresponds to the ≈10-fold induction observed in carp liver (10, 34) and in Synechocystis (11). Given the known rate-limiting role of Δ9-desaturation in the production of PUFAs including 20:5(n-3) (15, 21–23) it is reasonable to link the transient but substantial induction of fat-7 to the de novo synthesis of 18:1(n-9) and its rapid conversion to 20:5(n-3) with the rapid phase of change in unsaturation. The source of vaccenic acid, dietary or de novo synthesis, that becomes incorporated in the longer second phase is not known, but its significance might be in substituting for 20:5(n-3) in the sn-1 position of di-20:5(n-3) PC. This is an unusual complex lipid that has been found in C. elegans and whose level is enhanced in long-term 15°C-reared worms compared with 25°C-reared worms (35). We found that ω3 ablation by RNAi elicited no change in the cold tolerance phenotype (data not shown), suggesting that the proportion of 20:5(n-3) was not critical to cold tolerance and that other less unsaturated PUFAs can substitute.
Suppression of the cold-inducible expression of fat-7 using the RNAi technique was ≈90% successful, as judged by real-time PCR. Yet knockdown of fat-7 resulted in increased fat-5 transcript abundance suggesting that the transcript expression of these two desaturases is linked either directly, through a gene regulatory mechanism, or indirectly perhaps through the effect of changes in substrate levels. Recently, nuclear hormone receptors, in particular nhr-80, have been found to play a critical role in this compensatory response (25). To establish greater control over desaturase expression we imposed fat-7 knockdown by RNAi on the C. elegans strain containing a fat-5 loss-of-function mutant. The combined fat-5 mutant/fat-7 RNAi worms displayed much the largest increases in fatty acid saturation compared with either acting alone. This is consistent with the effective suppression of desaturase activity due in part to the effective knockdown of fat-6, which is 86% homologous to fat-7. As a consequence our procedures significantly inhibited all three Δ9-desaturase activities, permitting the generation of 10°C-treated worms with an increased SFA content to match that of 25°C control worms and beyond. Complete ablation of desaturase activity by mutation is lethal (25), but our combination of mutation and RNAi achieved lipid manipulation without causing mortality or any obvious deleterious effect.
Desaturase manipulation caused substantial changes to the time–mortality curves at 0°C. The manipulations causing the greatest changes in lipid saturation, and conversely unsaturated fatty acids, had the greatest effects on chill tolerance of the whole animal, resulting in a clear linear relationship between the proportion of SFAs and chill tolerance in 10°C-treated worms, and a similar but separate relationship for 25°C treated worms. These effects do not relate so clearly to the proportion of monounsaturates, because for 10°C worms the different kinds of desaturase manipulation resulted in no significant differences in MUFAs despite the worms displaying large and significant differences in LT50. Furthermore, the rescue experiment shows that (i) dietary lipids can influence lipid composition and (ii) the restoration of lipid saturation in desaturase-ablated worms coincided with expression of normal cold tolerance. All of this evidence is consistent with a causal link between desaturase activity, lipid saturation, and the changes in LT50 rather than some other off-target effect of desaturase manipulation. These conclusions support the potential for altering cold tolerance by lipid manipulation either through dietary or genetic means, although lipid therapy may incur functional tradeoffs as with longevity and fecundity.
A quite separate question is whether the change in lipid saturation accounts for the entirety of the cold-tolerant phenotype. We show that the differences in lipid saturation between wild-type C. elegans grown at 25°C and those transferred to 10°C were much smaller than those generated by desaturase manipulation at either culture temperature, yet manipulation was unable to elicit the full transition between the cold-tolerant and -susceptible phenotypes. We establish a quantitative relationship between SFA and LT50, which suggests that the cold-induced change in lipid saturation, contributes just 16% to the cold tolerance phenotype. Thus, although lipid manipulation affects LT50, the major proportion of the naturally acquired cold tolerance requires the intervention of other mechanisms of cold protection which are currently unknown but which may involve the production of compatible solutes (36, 37). This surprising conclusion has a caveat, in that the inactivation of desaturases may have consequences on cellular processes other than phospholipid unsaturation (i.e., balance of fat synthesis and oxidation, energy charge) and at this stage we cannot exclude them from interfering with the expression of the cold tolerance phenotype. However, it is supported by (i) the warm-transfer experiment where worms held at 10°C for at least 10 days and then transferred to 25°C showed a rapid loss of cold tolerance well before any substantial change in lipid composition; (ii) the different time course of change in fatty acid composition in individual phosphoglycerides and induction of cold tolerance; and (iii) the fact that oleic acid supplementation of desaturase-manipulated worms failed to change cold tolerance by as much as that displayed by cold-acclimated worms, although we have not determined the shift in unsaturation in these animals. A key unanswered question is whether the conclusions offered by this study apply to poikilotherms generally, and combined genetic and quantitative approaches will be needed to advance this issue.
Methods
Culture.
Standard methods were used to culture C. elegans N2 Bristol and the fat-5(tm420) strain (a gift from S. Mitani and Y. Kohara, National Institute of Genetics, Mishima, Japan) at 25°C, 17.5°C, and 10°C (38) except that E. coli strain NA22 was used as food source instead of the slower-growing OP50 strain to support the growth of the large numbers of worms that were required for the experiments. fat-5(tm420) is a deletion mutant with a loss-of-function phenotype, hereafter called the fat-5 mutant. Synchronized populations were prepared by harvesting eggs from gravid hermaphrodites grown on NGM plates and allowing eggs to hatch in the absence of food source. The following day (or 2 days later for 10°C worms) L1s were placed onto seeded plates.
Determination of Thermal Tolerance.
Synchronized populations of young adults were exposed to 0°C or 36°C for various times. After 60 min of recovery at the growth temperature, the total number of worms (range 50–150) and the number of dead worms were counted to give percentage mortality. Animals were scored as dead if they showed absence of pharyngeal pumping and failed to respond by movement to prodding.
Semiquantitative RT-PCR and α-Amanitin Experiments.
cDNA was reverse-transcribed from total RNA, and either end-point analysis using densitometry (39) or real-time quantification with LightCycler (Roche, Burgess Hill, U.K.) using SYBR green (40) was performed. Except for α-amanitin experiments where ≈50–1,000 worms were used, 30 worms were selected from each sample for analysis. Worms were exposed to 100 μg/ml α-amanitin for 24 h at 25°C before being transferred to 10°C; worms were collected 3 h later for analysis.
In Situ Hybridization.
Sense and antisense probes spanning nucleotides 196–793 from ATG of fat-7 cDNA were synthesized by using a standard protocol (41), and whole-mount in situ hybridization was performed as previously described (42), except worms were treated with 1 mmol/liter DTT for 5 min before prehybridization.
Fatty Acid Composition.
Lipids were extracted from synchronized cultures of young adults or E. coli and transmethylated as described previously (43). Fatty acid methyl esters were analyzed by gas–liquid chromatography and identified by comparing peak retention times with authentic standards whose identity had been confirmed by mass spectroscopy. Fatty acid compositions are presented on a percentage weight basis.
Sphingomyelin Composition.
Methods for isolation and molecular species analysis of this lipid fraction are described in SI Text.
RNAi.
Synchronized populations were fed bacteria expressing double-stranded fat-7 RNA from the L1 stage according to protocol 1 in ref. 44.
Longevity and Fecundity.
L4 worms were selected from a synchronized population and picked onto separate plates. During egg-laying worms were picked onto fresh plates daily and the number of offspring was counted. Day of death was later noted. The day that the synchronous L1 population was placed onto seeded plates is denoted day 0.
Supplementary Material
Acknowledgments
We thank Dr. D. Tocher, J. Dick, and M. Prescott for help with mass spectrometry; Drs. J. Watts, G. Lithgow, and A. Postle for helpful discussions; Dr. S. Mitani for cultures of the fat-5 mutant strain; and anonymous reviewers for insightful comments. This work was supported by a grant from Biotechnology and Biological Sciences Research Council (U.K.). P.M. is a Royal Society Dorothy Hodgkin Fellow (U.K.).
Abbreviations
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PUFA
polyunsaturated fatty acid
- MUFA
monounsaturated fatty acid
- SFA
saturated fatty acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609590104/DC1.
References
- 1.Cossins AR, Bowler K. Temperature Biology of Animals. London: Chapman & Hall; 1987. [Google Scholar]
- 2.Hoffman AA, Sorenen JG, Loeschcke V. J Thermal Biol. 2003;28:175–216. [Google Scholar]
- 3.Feder ME, Hofmann GE. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 4.Heilbrunn LV. Am J Physiol. 1924;69:190–199. [Google Scholar]
- 5.Hazel JR, Williams EE. Prog Lipid Res. 1990;29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 6.Hazel JR, Williams EE, Livermore R, Mozingo N. Lipids. 1991;26:277–282. doi: 10.1007/BF02537137. [DOI] [PubMed] [Google Scholar]
- 7.Cossins AR, Sinensky M. In: Physiology of Membrane Fluidity. Shinitzky M, editor. Vol 2. Boca Raton, FL: CRC; 1984. pp. 1–20. [Google Scholar]
- 8.Cossins AR. In: Temperature Adaptation of Biological Membranes. Cossins AR, editor. London: Portland; 1994. pp. 63–75. [Google Scholar]
- 9.Logue JA, de Vries AL, Fodor E, Cossins AR. J Exp Biol. 2000;203:2105–2115. doi: 10.1242/jeb.203.14.2105. [DOI] [PubMed] [Google Scholar]
- 10.Tiku PE, Gracey AY, Macartney AI, Beynon RJ, Cossins AR. Science. 1996;271:815–818. doi: 10.1126/science.271.5250.815. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki I, Los DA, Kanesaki Y, Mikami K, Murata N. EMBO J. 2000;19:1327–1334. doi: 10.1093/emboj/19.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizakinishizawa O, Fujii T, Azuma M, Sekiguchi K, Murata N, Ohtani T, Toguri T. Nat Biotechnol. 1996;14:1003–1006. doi: 10.1038/nbt0896-1003. [DOI] [PubMed] [Google Scholar]
- 13.Somerville C, Browse J. Trends Cell Biol. 1996;6:148–153. doi: 10.1016/0962-8924(96)10002-7. [DOI] [PubMed] [Google Scholar]
- 14.Kinney AJ, Cahoon EB, Hitz WD. Biochem Soc Trans. 2001;30:1099–1103. doi: 10.1042/bst0301099. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Ikita K, Ashida T, Motoyama Y, Yamaguchi Y, Satouchi K. Lipids. 1996;31:1173–1178. doi: 10.1007/BF02524292. [DOI] [PubMed] [Google Scholar]
- 16.Rabin Y, Podbilewicz B. J Microsc. 2000;199:214–223. doi: 10.1046/j.1365-2818.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 17.Imai H, Ohnishi M, Hotsubo K, Kojima M, Ito S. Biosci Biotech Biochem. 1997;61:351–353. [Google Scholar]
- 18.Kawaguchi M, Imai H, Naoe M, Yasui Y, Ohnishi M. Biosci Biotechnol Biochem. 2000;64:1271–1273. doi: 10.1271/bbb.64.1271. [DOI] [PubMed] [Google Scholar]
- 19.Satouchi K, Hirano K, Sakaguchi M, Takehara H, Matsuura F. Lipids. 1993;28:837–840. doi: 10.1007/BF02536239. [DOI] [PubMed] [Google Scholar]
- 20.Chitwood DJ, Lusby WR, Thompson MJ, Kochansky JP, Howarth OW. Lipids. 30:567–573. doi: 10.1007/BF02537032. [DOI] [PubMed] [Google Scholar]
- 21.Watts JL, Browse J. Biochem Biophys Res Commun. 2000;272:263–269. doi: 10.1006/bbrc.2000.2772. [DOI] [PubMed] [Google Scholar]
- 22.Beaudoin F, Michaelson LV, Hey SJ, Lewis MJ, Shewry PR, Sayanova O, Napier JA. Proc Natl Acad Sci USA. 2000;97:6421–6426. doi: 10.1073/pnas.110140197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Napier JA, Michaelson LV. Lipids. 2001;36:761–766. doi: 10.1007/s11745-001-0782-9. [DOI] [PubMed] [Google Scholar]
- 24.Polley SD, Tiku PE, Trueman RT, Caddick MX, Morozov IY, Cossins AR. Am J Physiol. 2003;284:R41–R50. doi: 10.1152/ajpregu.00263.2002. [DOI] [PubMed] [Google Scholar]
- 25.Brock TJ, Browse J, Watts JL. PLoS Genet. 2006;2:997–1005. doi: 10.1371/journal.pgen.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmons L, Fire A. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 27.Lithgow GJ, White TM, Melov S, Johnson TE. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yashin AI, Cypser JR, Johnson TE, Michalski AI, Boyko SI, Novoseltsev VN. Mech Ageing Dev. 2001;122:1477–1495. doi: 10.1016/s0047-6374(01)00273-1. [DOI] [PubMed] [Google Scholar]
- 29.Michalski AI, Johnson TE, Cypser JR, Yashin AI. Biogerontology. 2001;2:35–44. doi: 10.1023/a:1010091315368. [DOI] [PubMed] [Google Scholar]
- 30.Butov A, Johnson T, Cypser J, Sannikov I, Volkov M, Sehl M, Yashin A. Exp Gerontol. 2001;37:57–66. doi: 10.1016/s0531-5565(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 31.Munoz MJ. Mech Ageing Dev. 2003;124:43–48. doi: 10.1016/s0047-6374(02)00168-9. [DOI] [PubMed] [Google Scholar]
- 32.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 33.Sperling P, Heinz E. Biochim Biophys Acta. 2003;1632:1–15. doi: 10.1016/s1388-1981(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 34.Wodtke E, Cossins AR. Biochim Biophys Acta. 1991;1064:343–350. doi: 10.1016/0005-2736(91)90321-x. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka T, Izuwa S, Tanak K, Yamamoto D, Takimoto T, Matsuura F, Satouchi K. Eur J Biochem. 1999;263:189–194. doi: 10.1046/j.1432-1327.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- 36.Fields PG, Fleurat-Lessard F, Lavenseau L, Febvay G, Peypelut L, Bonnot G. J Insect Physiol. 1998;44:955–965. doi: 10.1016/s0022-1910(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 37.Misener SR, Chen C, Walker VK. J Insect Physiol. 2001;47:393–400. doi: 10.1016/s0022-1910(00)00141-4. [DOI] [PubMed] [Google Scholar]
- 38.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Squitti R, De Stefano ME, Edgar D, Toschi G. Neuroscience. 1999;91:707–722. doi: 10.1016/s0306-4522(98)00660-5. [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl MW. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray P, Edgar D. J Cell Biol. 2000;150:1215–1221. doi: 10.1083/jcb.150.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leibl MA, Ota T, Woodward MN, Kenny SE, Lloyd DA, Vaillant CR, Edgar DH. Gut. 1999;44:246–252. doi: 10.1136/gut.44.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JA, Cossins AR. Biochim Biophys Acta. 1990;1026:195–203. doi: 10.1016/0005-2736(90)90064-u. [DOI] [PubMed] [Google Scholar]
- 44.Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Genome Biol. 2001;2 doi: 10.1186/gb-2000-2-1-research0002. RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.