Abstract
Mechanical and physical features of the extracellular environment dramatically impact cell shape. Fibroblasts interacting with 3D relaxed collagen matrices appear much different from cells on 2D collagen-coated surfaces and form dendritic cell extensions that contain microtubule cores and actin-rich tips. We found that interfering with cellular microtubules caused cells in relaxed matrices to remain round and unable to form dendritic extensions, whereas fibroblasts on coverslips formed lamellipodial extensions and were spread completely without microtubules but were unable to become polarized. Fibroblasts in relaxed collagen matrices lack stress fibers, focal adhesions, and focal adhesion signaling. Fibroblasts on collagen-coated coverslips that were unable to develop stress fibers and focal adhesions, because of either adding blebbistatin to the cells or use of soft coverslips, also formed microtubule-dependent dendritic extensions. Conversely, fibroblasts interacting with precontracted collagen matrices developed stress fibers and lamellipodial extensions and required microtubules for polarization but not spreading. Our findings demonstrate an unexpected relationship between the role of microtubules in cell spreading and the tension state of cell–matrix interactions. At a low tension state (absence of stress fibers and focal adhesions) typical of fibroblasts in relaxed collagen matrices, cells spread with dendritic extensions whose formation requires microtubules; at a high tension state (stress fibers and focal adhesions) typical of cells on coverslips, cells spread with lamellipodial extensions and microtubules are required for cell polarization but not for spreading.
Keywords: adhesion, cell plasticity, cytoskeleton, extracellular matrix, mechanosignaling
Mechanistic explanations for the functions of hierarchical systems such as tissue cells require an understanding of cell shape and cell composition (1). Since the early days of cell culture, it has been clear that mechanical and physical features of the extracellular environment have a dramatic impact on cell shape. More than 80 years ago, Lewis and Lewis (2) observed mesenchymal cells on glass coverslips and reported that the cells were highly flattened with “tension striae” or stress fibers. Around the same time, Weiss (3) cultured mesenchymal cells in blood plasma clots and showed that cell shape varied from stellate to bipolar depending on the orientation of the fibrous network of the clot.
Subsequent work has confirmed differences in the shape of cells interacting with 2D rigid surfaces (glass or plastic) vs. 3D flexible matrices (e.g., collagen or fibrin) (4–6). Ironically, most research concerning regulation of cell shape has been carried out with 2D rigid surfaces although stress fibers can rarely be seen in fibroblasts in tissues except under conditions of wound repair and fibrosis (7, 8). On the other hand, the diversity of fibroblast shapes observed by Weiss (3) resembles the plasticity of cells in tissues (9–11).
Recently, it has become clear that differences between cell shape in 2D vs. 3D environments depend on specific regulatory mechanisms. For instance, 3D environments permit cell extensions to engage integrins on both dorsal and ventral cell surfaces simultaneously, which results in activation of unique signaling mechanisms (12), and matrix stiffness regulates cell migration differently in 3D environments vs. 2D surfaces (13). Moreover, 3D matrices permit cell extensions to become entangled with matrix fibrils, resulting in integrin-independent mechanical interactions that are not possible when cells attach to planar surfaces (14).
A striking feature of fibroblasts when they spread in 3D relaxed collagen matrices is their neuronal-like appearance, that is, formation of dendritic cell extensions that contain microtubule cores and actin-rich tips (15). Given the differences in microtubule organization of fibroblasts in 3D matrices vs. 2D surfaces and the observation that microtubule polymerization is required for neurite formation (16, 17), we wondered whether microtubules might play different roles in regulation of fibroblast shape in the 2D and 3D environments. To test this possibility, we analyzed changes in the shape of fibroblasts interacting with collagen-coated coverslips vs. collagen matrices in response to specific manipulation of microtubules and the actin cytoskeleton. Our findings demonstrate a relationship between the role of microtubules in cell spreading and the tension state of cell–matrix interactions. At a low tension state (absence of stress fibers and focal adhesions) typical of fibroblasts in relaxed collagen matrices, cells spread with dendritic extensions whose formation requires microtubules; at a high tension state (stress fibers and focal adhesions) typical of cells on coverslips, cells spread with lamellipodial extensions and microtubules are required for cell polarization but not spreading.
Results
Microtubules Are Required for Spreading of Fibroblasts Interacting with 3D Relaxed Collagen Matrices but Not 2D Collagen-Coated Coverslips.
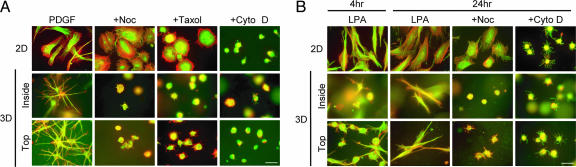
Using nocodazole and taxol to interfere with microtubules and cytochalasin D to interfere with the actin cytoskeleton, we compared the role of microtubules and the actin cytoskeleton in cell spreading in 3D vs. 2D environments. Spreading was visualized by fluorescence imaging of actin (red) and tubulin (green). In the 2D environment (Fig. 1A Top), interfering with microtubules (+Noc, +Taxol) altered cell shape but not cell spreading. That is, cells spread to the same extent based on projected cell surface area but had a round rather than elongated morphology. Interfering with microfilaments (+Cyto D), on the other hand, prevented cell spreading.
Fig. 1.
Interfering with microtubules prevents formation of dendritic extensions by fibroblasts inside or on top of relaxed collagen matrices. (A) Fibroblasts were incubated 4 h in medium containing 50 ng/ml PDGF and other additions as indicated after which samples were fixed and stained for microtubules (green) and actin (red). (Top) Cells incubated on collagen-coated coverslips spread in an elongated, flattened morphology. Nocodazole (Noc, 5 μM) or taxol (5 μM) inhibited cell polarization but not spreading; cytochalasin D (Cyto D, 10 μM) inhibited spreading. (Middle and Bottom) Cells incubated inside or on top of relaxed collagen matrices spread by formation of dendritic extensions with microtubule cores and actin-rich tips. Nocodazole, taxol, or cytochalasin D prevented formation of dendritic extensions. (B) The same as A except the medium contained LPA instead of PDGF and incubation times were adjusted to allow for reformation of dendritic extensions. (Top) Cells incubated on collagen-coated coverslips spread in an elongated, flattened morphology. Nocodazole (5 μM) decreased cell polarization but not spreading; cytochalasin D (10 μM) inhibited spreading. (Middle and Bottom) After 4–24 h, cells incubated inside or on top of relaxed collagen matrices form bipolar extensions. Nocodazole or cytochalasin D prevented formation of extensions. (Scale bar: 50 μm.)
Parallel experiments were carried out with fibroblast-collagen matrix cultures (Fig. 1A Middle and Bottom) that were prepared with cells either inside or on top of the matrix. As shown previously, fibroblasts formed dendritic extensions with microtubule cores and actin-rich tips regardless of whether the cells were completed surrounded by matrix or the cell bodies were resting on top of the matrix with cell extensions penetrating within (6, 14). Formation of dendritic extensions was completely blocked by interfering with microtubules and interfering with microfilaments. Dose–response studies showed that concentrations as low as ≈1–2 μM nocodazole or taxol inhibited dendritic extensions (data not shown).
The experiments in Fig. 1A were carried out in the presence of the physiological agonist platelet-derived growth factor (PDGF), which activates the small G protein Rac and stimulates the length and complexity of human fibroblast dendritic extensions, whereas the platelet-released agonist lysophosphatidic acid (LPA) activates the small G protein Rho and causes Rho kinase-dependent retraction of the extensions (15). Retraction is transient, however, and cells stimulated with LPA eventually reform extensions with bipolar morphology (18). Fig. 1B Middle and Bottom shows that fibroblasts interacting with relaxed collagen matrices in the presence of LPA were still mostly round after 4 h although some cells were beginning to develop bipolar extensions, whereas cells on collagen coverslips were completely spread. By 24 h, fibroblasts interacting with collagen matrices had developed bipolar morphology, which was prevented by interfering with microtubules or microfilaments. For cells on 2D surfaces in LPA-containing medium (Fig. 1B Top), interfering with microfilaments completely prevented spreading, and interfering with microtubules changed cell shape but not the extent of cell spreading.
Initial Spreading of Fibroblasts Occurs by Lamellipodial Extensions on 2D Collagen-Coated Coverslips but by Dendritic Extensions on 3D Relaxed Collagen Matrices.
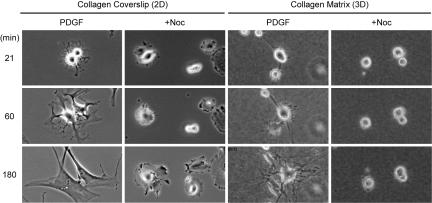
Dendritic extensions might have arisen by actin polymerization or extension and retraction of lamellipodia. To address this aspect of the problem, time-lapse microscopic studies were carried out. Fig. 2 shows representative images of cells in medium containing PDGF at various times up to 3 h, and the original films are available as supporting information (SI) Movies 1–4. On collagen-coated coverslips, cell protrusions rapidly developed into multiple lamellipodia that increased in size and merged. In the presence of nocodazole, lamellipodia occurred in a radial pattern around the cells followed by cell spreading into a round morphology. On relaxed collagen matrices, by contrast, cell protrusions developed into dendritic extensions, and lamellipodia were never observed. Also, marginal cell ruffling occurred in the presence of nocodazole, but stable extensions did not form. These findings indicated that dendritic extensions formed by actin polymerization and not by extension and retraction of lamellipodia.
Fig. 2.
Spreading of fibroblasts occurs by dendritic extensions on 3D relaxed collagen matrices but by lamellipodial extensions on 2D collagen-coated coverslips. Details are the same as in Fig. 1A except time-lapse images were collected every 3 min. Three to five films were made for each condition, and representative fields are shown for each condition at the times indicated. On collagen-coated coverslips, cell protrusions rapidly developed into multiple lamellipodia that increased in size and merged. With nocodazole added the lamellipodia protruded radially and cells spread into a round morphology. On relaxed collagen matrices, cell extensions maintained their dendritic character and lamellipodia were never observed. In the presence of nocodazole, stable extensions did not form.
Tension State of Cell–Matrix Interactions.
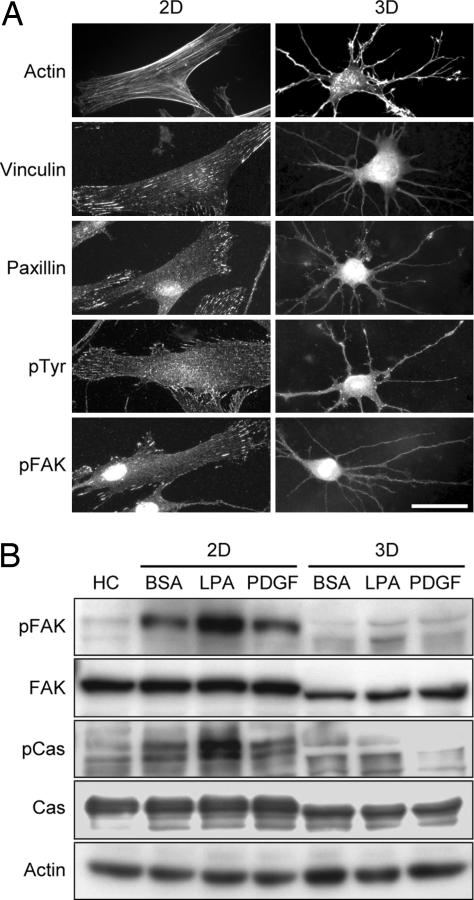
Fibroblasts interacting with 2D rigid surfaces typically develop tension as shown by the formation of stress fibers and focal adhesions (19–21). Fig. 3A shows that after 4 h, human fibroblasts on collagen coverslips (2D) had stress fibers and focal adhesion streaks containing vinculin, paxillin, phospho-tyrosine (pTyr), and phospho-focal adhesion kinase (pFAK). Fibroblasts in relaxed collagen matrices (3D), on the other hand, lacked stress fibers, and conventional focal adhesion proteins did not cluster in streaks along or at the tips of dendritic extensions. These differences were reflected in cell signaling behavior. Fig. 3B shows that with freshly harvested fibroblasts (HC) little phosphorylation could be detected in focal adhesion-associated proteins FAK or p130Cas, but FAK and p130Cas phosphorylation occurred in fibroblasts incubated on collagen-coated coverslips (2D) under control (BSA) or agonist stimulated (PDGF, LPA) conditions. Little phosphorylation of FAK or p130Cas occurred, however, when cells interacted with relaxed collagen matrices (3D).
Fig. 3.
Fibroblasts interacting with relaxed collagen matrices lack stress fibers, focal adhesion structures, and focal adhesion signaling. (A) Fibroblasts were incubated 4 h in medium containing 50 ng/ml PDGF on collagen-coated coverslips (2D; Left) or on top of relaxed collagen matrices (3D; Right) after which samples were fixed and stained for actin or focal adhesion proteins. Cells on coverslips but not matrices formed stress fibers and focal adhesions containing vinculin, paxillin, pTyr, and pFAK. (Scale bar: 50 μm.) (B) Fibroblasts were incubated 4 h in medium with or without 50 ng/ml PDGF or 10 μM LPA as indicated on collagen-coated coverslips (2D; Left) or inside relaxed collagen matrices (3D; Right) after which samples were extracted and subjected to immunoblotting for pFAK, FAK, p130Cas (Cas), phospo-Cas (pCas), and actin. Compared with freshly harvested cells (HC), fibroblasts incubated on coverslips but not within relaxed collagen matrices showed FAK and Cas activation (phosphorylation).
Contractile Tension Modulates Formation of Cell Extensions.
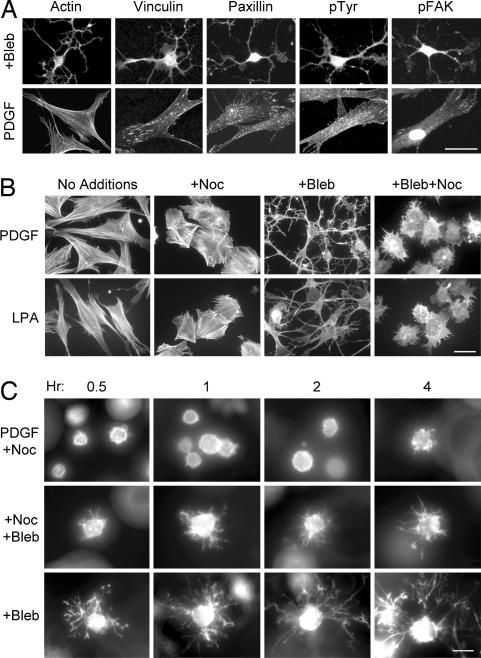
The foregoing observations led us to speculate that the role of microtubules in cell spreading might be modulated according to the tension state of cell–matrix interactions. To examine this possibility further, experiments were carried out with the myosin II inhibitor blebbistatin, which inhibits actin–myosin contractility (22). Fig. 4A shows that fibroblasts on collagen-coated coverslips no longer were able to form stress fibers or focal adhesions in the presence of blebbistatin and, under this low tension state, cells formed dendritic extensions. Time-lapse microscopic studies (SI Movie 5) showed that the development of dendritic extensions by fibroblasts on collagen coverslips in the presence of blebbistatin appeared similar to control cells interacting with relaxed collagen matrices. In addition, Fig. 4B shows that formation of these blebbistatin-induced dendritic extensions could be prevented by interfering with microtubules (+Bleb +Noc).
Fig. 4.
Blocking myosin II activity modulates microtubule dependence of fibroblast spreading. (A) Fibroblasts were incubated 4 h on collagen-coated coverslips in medium containing 50 ng/ml PDGF with 20 μM blebbistatin (Upper) or without blebbistatin (Lower) after which samples were fixed and stained for actin or focal adhesion proteins. In the presence of blebbistatin, cells lacked stress fibers or focal adhesions and developed dendritic extensions. (B) Fibroblasts were incubated 4 h on collagen-coated coverslips in medium containing 50 ng/ml PDGF (Upper) or 10 μM LPA (Lower) with 5 μM nocodazole and 20 μM blebbistatin. Nocodazole prevented blebbistatin-treated cells from forming dendritic extensions, and cells appeared round with short protrusions. (C) Fibroblasts were incubated within relaxed collagen matrices in DMEM/BSA containing 50 ng/ml PDGF, 20 μM blebbistatin (Bleb), and 5 μM nocodazole (Noc) as indicated. After 1 h, blebbistatin was added to half the nocodazole-treated samples. At the subsequent times indicated, samples were fixed and stained for actin. With blebbistatin treatment alone, cells developed dendritic extensions and after blebbistatin was added to nocodazole-treated cells, short protrusions developed, which were similar to those seen in B. (Scale bars: 30 μm, A and C; 50 μm, B.)
In other experiments, we found that addition of blebbistatin did not prevent formation of dendritic extensions by fibroblasts interacting with collagen matrices, but addition of blebbistatin to nocodazole-treated cells caused cells to form short protrusions (Fig. 4C). As a result, fibroblasts in relaxed collagen matrices or on collagen-coated coverslips developed a similar appearance in the presence of both nocodazole and blebbistatin.
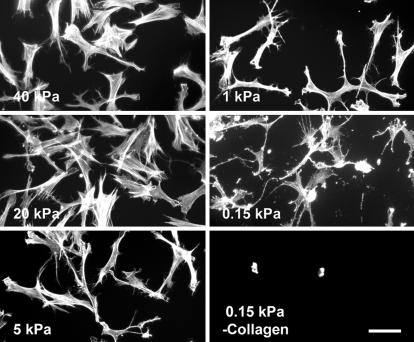
Studies also were carried out with collagen-coated, soft polyacrylamide gels on which the tension state of cell–matrix interactions is reduced (23). Collagen acrylamide gels were prepared with ratios of acrylamide and bisacrylamide previously shown to result in matrix stiffness ranging from 0.15 to 40 kPa as measured by atomic force microscopy (24). Fig. 5 shows that on softer gels fibroblasts developed fewer stress fibers and appeared more dendritic. Stiffness can vary somewhat depending on gel thickness and bonding to the underlying glass support and some variability occurred from experiment to experiment with the softer gels but, overall, the appearance of dendritic extensions correlated with decreased gel stiffness. The above experiments were carried out in medium containing PDGF. With LPA as the agonist, fibroblasts were still mostly round on collagen-coated acrylamide gels after 4 h (data not shown), as was the case for cells interacting with relaxed collagen matrices (Fig. 1B).
Fig. 5.
Fibroblasts on collagen-coated, soft polyacrylamide gels develop dendritic extensions. Fibroblasts were incubated 4 h in DMEM/BSA containing 50 ng/ml PDGF on polyacrylamide gels that had been prepared with ratios of acrylamide and bisacrylamide to produce the gel stiffness indicated and coated with or without collagen. At the end of the incubations, samples were fixed and stained for actin. On softer gels, cells formed dendritic extensions. (Scale bar: 100 μm.)
Collagen Matrix Precontraction Modulates the Function of Microtubules in Fibroblast Spreading.
As fibroblasts remodel and contract collagen matrices, cells change from dendritic to stellate/bipolar morphology and tension state increases as shown by the appearance of stress fibers and focal adhesions (18). If the role of microtubules in cell spreading was modulated according to cell-matrix tension, then fibroblasts interacting with remodeled collagen matrices should have become less dependent on microtubules for cell spreading.
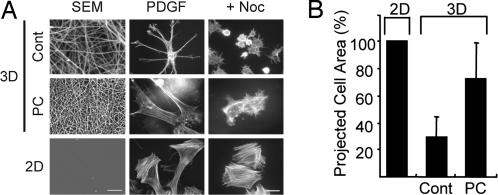
We tested the above possibility by using collagen matrices that were precontracted. Fig. 6Left shows the surfaces of the control (Cont) and precontracted (PC) matrices (3D) and collagen-coated coverslips (2D). Packing of the collagen fibrils in the precontracted matrices was much tighter compared with controls. Fibroblasts interacting with precontracted matrices developed prominent stress fibers and appeared more similar to cells on collagen-coated coverslips than to cells on relaxed matrices. Although interfering with microtubules still decreased the extent cell spreading somewhat, the projected surface area of nocodazole-treated fibroblasts achieved ≈75% of nocodazole-treated cells on coverslips (Fig. 6B). Therefore, when fibroblasts interacted with precontracted collagen matrices, microtubules became less important for cell spreading and more important for cell polarization.
Fig. 6.
Fibroblasts interacting with precontracted (PC) collagen matrices show decreased microtubule requirement for cell spreading. Floating collagen matrices were precontracted for 12 h after which cells within the matrices were removed by detergent extraction and the matrices were centrifuged onto the surfaces of coverslips. SEM showed tight collagen fibril packing of precontracted matrices vs. control (Cont) matrices. Fibroblasts were incubated on collagen-coated coverslips or collagen matrices for 4 h in DMEM/BSA containing 50 ng/ml PDGF. Fibroblasts on precontracted floating collagen matrices appeared less dendritic, had actin stress fibers similar to cells on collagen-coated coverslips, and were able to spread but not polarize in the presence of 5 μM nocodazole. (Scale bars: 10 μm, Left; 50 μm, Center and Right.) (B) Nocodazole-treated cells in A were photographed (≈25 cells per condition), and projected cell surface areas were measured by using MetaView. Data shown are averages and SDs normalized to 2D spreading (2D absolute value = 2,578 ± 842 μm2). Values were compared by using Student's t test and differed with P < 0.002 between each pair of samples. Cell spreading in the presence of nocodazole was ≈75% of cells on coverslips.
Discussion
Mechanical and physical features of the extracellular environment dramatically affect cell shape (2–6). Recently it has become clear that differences between cell shape in 2D vs. 3D environments depend on specific regulatory mechanisms. For instance, 3D environments permit cell extensions to engage integrins on both dorsal and ventral cell surfaces simultaneously, which results in activation of unique signaling mechanisms (12). Moreover, 3D matrices permit cell extensions to become entangled with matrix fibrils resulting in integrin-independent mechanical interactions that are not possible when cells attach to planar surfaces (14).
Fibroblasts in 3D relaxed collagen matrices appear much different from cells on 2D surfaces and have dendritic extensions that contain microtubule cores and actin-rich tips (15). The current studies were carried out to learn whether microtubules might play different roles in regulating cell form in the 2D and 3D environments. Interfering with cellular microtubules caused cells in relaxed collagen matrices to remain round and unable to form dendritic extensions, whereas fibroblasts on coverslips formed lamellipodial extensions and spread completely without microtubules but did not become polarized.
Either nocodazole or taxol blocked formation of fibroblast dendritic extensions in relaxed collagen matrices, which was important because nocodazole might have interfered with extensions by stimulating fibroblast contraction (25, 26). Taxol, on the other hand, can block microtubule dynamics (27, 28) without simultaneously increasing cell contraction (25, 26). Also, microtubules were required for fibroblast spreading in relaxed collagen matrices regardless of whether cells were stimulated with PDGF or LPA. Both agonists stimulate human fibroblast ruffling sufficient for collagen matrix remodeling (29) but have much different short-term effects on dendritic cell extensions. PDGF increases the length and complexity of the extensions, whereas LPA causes short-term retraction of the extensions followed by their reformation in a bipolar morphology (15, 18).
Fibroblasts could have interacted differently with collagen-coated coverslips vs. relaxed collagen matrices for several reasons: coverslips are stiffer than matrices; adhesion sites are organized differently, adhesion fields on coverslips vs. linear arrays on matrix fibrils; adhesion site density is likely different between collagen-coated coverslips vs. matrices; and cell processes can penetrate into matrices but not coverslips. Because fibroblasts within or on top of relaxed collagen matrices developed dendritic extensions, formation of the extensions appeared to be a characteristic of the cell-collagen matrix interface that does not require the entire cell to be surrounded by matrix.
Unlike cells on collagen coverslips, fibroblasts interacting with relaxed collagen matrices did not develop stress fibers, focal adhesions, and focal adhesion signaling. Studies with the myosin II inhibitor blebbistatin showed that when cells no longer were able to develop a high tension state they spread by formation of microtubule-dependent dendritic extensions similar to control cells interacting with relaxed collagen matrices. Moreover, fibroblasts also formed dendritic extensions on collagen-coated, soft polyacrylamide gels. Conversely, when fibroblasts interacted with precontracted collagen matrices on which the cells could develop prominent stress fibers, then the cells appeared more like fibroblasts on collagen-coated coverslips and became more dependent on microtubules for polarization than spreading. Taken together, these findings suggest that the tension state of the cell–matrix interactions is the key feature that determines the role of microtubules in formation of cell extensions and not the organization or concentration of collagen adhesion sites or penetration of cells into the matrix.
According to the tensegrity hypothesis of cell shape (30), microtubules act as noncompressive structures to resist contractile tension of the actin cytoskeleton. Therefore, for cells in 3D matrices, microtubules might have been necessary for formation of dendritic extensions to resist contractile tension, whereas cells on 2D surfaces could transfer contractile tension to the noncompressive rigid culture surface. This explanation did not appear to be the case, however, because blocking contractile tension failed to make cells in matrices less dependent on microtubules for cell spreading. Indeed, blocking contractile tension of cells on coverslips caused fibroblasts to form dendritic extensions that were microtubule-dependent.
Rather than tensegrity, our findings are consistent with the clutch hypothesis (31, 32). Fibroblasts treated with nocodazole and blebbistatin developed short cell protrusions in relaxed collagen matrices or on collagen-coated coverslips as if under these conditions actin polymerization and depolymerization were balanced (33, 34), and different mechanisms were required to achieve further actin polymerization and formation of cell extensions depending on the tension state of cell–matrix interactions. At a high tension state (stress fibers and focal adhesions) the clutch would be external, e.g., adhesion-dependent mechanisms to stimulate actin polymerization and cell spreading (35–39) and microtubules to determine cell polarity (40–42). At a low tension state (absence of stress fibers and focal adhesions) the clutch would be internal, that is, microtubule-dependent mechanisms such as Rac1 activation (43, 44) to stimulate further actin polymerization and cell spreading. Differences in the clutch mechanism may explain why fibroblast protrusions become lamellipodia on collagen coverslips but dendritic with collagen matrices and also may be related to the two distinct actin networks observed in epithelial protrusion, only one of which is coupled to actin–myosin contraction (45, 46).
Fibroblasts in connective tissues are highly plastic cells. Under resting conditions, they are organized in a dendritic network (9–11). Stretching the tissue causes dendritic fibroblasts to become more stellate (10), whereas aging and sun damage causes the cells to lose their normal mechanical interactions (47). During wound repair and fibrotic disease, fibroblasts increasingly develop tension as indicated by the formation of actin stress fibers and fibronexus junctions and switch from a resting to a proliferative, biosynthetically active and contractile phenotype (8, 48). The mechanisms by which cells regulate their form in the 3D environment are only beginning to be understood. Our findings demonstrate that the role of microtubules in cell spreading is modulated according to the tension state of cell–matrix interactions, and that at a low tension state microtubules are required for actin-dependent formation of dendritic cell extensions. We suggest that the ability of fibroblasts to modulate microtubule function according to the tension state of cell–matrix interactions is an important determinant of cell plasticity in tissues.
Methods
Cells, Collagen Matrix Cultures, and Polyacrylamide Gels.
Early passage human foreskin fibroblasts (immortalized with human telomerase reverse transcriptase and selected with 5 μg/ml blastocidin) were cultured in DMEM supplemented with 10% FBS. Cell culture and experimental incubations were carried out at 37°C in a 5% CO2 humidified incubator. Experimental incubation medium was DMEM containing 5 mg/ml BSA (fatty acid free) with PDGF (Upstate Biotechnology, Lake Placid, NY), LPA (Sigma, St. Louis, MO), nocodazole (Sigma), cytochalasin D (Sigma), taxol (Cytoskeleton, Denver), and blebbistatin (Toronto Research Chemicals, Downsview, ON, Canada) added as indicated in the figure legends.
Methods for preparing collagen matrix cultures (Vitrogen 100 type I collagen, 1.5 mg/ml; Angiotech Biomaterials, Palo Alto, CA) have been described (15, 18). Cells were added to the collagen solution and polymerized inside the matrices (2 × 104 cells per matrix) or seeded on top of collagen matrices after polymerization (104 cells per matrix), or 104/ml was incubated on glass coverslips that had been coated 15 min at 37°C with Vitrogen 100 type I collagen (50 μg/ml in DMEM).
For precontraction experiments, collagen matrices containing 2 × 105 cells per matrix were incubated 12 h floating in culture medium. Cells were removed by treating the matrices with 0.5% sodium deoxycholate as described (49). Precontracted matrices were collected by centrifugation onto coverslips at 22°C for 2 h at 693 × g in a GLC-2B centrifuge (Sorvall, Newton, CT) using a modified HL-4 rotor (49).
Thin polyacrylamide gels ranging from 3% acrylamide/0.03% bis to 8% acrylamide/0.48% bis were prepared and bonded to 3-aminopropyltriethoxy-silane (Sigma)-treated 12-mm glass coverslips, treated with 0.5 mg/ml of sulfo-sulfosuccinimidyl-6-[4′-azido-2′-nitrophenylamino] hexanoate (Pierce, Rockford, IL) and activated with 365 nm UV light as described (23, 24). Activated gels were coated with either 1 mg/ml of heat-denatured BSA or 50 μg/ml of neutralized collagen solution overnight at 4°C. The gels were then washed with PBS extensively and equilibrated in DMEM.
Immunostaining, Immunoblotting, and Time-Lapse and Scanning Electron Microscopy.
Immunostaining of matrices was carried out as described (14, 15, 18, 29). Primary antibodies used were against β-tubulin (Sigma), vinculin (Sigma), paxillin (BD Transduction Laboratories, Franklin Lakes, NJ), pTyr (4G10, Upstate Biotechnology), and pFAK (Biosource International, Camarillo, CA). For actin staining, we used Alexa Fluor 594-conjugated phalloidin (Molecular Probes, Carlsbad, CA). Images were collected at 22°C with an Elipse 400 fluorescent microscope (Nikon, Tokyo, Japan) using ×10/0.45, ×20/0.75, and ×40/0.75 Plan Apo infinity-corrected objectives (Nikon), a SenSys camera (Photometrics, Tucson, AZ), and MetaView acquisition software. Subsequent image processing was carried out with Photoshop 5.5 or 7.0 (Adobe, San Jose, CA). Time-lapse microscopy was carried out with glass bottom culture dishes (MatTek, Ashland, MA) placed in a 37°C environment chamber. Images were collected every 3 min for 4 h with a Axiovert 200M inverted microscope (Zeiss, Thornwood, NY) using a ×10/0.25 Achroplan objective (Zeiss), DXM1200F digital camera (Nikon), and Metamorph acquisition software.
Immunoblotting analyses were carried out as described (15, 29). Primary antibodies used were anti-actin (Sigma), anti-phospho-p130Cas (BD Transduction Laboratories), anti-p130Cas (Cell Signaling Technology, Beverly, MA), anti-FAK (BD Transduction Laboratories), and anti-pFAK antibodies. Secondary antibodies were HRP-conjugated goat-anti-rabbit or anti-mouse (ICN Biomedicals, Irvine, CA).
Preparation and analysis of samples by scanning electron microscopic analysis was carried out as described (14). Specimens were viewed and photographed with a 840A scanning electron microscope (JEOL, Tokyo, Japan).
Supplementary Material
Acknowledgments
We thank Dr. Woodring Wright for helping to prepare human telomerase reverse transcriptase-immortalized human foreskin fibroblasts; Drs. Yu-li Wang and Florian Rehfeldt for advice regarding polyacrylamide gels; and Drs. Matthew Petroll and William Snell for helpful discussions. Scanning electron microscopy was carried out with the assistance of the University of Texas Southwestern Molecular and Cellular Imaging Facility. This research was supported by National Institutes of Health Grant GM31321.
Abbreviations
- LPA
lysophosphatidic acid
- pTyr
phospho-tyrosine
- pFAK
phospho-focal adhesion kinase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608030104/DC1.
References
- 1.Polanyi M. Science. 1968;160:1308–1312. doi: 10.1126/science.160.3834.1308. [DOI] [PubMed] [Google Scholar]
- 2.Lewis WH, Lewis MR. In: General Cytology. Cowdry EV, editor. Chicago: Univ Chicago Press; 1924. pp. 384–447. [Google Scholar]
- 3.Weiss P. Rev Mod Phys. 1959;31:11–20. [Google Scholar]
- 4.Elsdale T, Bard J. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cukierman E, Pankov R, Yamada KM. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 6.Grinnell F. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 7.Byers HR, White GE, Fujiwara K. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- 8.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 9.Salomon D, Saurat JH, Meda P. J Clin Invest. 1988;82:248–254. doi: 10.1172/JCI113578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK. Am J Physiol. 2005;288:C747–C756. doi: 10.1152/ajpcell.00420.2004. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 12.Beningo KA, Dembo M, Wang YL. Proc Natl Acad Sci USA. 2004;101:18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaman MH, Trapani LM, Siemeski A, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Proc Natl Acad Sci USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Grinnell F. Mol Biol Cell. 2005;16:5070–5076. doi: 10.1091/mbc.E05-01-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Mol Biol Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keith CH. Cell Motil Cytoskeleton. 1990;17:95–105. doi: 10.1002/cm.970170205. [DOI] [PubMed] [Google Scholar]
- 17.Daniels MP. J Cell Biol. 1972;53:164–176. doi: 10.1083/jcb.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamariz E, Grinnell F. Mol Biol Cell. 2002;13:3915–3929. doi: 10.1091/mbc.E02-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 20.Bershadsky AD, Balaban NQ, Geiger B. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 21.Giannone G, Sheetz MP. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 23.Guo WH, Frey MT, Burnham NA, Wang YL. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Kolodney MS, Elson EL. Proc Natl Acad Sci USA. 1995;92:10252–10256. doi: 10.1073/pnas.92.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown RA, Talas G, Porter RA, McGrouther DA, Eastwood M. J Cell Physiol. 1996;169:439–447. doi: 10.1002/(SICI)1097-4652(199612)169:3<439::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Mikhailov A, Gundersen GG. Cell Motil Cytoskeleton. 1998;41:325–340. doi: 10.1002/(SICI)1097-0169(1998)41:4<325::AID-CM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Liao G, Nagasaki T, Gundersen GG. J Cell Sci. 1995;108:3473–3483. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- 29.Rhee S, Grinnell F. J Cell Biol. 2006;172:423–432. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingber DE. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 31.Jay DG. J Neurobiol. 2000;44:114–125. doi: 10.1002/1097-4695(200008)44:2<114::aid-neu3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Mitchison T, Kirschner M. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 33.Pollard TD, Blanchoin L, Mullins RD. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 34.Borisy GG, Svitkina TM. Curr Opin Cell Biol. 2000;12:104–112. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 35.Kodama A, Lechler T, Fuchs E. J Cell Biol. 2004;167:203–207. doi: 10.1083/jcb.200408047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox EA, Sastry SK, Huttenlocher A. Mol Biol Cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burridge K, Wennerberg K. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 38.Raftopoulou M, Hall A. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Fukata M, Nakagawa M, Kaibuchi K. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 40.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Proc Natl Acad Sci USA. 2002;99:10452–10457. doi: 10.1073/pnas.152339899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small JV, Kaverina I. Curr Opin Cell Biol. 2003;15:40–47. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Noritake J, Kaibuchi K. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 45.Danuser G. Biochem Soc Trans. 2005;33:1250–1253. doi: 10.1042/BST0331250. [DOI] [PubMed] [Google Scholar]
- 46.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 47.Varani J, Schuger L, Dame MK, Leonard C, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. J Invest Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- 48.Desmouliere A, Darby IA, Gabbiani G. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 49.Guidry C, Grinnell F. Collagen Relat Res. 1987;6:515–529. doi: 10.1016/s0174-173x(87)80050-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.