Abstract
Many animals recognize the alarm calls produced by other species, but the amount of information they glean from these eavesdropped signals is unknown. We previously showed that black-capped chickadees (Poecile atricapillus) have a sophisticated alarm call system in which they encode complex information about the size and risk of potential predators in variations of a single type of mobbing alarm call. Here we show experimentally that red-breasted nuthatches (Sitta canadensis) respond appropriately to subtle variations of these heterospecific “chick-a-dee” alarm calls, thereby evidencing that they have gained important information about potential predators in their environment. This study demonstrates a previously unsuspected level of discrimination in intertaxon eavesdropping.
Keywords: eavesdropping, heterospecific recognition, interspecific communication
Many animals produce alarm signals when they encounter predators. These signals typically are directed at certain conspecific receivers, such as kin or mates, yet they are often conspicuous, allowing other animals in the environment the opportunity for eavesdropping. There is a long history in the use of signals by third-party receivers and a growing recognition that animal communication networks can be extremely complex, consisting of many signalers and many receivers (1–8). If an individual intercepts information from the alarm calls directed toward another individual, this is considered “interceptive eavesdropping.” (9) This differs from “social eavesdropping,” (3) which involves obtaining information from an interaction between two signalers (9). Alarm signals contain some of the most pertinent information an animal can learn about its environment: the presence of a predator. Thus, an animal that is able to glean information by eavesdropping on the alarm signals of another species may obtain considerable, potentially even life-saving, information. There is growing evidence that alarm signals are recognized and used not only by conspecifics but also by other species that have shared or similar predators. Numerous studies have shown that mammals, birds, amphibians, and fish recognize alarm signals of other species (10–14), and interceptive eavesdropping can even occur between taxa that are not closely related. For example, vervet monkeys (Cercopithecus aethiops) respond to the alarm calls of superb starlings (Spreo superbus) (15), dwarf mongooses (Helogale parvula) recognize hornbill (Tockus spp.) alarm calls (16), and red squirrels (Sciurus vulgaris) respond to the alarm calls of jays (Garrulus glandarius) (17).
Instead of using a general type of alarm call, some species have categorically distinct vocalizations that are each associated with a different type of predator encounter (e.g., different calls that refer to aerial vs. terrestrial predators) (18). There are a few studies that show that birds and mammals discriminate among categorically distinct types of heterospecific vocalizations. For example, the closely related Diana monkeys (Cercopithecus diana) and Campbell's monkeys (Cercopithecus campbelli) recognize each other's acoustically distinct leopard and eagle alarm calls and treat them similarly to conspecific alarm calls (19). A sympatric bird, the yellow-casqued hornbill (Ceratogymna elata), also differentiates between these two types of monkey alarm calls (20). Similarly, vervet monkeys discriminate among the aerial and terrestrial predator alarm calls of superb starlings (15).
Instead of or in addition to using acoustically distinct vocalizations, some animals encode considerable information about predators in more subtle variations of a single type of call. Black-capped chickadees (Poecile atricapillus) transmit complex information about the size of potential predators in variations of their mobbing alarm call (21). When chickadees discover a perched raptor or terrestrial predator, they produce a “chick-a-dee” alarm call. Unlike the high-frequency “seet” alarm calls that are produced by many bird species in response to flying raptors and that cause other individuals to dive for cover or become immobile, mobbing alarm calls are produced in response to perched raptors or terrestrial predators. Unlike seet alarm calls, mobbing calls typically cause flock mates to fly toward the caller, looking for the nearby perched predator to mob (22). It may seem counterintuitive, but when mobbing perched raptors, birds tend to approach more dangerous stationary predators more closely than less dangerous ones. Mobbing behavior may have several different functions (23), but one of the most important is that the mobbing is likely to harass the predator enough to drive it from the area so that it does not surprise the birds later. The chickadee is one of the principal sentinels in mixed-species winter flocks in North America, and 24–50 different species are known to respond to its chick-a-dee mobbing alarm calls (24, 25).
We recently showed that chick-a-dee alarm calls encode a surprising amount of information about the size and threat of a given predator through variations in the acoustic structure of the calls (21), demonstrating that chickadees have one of the most sophisticated alarm call systems yet discovered. In comparison with larger raptors, small raptors have greater maneuverability (26) and are more likely to prey heavily on small species of birds (27, 28). Thus, small raptors should pose more of a threat than large raptors to small birds such as chickadees. Because chickadees encode information about the size of raptors in their mobbing calls, other potential prey species attentive to the content of these vocalizations could gain critical information about the relative danger posed by a nearby predator.
Although it has been shown that animals respond to categorically different types of heterospecific alarm signals, the ability to decode predator information from variations within a single type of call of another species has not been investigated. We experimentally tested whether red-breasted nuthatches (Sitta canadensis) could discriminate among different variations of the chick-a-dee call that were recorded in response to small (high threat) or large (low threat) predators (Fig. 1). Nuthatches cooccur with chickadees throughout much of the northern United States and Canada and are commonly found together in mixed-species flocks with black-capped chickadees, especially in winter. In some areas, it has been estimated that more than half of the individual red-breasted nuthatches join chickadee flocks (22, 29). We recorded chickadee alarm calls in controlled predator encounters with a live great horned owl, Bubo virginianus (large, low threat), and a northern pygmy owl, Glaucidium gnoma (small, high threat), and we used a contact call of a house sparrow (Passer domesticus) as a control. These calls were played back to pairs of wild, free-living nuthatches in the field from a speaker hidden at the bottom of a tree. To ensure that the nuthatches responded only to the broadcast stimuli rather than the behavior of chickadees that might also respond to the playbacks, we conducted playback trials only when chickadee flocks were not present. We predicted that nuthatches should respond more strongly to the mobbing calls that denote small predators, which would pose considerably greater danger (21, 29).
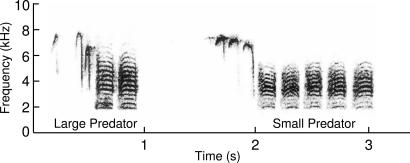
Fig. 1.
Chickadee alarm calls encode information about predator size and risk. The figure shows two chick-a-dee calls from the same individual chickadee: The first call was recorded in response to a large, low-threat, great horned owl, and the second was in response to a small, high-threat, northern pygmy owl. These signals vary in a number of acoustic features, including the calling rate, the number of broadband terminal D syllables in the call, and subtle variations in acoustic features related to the frequency and timing of the call (see ref. 20 for more quantitative details on the acoustic differences).
Results
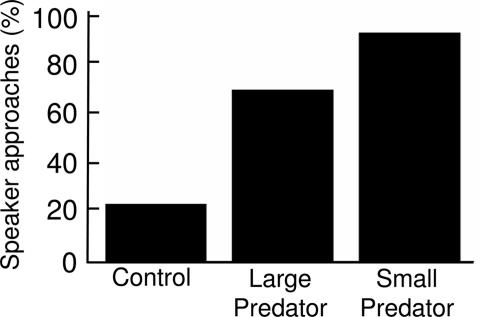
Although nuthatches responded to both variations of chick-a-dee alarm call, they responded with much stronger mobbing behavior to playback of the small-predator alarm calls. Nuthatches consistently approached the speaker during the small-predator chick-a-dee playback (92% of trials), frequently approached it during the large-predator chick-a-dee trials (69%), and infrequently approached it during control trials (23%) (Cochran's test: n = 13, C = 9.80, P = 0.003; all statistics reported are from one-tailed tests) (Fig. 2). Both the large-predator and small-predator alarm call treatments were significantly different from those of the control trials (McNemar's test: n = 13, P = 0.019 and P = 0.006, respectively). Nuthatches were more likely to approach the speaker during the small-predator than large-predator playback trials, although this difference was not statistically significant (McNemar's test: n = 20, P = 0.062).
Fig. 2.
Nuthatch approach behavior in response to different alarm call variants: chick-a-dee calls denoting large and small predators and control calls from a house sparrow (Cochran's test: P = 0.003). Each of the three playback treatments was standardized for length and amplitude (≈75 dB at 1 m).
Further examination of the behavior of the birds approaching the speaker during the alarm call playbacks revealed that nuthatches moved closer to the tree with the hidden speaker during the small-predator trials (median and semiinterquartile range showing 25% and 75% of the data: 0 m, 0–0 m) than large-predator (0 m, 0–13.8 m) or control trials (15 m, 7.5–15 m) (Kendall's W: n = 13, W = 0.480, P = 0.001). Post hoc analyses revealed significant differences between the two predator treatments [Wilcoxon signed ranked test (WSR): n = 20, Z = −2.410, P = 0.008] and between the small predator and control treatments (WSR: n = 13, Z = −2.970). Birds tended to move closer to the speaker during the large-predator playback trials than control trials, although this difference was not statistically significant (WSR: n = 13, Z = −1.590, P = 0.061).
Not only did they approach closer during small-predator trials, but nuthatches also were more likely to fly to the tree with the speaker for those trials (95% for small-predator trials vs. 55% of large-predator trials and 7.9% of control trials; Cochran's test: n = 13, P = 0.00018; McNemar's test: P < 0.01 for all pairwise comparisons).
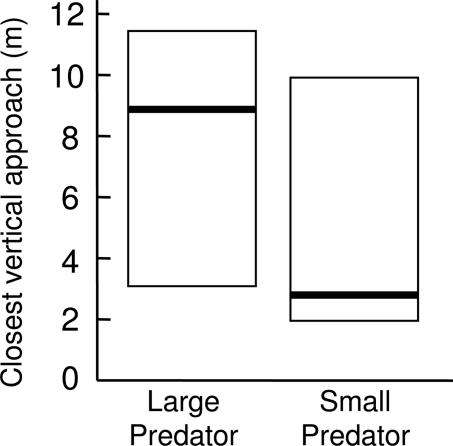
Of the birds that flew to the tree with the speaker, nuthatches moved farther down the tree vertically toward the speaker during the small-predator playback (3 m, 2–10 m) than the large-predator playback (9 m, 3–12 m; WSR: n = 12, Z = −2.493, P = 0.006) (Fig. 3). There was also no significant difference in the latency to fly to the tree with the hidden speaker (small-predator playback: 24 sec, 15–39 sec; large-predator playback: 88 sec, 32–100 sec; WSR: n = 20, Z = −1.423, P = 0.078).
Fig. 3.
Closest vertical-approach distance to the speaker varies with alarm call treatment for nuthatches that landed in the tree above the playback speaker (WSR: P = 0.006). Bars represent medians, and boxes delineate semiinterquartile ranges (25 and 75% of the data).
More birds were attracted to the playback of the predator alarm calls (small-predator calls: 2, 1–2.7 birds; large-predator calls: 2, 2–3 birds) than the control trials (0, 0–1 birds; Kendall's test: n = 13, W = 0.387, P = 0.004). There was no difference between the two alarm call treatments (WSR: n = 20, Z = −0.498, P = 0.31), although both treatments differed from the control playbacks (WSR: n = 13, P < 0.03 for both pairwise comparisons).
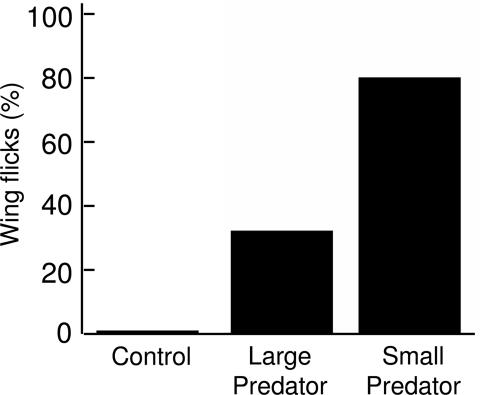
In addition to differentially approaching the speaker, other behaviors of nuthatches also varied between the playback treatments. We found a strong difference in the likelihood of nuthatches to exhibit wing-flick displays, which are associated with high levels of agitation (29) (Cochran's test: n = 13, C = 14.0, P = 0.0004) (Fig. 4). Nuthatches exhibited more wing flicks during playback of the large-predator alarm calls (35% of trials) than during the control trials (0%), although this difference was not significant (McNemar's test: n = 12, P = 0.062). The small-predator treatment elicited considerably more wing-flick displays by the nuthatches (79% of trials) than either of the other two treatments (McNemar's test: P = 0.002 for both).
Fig. 4.
Percentage of trials in which nuthatches exhibited wing-flick behavior after the playback of large-predator and small-predator chick-a-dee alarm calls and control trials (Cochran's test: P = 0.0004).
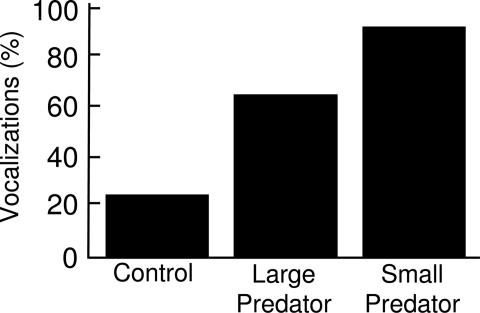
Nuthatches were also more likely to vocalize when chick-a-dee alarm calls were broadcasts than during control trials (Cochran's test: C = 14.2, P = 0.0004) (Fig. 5). Nuthatches vocalized during 91% of small-predator alarm call playbacks and 65% of large-predator alarm call playbacks, compared with only 25% of control trials. The large-predator alarm call treatments (McNemar's test: n = 13, P = 0.008) and small-predator alarm call treatments (McNemar's test: n = 13; P = 0.004) were significantly different from the control treatment, but the difference between the two alarm call treatments was not statistically significant (McNemar's test: n = 20, P = 0.062).
Fig. 5.
Percentage of nuthatches that vocalized after the playback of control calls from a house sparrow and large-predator and small-predator chick-a-dee alarm calls (Cochran's test: P = 0.0004).
The amount of time nuthatches spent mobbing the playback speaker varied considerable among treatments (Kendall's test: n = 13, W = 0.720, P = 0.00004). Nuthatches spent longer mobbing the speaker during the alarm call playbacks than during the control trials (WSR: P < 0.004 for both pair-wise comparisons). In addition, nuthatches hearing small-predator alarm calls spent considerably longer mobbing the speaker before returning to normal foraging behavior than those hearing large-predator playback (control: 0 sec, 0–0 sec; small-predator playbacks: 210 sec, 130–368 sec; large-predator playbacks: 83 sec, 13–170 sec; WSR: n = 20, Z = −2.536, P = 0.0005).
Discussion
Nuthatches respond to playback of chick-a-dee alarm calls by approaching the speaker, showing that they recognize this heterospecific mobbing signal. Because nuthatches (family Sittidae) are not closely related to chickadees (family Paridae), this is an interesting example of interspecific interceptive eavesdropping. Moreover, nuthatches discriminate between subtle differences in chickadee alarm calls that contain information about the size of potential predators. The data in this study show that animals can make sophisticated antipredator decisions from information contained in variations within a single type of heterospecific alarm call.
The adaptive value of eavesdropping on the chickadee alarm call signaling system seems clear for nuthatches. Because nuthatches are very similar in size to chickadees, occupy many of the same habitats, and cooccur with chickadees in mixed-species flocks during much of the winter, they are attacked by most of the same predators as chickadees (22, 29). In addition, nuthatches maintain year-long pair bonds and occupy smaller wintering home ranges than chickadees; thus, they have an even stronger incentive to attempt to drive predators away (23) through active mobbing. However, not all potential predators pose the same degree of threat to nuthatches. Because small birds like nuthatches may be severely food-limited in the winter as the result of low prey abundance and high energetic demands (22, 30–32), discriminating among different species of potential predators and mobbing dangerous predators most intensely may help conserve energy. Because they can differentiate among within-category variations of the chick-a-dee alarm calls that contain information about predator risk, nuthatches can selectively mob only the most dangerous predators.
This study raises some interesting additional questions. Although it is clear that chickadees encode information in their calls, which convey predator risk to conspecific and heterospecific receivers, it is difficult to know which feature(s) of the calls are used for this purpose. Because a number of acoustic features in these calls vary predictably with predator risk, it is possible that nuthatches assess a combination of calling rate, number of D notes, and fine-scale acoustic features when making decisions about how to respond to an alarm call. It also seems likely that this discriminating among chick-a-dee call variants is learned, rather than simply the result of similarities with their own call structure (33), because nuthatch alarm calls are very different from those of chickadees (22, 29).
The results from this study may have important implications for currently developing census and monitoring techniques. In lieu of the more demanding point count method of sampling birds, recent studies have advocated using playback of chickadee mobbing calls to census bird abundance (24, 34). This method has been proposed because it increases the number of birds detected during a given observation, provides a mechanism for surveying nonsinging birds during the winter, and potentially allows for insights into reproductive behavior. However, our results indicate that the likelihood of other species approaching a speaker broadcasting chick-a-dee mobbing calls depends on the specific type of chick-a-dee mobbing calls being broadcast. Therefore, it is extremely important that all studies employing this methodology exercise great caution to only use chickadee calls recorded under similar predator conditions so that results from various studies can be compared.
Chickadees have one of the most sophisticated alarm call systems yet discovered and encode complex information in subtle variations within their calls. Although there have been previous examples of some primates and birds distinguishing between acoustically distinct types of alarm calls (14), this study shows that an animal can discriminate among variations within a single type of heterospecific alarm call. Our results reveal the remarkable amount of information that animals can learn about their environment by eavesdropping on the vocalizations of other species.
Materials and Methods
For this study, we constructed playback stimuli from chick-a-dee calls that had been previously recorded from captive black-capped chickadees during controlled predator encounters with live, perched raptors (21). We used calls recorded in response to a great horned owl (large body size, low threat) and a northern pygmy owl (small body size, high threat), and we used contact calls of a sympatric house sparrow as a control. The chick-a-dee calls produced in response to these predators varied in a number of acoustic features, including the calling rate, number of D syllables, and the shape and timing of the D notes (Fig. 1) (21). Our playback stimuli maintained all of these differences to provide the most realistic auditory conditions. We used three different playback stimuli for each treatment to limit pseudoreplication (35). The alarm call stimuli consisted of 1 min of unique exemplars recorded from one individual chickadee, with a different chickadee's calls used for each of the three versions of the playback stimuli. The same individual chickadee's calls were used for the small-predator and large-predator alarm call stimuli, allowing us to create matched playback stimuli. After the 1-min playback period, we observed the nuthatches until they returned to normal foraging behavior or for at least 5 min if they did not initially respond.
Playback trials were conducted in the field near Missoula, MT, (46°, 50′ N; 114°, 02′ W) with pairs of wild nuthatches. Although the focal pairs of nuthatches were likely mated pairs, the plumage differences between male and female nuthatches are very subtle and we did not attempt to identify the sex of each bird for this study. Although we conducted this study during the winter, when nuthatches often associate in mixed-species flocks with chickadees, we were careful to perform our playback experiments only at times when there were no chickadees present, so that we could be sure that nuthatches were responding only to the acoustic stimuli of the playback and not to the behavior of live chickadees. Once a pair of nuthatches was located in the absence of chickadees, we placed a speaker (Pignose Model 7–100; Pignose-Gorilla, North Los Vegas, NV) at the base of a tree, ≈15 m away from them. Once the speaker was positioned, we moved ≈20 m from the birds and immediately began the playback trial. These experiments were conducted in areas with considerable human activity where nuthatches were habituated to human presence. We observed no evidence that our activities setting up the speakers disturbed the behavior of the nuthatches before the playbacks. We played vocalizations from uncompressed .wav files recorded to compact disk. Each stimulus was played at the same volume and approximated natural calling amplitudes (sound pressure level at 1 m = ≈75 dB for all treatments).
We carefully designed a playback experiment that would be powerful in detecting differences in responses to the two different types of chickadee mobbing stimuli. We set stringent experimental conditions (conducting trials in the absence of any chickadees and relocating the same pair of nuthatches in a season when they are not particularly conspicuous or vocal). Thus, it was challenging to meet all of the required conditions, and many trails had to be aborted. We presented all three treatments for the first 13 pairs of nuthatches. However, it became clear early in the trials that the birds' response to the control playback was quite different from the predator treatments. Therefore, we chose to focus the rest of our efforts on playback presentations of the two different predator treatments to increase the sample sizes to give us more power to detect differences in response to these treatments.
To control for any within-subject variation in prior experience with chickadees or likelihood to respond to playback, we conducted paired playback trials with each pair of nuthatches. Each pair of nuthatches was tested by using both the small-predator and large-predator alarm call treatments, randomly ordered among pairs (n = 20). This repeated-measures experimental design allowed us to use paired comparisons, providing more statistical power than a nonpaired design because it accounts for behavioral variation between different pairs of nuthatches. Trials on the same pair of nuthatches were separated by at least 1 hr. In addition, we were able to conduct control trials with the majority of these pairs (n = 13), which were also randomly ordered and spaced at least 1 hr from other trials. The two alarm call treatments were matched so that each pair of nuthatches was played small-predator and large-predator alarm calls recorded from the same individual chickadee.
We observed a number of different behaviors of the nuthatches during and immediately after each playback to determine whether they responded differently to each treatment. In response to mobbing alarm calls, nuthatches and other species typically approach the warning signals, inspecting for the perched predators (25). Thus, we recorded whether birds approached the speaker (moving in the direction of the speaker, regardless of distance moved) after the initial playback sounds. For all birds, we estimated the closest horizontal distance to the speaker. For birds that flew to the tree with the hidden speaker, we also measured the latency to fly to the tree and the closest vertical distance to the speaker. We tallied the occurrence of any vocalizations and wing-flick behaviors, which signal agitation. Last, we measured data on the time to when birds returned to normal foraging behavior (return time was set at 0 for nonresponding birds).
Because we had a priori predictions about the direction of the data and because some of the variables violated the assumptions of parametric statistics, we used one-tailed, nonparametric, paired statistical tests for all comparisons.
Acknowledgments
We thank Kate Davis of Raptors of the Rockies for help recording the alarm calls with live raptors, Swift Instruments Inc. (Aliso Viejo, CA) for donating binoculars for the study, and Ken Dial for stimulating discussions. This research was supported in part by a National Institutes of Health Auditory Neuroscience Training Grant (to C.N.T.).
Abbreviation
- WSR
Wilcoxon's sign ranked test.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.B. is a guest editor invited by the Editorial Board.
References
- 1.Marler P. Nature. 1955;176:6. [Google Scholar]
- 2.McGregor PK. Philos Trans R Soc London Ser B. 1993;340:237–244. [Google Scholar]
- 3.McGregor PK, Dabelsteen T. In: Ecology and Evolution of Acoustic Communication in Birds. Kroodsma DE, Miller EH, editors. Ithaca, NY: Cornell Univ Press; 1996. pp. 409–425. [Google Scholar]
- 4.Mennill DJ, Ratcliffe LM, Boag PT. Science. 2002;296:873. doi: 10.1126/science.296.5569.873. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira RF, McGregor PK, Latruffe C. Proc R Soc London Ser B. 1998;265:1045–1049. [Google Scholar]
- 6.Peake TM, Matessi G, McGregor PK, Dabelsteen T. Anim Behav. 2005;69:1063–1068. [Google Scholar]
- 7.Deecke VB, Ford JKB, Slater PJB. Anim Behav. 2005;69:395–405. [Google Scholar]
- 8.Earley RL, Dugatkin LA. Proc R Soc London Ser B. 2002;269:943–952. doi: 10.1098/rspb.2002.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peake TM. McGregor PK. Animal Communication Networks. Cambridge, UK: Cambridge Univ Press; 2005. pp. 13–37. [Google Scholar]
- 10.Brown GE. Fish Fisheries. 2003;4:227–234. [Google Scholar]
- 11.Sullivan AM, Madison DM, Rohr JR. Behaviour. 2003;140:553–564. [Google Scholar]
- 12.Adams MJ, Claeson S. Ethology. 1998;104:955–961. [Google Scholar]
- 13.Shriner WM. Anim Behav. 1998;55:529–536. doi: 10.1006/anbe.1997.0623. [DOI] [PubMed] [Google Scholar]
- 14.Caro T. Antipredator Defenses in Birds and Mammals. Chicago: Univ Chicago Press; 2005. [Google Scholar]
- 15.Seyfarth R, Cheney D. Anim Behav. 1990;40:754–764. [Google Scholar]
- 16.Rasa OAE. Behav Ecol Sociobiol. 1983;12:181–190. [Google Scholar]
- 17.Randler C. Ethology. 2006;112:411–416. [Google Scholar]
- 18.Macedonia JM, Evans CS. Ethology. 1993;93:177–197. [Google Scholar]
- 19.Zuberbuhler K. Proc R Soc London Ser B. 2000;267:713–718. doi: 10.1098/rspb.2000.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainey HJ, Zuberbuhler K, Slater PJB. Proc R Soc London Ser B. 2004;271:755–759. doi: 10.1098/rspb.2003.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templeton CN, Greene E, Davis K. Science. 2005;308:1934–1937. doi: 10.1126/science.1108841. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM. The Black-Capped Chickadee: Behavioral Ecology and Natural History. Ithaca, NY: Cornell Univ Press; 1991. [Google Scholar]
- 23.Curio E. Z Tierpsychol J Comp Ethol. 1978;48:175–183. [Google Scholar]
- 24.Gunn JS, Desrochers A, Villard MA, Bourque J, Ibarzabal J. J Field Ornithol. 2000;71:472–483. [Google Scholar]
- 25.Hurd CR. Behav Ecol Sociobiol. 1996;38:287–292. doi: 10.1007/s002650050219. [DOI] [PubMed] [Google Scholar]
- 26.Howland HC. J Theor Biol. 1974;47:333–350. doi: 10.1016/0022-5193(74)90202-1. [DOI] [PubMed] [Google Scholar]
- 27.Johnsgard PA. Hawks, Eagles, and Falcons of North America. Washington, DC: Smithso-nian Inst Press; 1990. [Google Scholar]
- 28.Johnsgard PA. North American Owls: Biology and Natural History. Washington, DC: Smithsonian Inst Press; 2002. [Google Scholar]
- 29.Ghalambor CK, Martin TE. In: The Birds of North America. Poole A, Gill F, editors. Philadelphia, PA: Birds of N Am; 1999. [Google Scholar]
- 30.Brittingham MC, Temple SA. Ecology. 1988;69:581–589. [Google Scholar]
- 31.Doherty PF, Grubb TC. Ecology. 2002;83:844–857. [Google Scholar]
- 32.Grubb TC, Cimprich DA. Ornis Scand. 1990;21:277–281. [Google Scholar]
- 33.Johnson FR, McNaughton EJ, Shelley CD, Blumstein DT. Aust J Zool. 2003;51:577–585. [Google Scholar]
- 34.Turcotte Y, Desrochers A. J Field Ornithol. 2002;73:303–307. [Google Scholar]
- 35.Kroodsma DE. Anim Behav. 1989;37:600–609. [Google Scholar]