Abstract
Plants respond to herbivore attack with the release of volatile organic compounds (VOCs), which can attract predatory arthropods and/or repel herbivores and thus serve as a means of defense against herbivores. Such VOCs might also be perceived by neighboring plants to adjust their defensive phenotype according to the present risk of attack. We exposed lima bean plants at their natural growing site to volatiles of beetle-damaged conspecific shoots. This reduced herbivore damage and increased the growth rate of the exposed plants. To investigate whether VOCs also can serve in signaling processes within the same individual plant we focused on undamaged “receiver” leaves that were either exposed or not exposed to VOCs released by induced “emitter” leaves. Extrafloral nectar secretion by receiver leaves increased when they were exposed to VOCs of induced emitters of neighboring plants or of the same shoot, yet not when VOCs were removed from the system. Extrafloral nectar attracts predatory arthropods and represents an induced defense mechanism. The volatiles also primed extrafloral nectar secretion to show an augmented response to subsequent damage. Herbivore-induced VOCs elicit a defensive response in undamaged plants (or parts of plants) under natural conditions, and they function as external signal for within-plant communication, thus serving also a physiological role in the systemic response of a plant to local damage.
Keywords: ant–plant interaction, extrafloral nectar, indirect defense, lima bean, plant–plant communication
In response to herbivore attack, many plants release volatiles (1), a response that is mediated via the plant hormone jasmonic acid (JA) (2–5). Volatile organic compounds (VOCs) can attract predatory arthropods (6–9) and/or repel herbivores (10, 11) and thus serve as a means of plant defense to herbivores (12). However, volatiles carry information on the attack of the plant and thus can also be used by herbivores to localize their host plants (13–15), and they may be used by neighboring, yet-undamaged plants to adjust their defensive phenotype accordingly. Since Baldwin & Schultz in 1983 for the first time reported “plant–plant communication” (16), it has been controversially debated whether this phenomenon plays a role in nature (17–19). Studies reporting changes in the expression of defense-related genes (20–22), increased production rates of JA and of defensive compounds (16, 23, 24), or increased net defense (19, 25) in volatile-exposed plants were conducted in general under laboratory conditions, used artificially accelerated volatile concentrations, or damaged plants mechanically instead of using natural herbivores, thereby strongly compromising the direct applicability of the reported results to the natural field (26).
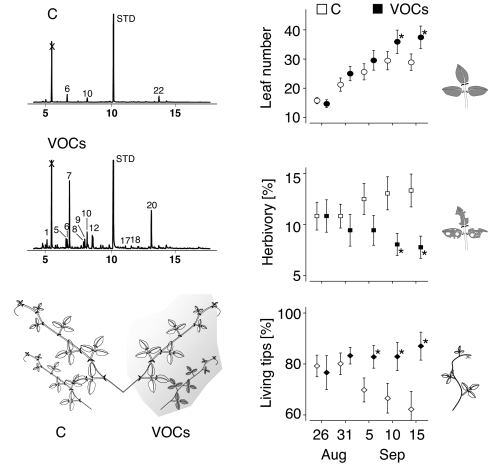
To investigate whether herbivore-induced VOCs can elicit defenses of neighboring plants in nature we made use of the existence of two types of indirect defense in lima bean (Phaseolus lunatus L., Fabaceae) (27). In response to damage or increased JA levels, lima bean releases VOCs and secretes extrafloral nectar (EFN), a nectar that attracts predatory arthropods and therefore serves as an indirect defense (28). Wild lima bean significantly benefits from increased EFN production in terms of decreased leaf damage and increased growth rates and seed production (27, 29). We divided plants growing at a site ≈15 km west of Puerto Escondido (state of Oaxaca, Mexico) into two parts and exposed one part repeatedly to shoots that had been damaged by herbivorous beetles. After <3 weeks, these plant parts grew faster and suffered less from herbivore damage than control parts (Fig. 1).
Fig. 1.
Protection of volatile-exposed plants in the field (experiment 1). (Left) Representative gas chromatic profiles of headspaces of tendrils exposed to herbivore-induced emitter tendrils (VOCs) and to undamaged emitters (C). (Right) Development of leaf number, herbivory (percent missing leaf area), and percentage of living shoot tips (means ± SE) during the experiment (August 26 until September 15). Asterisks indicate significant differences (P < 0.05 according to Wilcoxon pair test) between C and VOC tendrils. See SI Fig. 5 for detailed results of headspace analyses and Table 1 for identity of volatile compounds.
This result, while demonstrating that VOCs released by a plant in response to natural damage can indeed benefit its neighbors, opened up the question whether VOCs released by damaged leaves are perceived also by other leaves of the same plant. Do VOCs mediate signaling among different organs of the same plant individual (19, 30), and do neighboring plants only “eavesdrop” on what is within-plant signaling “worn on the outside”?
To answer this question we induced only a few leaves per shoot and then experimentally controlled air flow from these to other organs of the same plant, or other plants, to compare natural VOCs concentrations with reduced ones instead of artificially concentrating VOCs in the atmosphere. Indeed, VOCs released from damaged lima bean leaves induced EFN secretion by undamaged leaves of the same shoots, and they primed EFN secretion to show an augmented response to future mechanical damage. These results demonstrate that VOCs can serve as an external signal for within-plant signaling.
Results and Discussion
Defense Induction in Nature.
Field-grown lima beans were divided into two parts and trained along ropes to expose one part per plant every 4 days to shoots that had been damaged on the day before by the herbivorous leaf beetles Cerotoma ruficornis and Gynandrobrotica guerreroensis (Chrysomelidae). Over a 3-week field experiment, these parts suffered less from herbivory and grew faster than controls (Fig. 1). In the end of the experiment, exposed parts had significantly more leaves and more growing shoot tips and a significantly lower level of herbivory than controls (P < 0.05 for all three parameters according to Wilcoxon pair test; n = 18 pairs). Amounts of EFN secreted by and numbers of ants observed on exposed plant parts were higher than on controls [supporting information (SI) Fig. 4], a result consistent with earlier observations on lima bean plants exposed to artificial VOCs (31). It is therefore most likely that at least a part of this defensive effect was due to an EFN-mediated increase in ant numbers (27, 29, 32).
Induction of EFN Secretion by VOCs in the Field.
While demonstrating that VOCs indeed can induce defenses in neighboring plants under natural conditions, the above observation raised the question whether and how VOCs are perceived not only by a plant's neighbors but also by the emitter itself. Can VOCs serve as a means of within-plant signaling? Because earlier studies showed that VOCs induce and prime EFN secretion in undamaged lima beans (31, 33, 34) we focused on EFN secretion, which was quantified in all experiments 24 h after starting the induction of the emitter leaves as amounts of soluble solids per leaf dry weight.
Table 1.
Volatile compounds of wild lima bean
| Number | Retention time, min | Compound |
|---|---|---|
| 1 | 5.24 | cis-Hexenylacetate* |
| 2 | 5.60 | 2-Ethylhexanol* |
| 3 | 5.71 | cis-β-Ocimene* |
| 4 | 5.87 | trans-β-Ocimene |
| 5 | 6.66 | Linalool* |
| 6 | 6.74 | Nonanal* |
| 7 | 6.91 | 2-Ethenyl-cyclohexan |
| 8 | 7.10 | C11 homoterpene* |
| 9 | 7.95 | cis-3-Hexen-1-yl-butyrat |
| 10 | 8.04 | MeSA* |
| 11 | 8.21 | Decanal* |
| 12 | 8.59 | Unidentified |
| 13 | 9.37 | Unidentified |
| 14 | 10.57 | cis-3-Hexenylhexoate |
| 15 | 10.64 | cis-3-Hexenyl-format |
| 16 | 10.80 | cis-Jasmone* |
| 17 | 11.04 | β-Caryophyllene* |
| 18 | 11.47 | trans-Geranylacetone* |
| 19 | 12.87 | cis-3-Hexen-1-ol-benzoate |
| 20 | 12.95 | 4,8,12-trimethyltrideca,1,3,7,11-tetraene* |
| 21 | 13.75 | MeJA* |
| 22 | 15.07 | Unidentified |
| 23 | 15.74 | 4,8,12-Tetradecatrienal,5,9,13-trimethyl |
| 24 | 17.39 | Stearyl acetate |
| 25 | 17.53 | Palmitinic acid-isopropylester |
| STD | 10.14 | n-Bromodecane (as standard)* |
Identities and retention times of volatile compounds appearing in headspaces of wild lima bean plants in response to the different treatments applied in this study. Substances marked with ∗ are identified by comparison with pure standards, and the other compounds were identified by using the NIST 05 library.
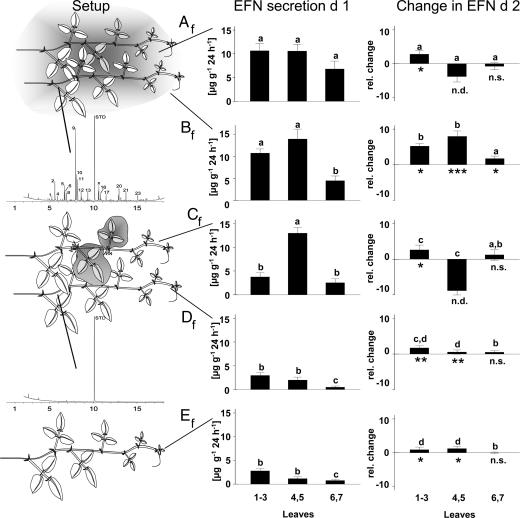
Leaves 4 and 5 of shoots growing in the field were induced by mechanical damage and application of JA (Fig. 2). The volatile bouquet released in response to this treatment shared most compounds with the bouquet released in response to herbivore feeding (Figs. 1 and 3; see also SI Figs. 5–7). After treating the leaves of this emitter shoot (Af for treatment A in field experiment), a second, undamaged “receiver” shoot (Bf) was wound around the emitter to simulate the natural, tangled growth of lima bean. A third shoot (Cf) was treated as was the emitter shoot, but volatiles were prevented from being set free by applying plastic bags around the induced leaves, which successfully reduced the amount of volatiles appearing in the headspace of such shoots (Fig. 2). A fourth shoot (Df) was wound around this shoot to be exposed to the reduced volatile bouquet, while a last shoot (Ef) remained untreated and served as control.
Fig. 2.
Induction and priming of EFN secretion by volatiles (experiment 2). (Left) The experimental setup with receiver tendrils being exposed (Bf) or not exposed (Df) to VOCs of artificially induced emitter tendrils (Af and Cf) and the respective GC profiles are displayed. (Center) EFN secretion (in micrograms of soluble solids secreted per gram of leaf dry mass and 24 h +SE) of different leaf age classes (leaves 1–3, leaves 4 and 5, and leaves 6 and 7) on day 1. (Right) Change in EFN secretion on day 2 relative to day 1 (a value of +2 indicating a 2-fold-higher secretion on day 2 than on day 1). Asterisks indicate significant (∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05) differences in EFN secretion on day 2 as compared with day 1 as tested by paired t tests within each leaf age class and treatment (n.s., not significant; n.d., not determined). Bars marked by different letters within the same leaf age group are significantly different (P < 0.05; LSD post hoc analysis conducted on effects of treatment separately for each leaf group after univariate ANOVA). See SI Fig. 6 for detailed results of headspace analyses and Table 1 for identity of volatile compounds.
Fig. 3.
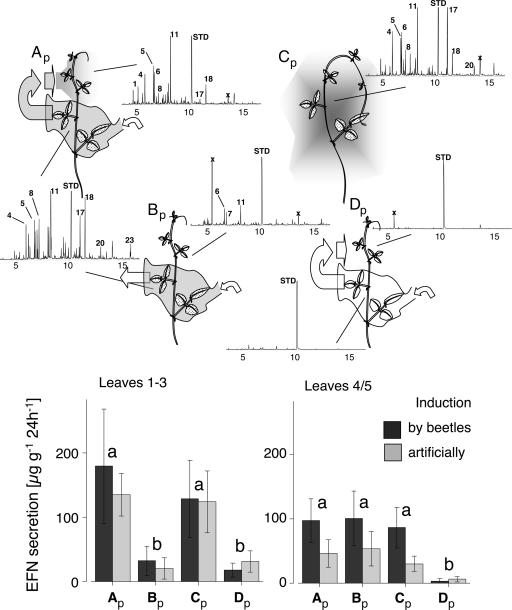
Within-plant signaling by volatiles (experiment 3). (Upper) The experimental setup (Ap, redirection of VOCs released by the induced leaves 4 and 5 to untreated leaves 1–3; Bp, removing VOCs from the plant; Cp, gas flow unaffected; Dp, air from uninduced leaves 4 and 5 redirected to leaves 1–3) and representative GC profiles. The resulting rates in EFN secretion (in micrograms of soluble solids secreted per gram of leaf dry mass and 24 h ± SE) are displayed separately for leaves 1–3 and leaves 4 and 5 and separately for plants whose leaves 4 and 5 were induced by beetles or artificially. See SI Fig. 7 for detailed results of headspace analyses, Table 1 for identity of volatile compounds, and Table 2 for results of ANOVA of EFN secretion rates. Treatments marked by different letters are significantly different (P < 0.05; LSD post hoc analysis on effects of treatment separately for each leaf group after univariate ANOVA).
EFN secretion by undamaged leaves responded significantly to the different treatments (P < 0.001 for an effect of treatment on EFN secretion; univariate ANOVA conducted separately for each leaf age class). EFN secretion by the youngest, undamaged leaves 1–3 was significantly higher when they were exposed to VOCs released from induced leaves (Af and Bf; see Fig. 2), which demonstrates that VOCs indeed have a role in within-plant signaling required for a systemic response. Although the systemic spread of information in a plant is generally assumed to occur via the xylem (35) or the phloem (36), recent studies already pointed to a role of VOCs in the information transfer among parts of the same plant (19). This is confirmed by the results of the present study. Our experiment allows separating the effects of volatiles from the effects of an internal signal. Undamaged leaves of shoot Af could receive putative internal signals from the induced leaves 4 and 5 as well as VOCs as an external signal, whereas those of shoot Cf could only receive the putative internal signal. Undamaged leaves of shoot Af secreted significantly more EFN than those of shoot Cf (Fig. 2). The largest part of information transfer among the leaves of the same shoot thus was obviously due to VOCs rather than a plant-internal signal.
Priming of EFN Secretion.
Expression of resistance traits in response to damage affecting neighbors comes with the risk that resources are allocated to a resistance that finally is not needed. Therefore, priming for defense may combine the advantages of induced protection and low costs (26, 37). Primed plants respond stronger once they are attacked or infected themselves, yet they do not show detectable expression of resistance traits before damage occurs (23, 38, 39). For instance, primed corn plants were significantly more attractive to parasitic Cotesia marginiventris wasps when damaged, HI-VOC-induced priming thus in fact can lead to enhanced direct and indirect resistance against insect attack (40).
We damaged mechanically all leaves after EFN collection and quantified EFN secretion after another 24 h. A significant induction due to mechanical damage was observed in most of the leaf groups besides those that had already been damaged on day 1 (Fig. 2). The presence of VOCs, however, significantly augmented the leaves' response to mechanical damage. EFN secretion by leaves 1–3 of the emitter shoot Af as well as leaves 1–3 and 4–5 of the receiver shoot Bf was more strongly induced than in the other leaves, and among the oldest leaves (number six and seven) only those that had been exposed to volatiles (shoot Bf) showed a significant response. The strongest effect by far was observed in leaves 4 and 5 of the receiver shoot Bf, i.e., in those leaves that had been exposed to the highest dose of volatiles released from the induced leaves of shoot Af.
VOCs as Means of Within-Plant Signaling.
The above results indicated that VOCs can be more important for within-plant signaling than internal signals. This appears surprising because the systemic response to local damage usually is regarded as resulting from signals transported within the plant (35, 36, 41). Air was enclosed in plastic bags around the induced leaves 4 and 5, thus leading to a microclimate that might have affected the synthesis and/or release of an internal signal. Moreover, the use of field-grown plants did not allow the exclusion of volatiles released by other plants (both con- and heterospecific) in the background vegetation, or of signals coming from the rhizosphere (42). To solve these problems we repeated the experiment with plantlets cultivated in pots and an open-flow system.
Leaves 4 and 5 were induced, either artificially with mechanical damage and JA as described above, or by placing 20 beetles (G. guerreroensis) on them. These leaves were placed in a plastic bag which was left open on one end. Air was continuously moved over these leaves by means of a tube and a ventilator and was either re-directed to the undamaged leaves 1–3 of the same plant (treatment Ap for treatment A on potted plants) or blown away from the plant (Bp; see Fig. 3). As controls, we induced leaves 4 and 5 and placed leaves 1–3 close to them without any active movement of the air (Cp), and we installed the same air flow as in treatment Ap for uninduced plants (Dp). The experiment was conducted outside under shady natural climatic conditions, and temperatures in the bags were not higher than outside because of the continuous air flow.
EFN secretion of undamaged leaves of plants induced with beetles or artificially (n = 5 groups each) responded significantly to the different treatments (Table 2). Undamaged leaves that were exposed to VOCs from induced leaves of the same plant (treatments Ap and Cp) secreted significantly more EFN than leaves of plants from which VOCs were blown away (Bp), or leaves exposed to VOCs from uninduced leaves (Dp) (Fig. 3). There was no significant difference between Ap and Cp or between Bp and Dp. Because undamaged leaves in treatment Bp still could have been affected by a putative internal signal, this confirms that VOCs in our study system are more important in within-plant communication than internal signaling.
Table 2.
ANOVA analysis on EFN secretion in experiment 3
| Age class | Factors | df | F value | P value |
|---|---|---|---|---|
| Leaves 1–3 | Treatment | 3 | 7.871 | 0.004 |
| Induction type | 1 | 0.230 | n.s. | |
| Date | 4 | 5.314 | 0.033 | |
| Leaves 4 and 5 | Treatment | 3 | 6.367 | 0.008 |
| Induction type | 1 | 3.004 | n.s. | |
| Date | 4 | 3.232 | n.s. | |
| Induction type × date | 4 | 3.847 | 0.030 |
ANOVA was conducted separately for the two leaf age classes on the effects of treatment and type of induction (fixed factors) and date (random factors) of EFN secretion. Of the possible interactions among factors, only significant ones are displayed.
This phenomenon appears adaptive, particularly when considering the morphology of lima bean. Karban et al. (19) recently discussed that sagebrush might rely on VOC-mediated within-plant signaling because of the lack of vascular connections among different parts of this plant. Here we demonstrate that communication even among neighboring leaves of the same shoot is mediated by VOCs. Because of the tangled growth of very long shoots, leaves (even of the same plant) that are growing adjacent to damaged leaves are not necessarily the consecutive leaves on the shoot. This makes a plant-internal signal less efficient as long as it is transported within the shoots. VOCs, in contrast, serve as a cue to elicit EFN secretion in exactly those parts of the plant, where resistance actually is required: in the spatially (but not necessarily anatomically) neighboring parts.
Conclusion
We demonstrate here that volatiles released from damage-induced lima bean leaves can induce and prime EFN secretion by yet-undamaged plants or parts of plants. VOCs affecting the plants in the different experiments were not identical, because they were induced by beetle feeding on the day before (experiment 1), artificially by mechanical damage and JA application (experiments 2 and 3), or by feeding by beetles actually present on the leaves (experiment 3). Still, the response of EFN secretion reproducibly occurred when leaves were exposed to those volatiles. Our study not only demonstrates that the quality and quantity of volatiles released by induced leaves are sufficient to elicit another indirect defense in yet-undamaged plants under natural conditions. It also underlines that VOCs might serve as a rapid and efficient external signal for within-plant signaling and thus adds a new facet to the spectrum of known biological effects of VOCs (43, 44). Future studies on systemic responses of plants to local damage events should control airflow among different organs to disentangle internal and external signals.
Methods
Experiment 1.
Lima bean plants (P. lunatus L., Fabaceae) growing ≈15 km west of Puerto Escondido (state of Oaxaca, Mexico; 15°55.596 N and 97°09.118 W, elevation 15 m above sea level) were used. In August 2006 we selected 18 plants and trained them in equal parts along two ropes (see drawing in Fig. 1). Numbers of leaves and living and dead shoots tips were counted, and the rate of herbivory was estimated as missing leaf area to the nearest 5% for all plant parts, and parts were randomly assigned to serve as exposed to damaged or control tendrils. Shoot tips of other lima beans were placed in nets containing 15 beetles (G. guerreroensis and C. ruficornis in approximately equal numbers) for 24 h. They were then removed from the nets, cleaned from beetles and their frass, and wound gently around the “exposed” plants parts for another 24 h, whereas undamaged shoots were wound around the controls. This treatment was repeated every 4 days from August 26 until September 15.
Experiment 2.
We selected 10 groups consisting of five field-grown plants each in March 2006. All shoots were characterized by still-growing tips and no visible damage on the youngest 10 leaves. The experimental design is graphically presented in Fig. 2. Of the first shoot per group (Af) we induced the leaves 4 and 5 with mechanical damage (punching holes into the blades of all three leaflets per leaf with a metal brush resulting in an average damage of ≈15 holes per cm−2, diameter of individual holes ≈0.2 mm) and application of 0.5 ml of a 0.5 mmol aqueous solution of JA. After treating the leaves of this emitter shoot (Af), a second, undamaged receiver shoot (Bf) was wound around the emitter to simulate the natural, tangled growth of lima bean. Care was taken that leaves of equal age were closest to each other. Of a third shoot (Cf), leaves 4 and 5 were treated as were the leaves of shoot Af, but volatiles were prevented from being set free by applying plastic bags [Bratenschlauch, a PET foil that does not emit detectable amounts of volatiles by itself (Toppits, Minden, Germany)] around the induced leaves. These bags successfully reduced the amount of volatiles appearing in the headspace of such shoots (Fig. 2 and SI Fig. 6). A fourth shoot (Df) was wound around this shoot to be exposed to the reduced volatile bouquet, and a last shoot (Ef) remained untreated and served as control.
All shoots then were placed in mesh bags (mesh size 0.5 mm), and a ring of sticky resin (Tangletrap; Tanglefoot, Grand Rapids, MI) was applied at their base to protect them from flying and crawling nectar consumers. The production rate of EFN was quantified after 24 h separately for the leaf groups 1–3, 4 and 5, and 6 and 7 by quantifying nectar volume with microcapillaries and nectar concentration with a portable refractometer to calculate amounts of soluble solids as described previously (45).
To study putative priming effects, all leaves were damaged mechanically as described above after quantifying EFN secretion and were kept in mesh bags for a second day. EFN secretion in response to this treatment then was quantified as described above.
Experiment 3.
Plants were collected as seedlings from the natural population and cultivated for 8 weeks (August and September 2006) in plastic pots filled with 250 ml of natural soil. Plants were watered daily until runoff. By the time of use these plants had three to five shoots and a total of 20–40 leaves. Leaves 4 and 5 of the major shoot of plants assigned to treatment Ap were induced either artificially with mechanical damage and JA as described above or by placing 20 beetles (G. guerreroensis) on them. Leaves then were enclosed in PET foil with a hole of ≈1 cm2. To create an open-flow system, a plastic tube (30 × 2 cm; inner surface lined with Bratenschlauch) was placed on the side opposite to the hole and a continuous air flow was guaranteed by one ventilator [video card cooler Evercool EC-4010 (Steren, Mexico City, Mexico) supplied with 4.5 V] placed on the upper end of the tube. In treatment Ap, the air leaving the induced leaves then passed the untreated leaves 1–3 of the same shoot. In treatment Bp the same installation was repeated with the air leaving the plant entirely. A third group of plants were induced only on leaves 4 and 5 without any artificial air stream (Cp), and the fourth group (Dp) was installed as Ap, but without any induction. The number of groups was five each for the two types of induction (beetles and artificially). Volatiles released from leaves 4 and 5 and leaves 1–3 in the experimental setup were collected in parallel from identically treated plants in an open-flow design on charcoal traps and analyzed as described below.
Gas-Chromatographic Profiles of Headspaces.
To characterize VOCs released from emitter shoots in the first field experiment, shoots were treated for 24 h with the same groups of 15 beetles as used in the field or were left untreated. After 24 h, beetles and their debris were removed and shoots were bagged in Bratenschlauch over the next 24 h (n = 5). For field experiment 2, each four shoots were treated as were shoots Af and Cf and were bagged immediately in Bratenschlauch over the next 24 h. The emitted VOCs were collected continuously over 24 h on charcoal traps (1.5 mg of charcoal, CLSA-Filters, Le Ruissaeu de Montbrun, France) using a closed-loop stripping system (46). From potted plants we induced leaves 4 and 5 either artificially or with the same beetle groups as used in the experiment (n = 4 per type of induction and leaf age class). Volatiles were collected on the same type of filters, but in an open-loop design with air entering the leaf-containing plastic bags via charcoal filters.
Organic compounds were eluted from the charcoal traps after 24 h with dichloromethane (40 μl) containing 1-bromodecane (200 ng·μl−1) as a standard. Samples were then transferred to glass capillaries, sealed by melting the open end, and stored at <5°C for transport to Germany. Samples were analyzed on a GC-Trace mass spectrometer (Trace GC Ultra DSQ; Thermo Electron, Austin, TX). The program for separation [Rtx5-MS column (Restek, Philadelphia, PA), 15 m × 0.25 mm; 0.25-μm coating] was 40°C initial temperature (2 min), 10°C·min−1 to 200°C, then 30°C·min−1 to 280°C with He (constant flow 1.5 ml·min−1) as carrier gas. Identification of compounds was done by comparison with standard substances and with the Nist 05 library. Individual compounds (peak areas) were quantified with respect to the peak area of the internal standard, and quantities are presented as a percentage of the internal standard's area. Plants used in the various repetitions of experiments 1 and 3 did not have identical sizes; therefore, we corrected the quantitative data on volatile production by the leaf dry mass of the plant (i.e., divided relative peak areas in percentage by the dry weight in grams) that yielded the respective sample.
Supplementary Material
Acknowledgments
We thank Raul F. Medina, Wilhelm Boland, Perla Martinez Cruz, and four anonymous referees for valuable comments on earlier versions of the manuscript and Ulrich Lion and Susann Schiwy for help with GC analyses. This work was supported by Deutsche Forschungsgemeinschaft Grant He 3169/4-2.
Abbreviations
- EFN
extrafloral nectar
- VOC
volatile organic compound
- JA
jasmonic acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
See Commentary on page 5257.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610266104/DC1.
References
- 1.Paré PW, Tumlinson JH. Nature. 1997;385:30–31. [Google Scholar]
- 2.Boland W, Hopke J, Donath J, Nüske J, Bublitz F. Angew Chem Int Ed. 1995;34:1600–1602. [Google Scholar]
- 3.Schmelz EA, Alborn HT, Banchio E, Tumlinson JH. Planta. 2003;216:665–673. doi: 10.1007/s00425-002-0898-y. [DOI] [PubMed] [Google Scholar]
- 4.Farmer EE, Alméras E, Krishnamurthy V. Curr Opin Plant Biol. 2003;6:372–378. doi: 10.1016/s1369-5266(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 5.Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan TM, Müller MJ, Xia ZQ, Zenk MH. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Nature. 1998;393:570–573. [Google Scholar]
- 8.Thaler JS. Nature. 1999;399:686–688. [Google Scholar]
- 9.Kessler A, Baldwin IT. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 10.De Moraes CM, Mescher MC, Tumlinson JH. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 11.Birkett MA, Campbell CAM, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, et al. Proc Natl Acad Sci USA. 2000;97:9329–9334. doi: 10.1073/pnas.160241697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karban R, Baldwin IT. Induced Responses to Herbivory. Chicago: Univ of Chicago Press; 1997. [Google Scholar]
- 13.Loughrin JH, Potter DA, HamiltonKemp TR, Byers ME. Environ Entomol. 1996;25:1188–1191. [Google Scholar]
- 14.Carroll MJ, Schmelz EA, Meagher RL, Teal PEA. J Chem Ecol. 2006;32:1911–1924. doi: 10.1007/s10886-006-9117-9. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi JI, Arimura GI, Ozawa R, Shimoda T, Dicke M, Takabayashi J, Nishioka T. Appl Entomol Zool. 2003;38:365–368. [Google Scholar]
- 16.Baldwin IT, Schultz JC. Science. 1983;221:277–279. doi: 10.1126/science.221.4607.277. [DOI] [PubMed] [Google Scholar]
- 17.Dicke M, Bruin J. Biochem Syst Ecol. 2001;29:981–994. [Google Scholar]
- 18.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 19.Karban R, Shiojiri K, Huntzinger M, McCall AC. Ecology. 2006;87:922–930. doi: 10.1890/0012-9658(2006)87[922:drisva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Arimura G-i, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 21.Paschold A, Halitschke R, Baldwin IT. Plant J. 2006;45:275–291. doi: 10.1111/j.1365-313X.2005.02623.x. [DOI] [PubMed] [Google Scholar]
- 22.Farag MA, Fokar M, Zhang HA, Allen RD, Pare PW. Planta. 2005;220:900–909. doi: 10.1007/s00425-004-1404-5. [DOI] [PubMed] [Google Scholar]
- 23.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Proc Natl Acad Sci USA. 2004;101:1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruther J, Kleier S. J Chem Ecol. 2005;31:2217–2222. doi: 10.1007/s10886-005-6413-8. [DOI] [PubMed] [Google Scholar]
- 25.Tscharntke T, Thiessen S, Dolch R, Boland W. Biochem Syst Ecol. 2001;29:1025–1047. [Google Scholar]
- 26.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 27.Heil M. J Ecol. 2004;92:527–536. [Google Scholar]
- 28.Heil M, McKey D. Annu Rev Ecol Evol Syst. 2003;34:425–453. [Google Scholar]
- 29.Kost C, Heil M. Basic Appl Ecol. 2005;6:237–248. [Google Scholar]
- 30.Orians C. J Chem Ecol. 2005;31:2231–2242. doi: 10.1007/s10886-005-7099-7. [DOI] [PubMed] [Google Scholar]
- 31.Kost C, Heil M. J Ecol. 2006;94:619–628. [Google Scholar]
- 32.Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE. Proc Natl Acad Sci USA. 2001;98:1083–1088. doi: 10.1073/pnas.031563398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choh Y, Kugimiya S, Takabayashi J. Oecologia. 2006;147:455–460. doi: 10.1007/s00442-005-0289-8. [DOI] [PubMed] [Google Scholar]
- 34.Heil M, Kost C. Ecol Lett. 2006;9:813–817. doi: 10.1111/j.1461-0248.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 35.de Bruxelles GL, Roberts MR. Crit Rev Plant Sci. 2001;20:487–521. [Google Scholar]
- 36.Gómez S, Stuefer JF. Oecologia. 2006;147:461–468. doi: 10.1007/s00442-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 37.van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J. Proc Natl Acad Sci USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman FA, Pieterse CMJ, Poinssot B, Pozo MJ, et al. Mol Plant-Microbe Interact. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerli L, Jakab G, Métraux J-P, Mauch-Mani B. Proc Natl Acad Sci USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ton J, D'Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 41.Dicke M, van Baarlen P, Wessels R, Dijkman H. J Chem Ecol. 1993;19:581–599. doi: 10.1007/BF00994327. [DOI] [PubMed] [Google Scholar]
- 42.Bezemer TM, van Dam NM. Trends Ecol Evol. 2005;20:617–624. doi: 10.1016/j.tree.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Pichersky E, Noel JP, Dudareva N. Science. 2006;311:808–811. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudareva N, Negre F, Nagegowda DA, Orlova I. Crit Rev Plant Sci. 2006;25:417–440. [Google Scholar]
- 45.Heil M, Fiala B, Baumann B, Linsenmair KE. Funct Ecol. 2000;14:749–757. [Google Scholar]
- 46.Donath J, Boland W. Phytochemistry. 1995;39:785–790. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.