Abstract
T7 gene 5 DNA polymerase (gp5) and its processivity factor, Escherichia coli thioredoxin, together with the T7 gene 4 DNA helicase, catalyze strand displacement synthesis on duplex DNA processively (>17,000 nucleotides per binding event). The processive DNA synthesis is resistant to the addition of a DNA trap. However, when the polymerase–thioredoxin complex actively synthesizing DNA is challenged with excess DNA polymerase–thioredoxin exchange occurs readily. The exchange can be monitored by the use of a genetically altered T7 DNA polymerase (gp5-Y526F) in which tyrosine-526 is replaced with phenylalanine. DNA synthesis catalyzed by gp5-Y526F is resistant to inhibition by chain-terminating dideoxynucleotides because gp5-Y526F is deficient in the incorporation of these analogs relative to the wild-type enzyme. The exchange also occurs during coordinated DNA synthesis in which leading- and lagging-strand synthesis occur at the same rate. On ssDNA templates with the T7 DNA polymerase alone, such exchange is not evident, suggesting that free polymerase is first recruited to the replisome by means of T7 gene 4 helicase. The ability to exchange DNA polymerases within the replisome without affecting processivity provides advantages for fidelity as well as the cycling of the polymerase from a completed Okazaki fragment to a new primer on the lagging strand.
Keywords: processivity, replisome, gp5–gp4, dynamic
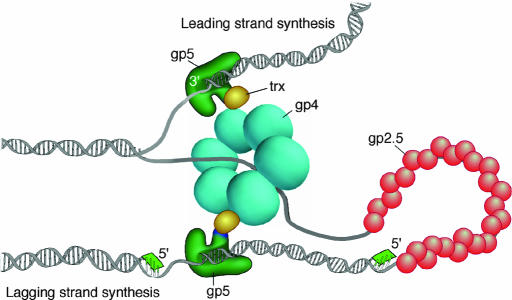
The replisome (Fig. 1) of bacteriophage T7 consists of three phage-encoded proteins and one host protein (1). DNA synthesis is carried out by the gene 5 DNA polymerase (gp5) after forming a tight one-to-one complex with Escherichia coli thioredoxin (trx), the processivity factor (2, 3). The multifunctional gene 4 protein (gp4) provides both primase and helicase activities (4–7), and the gene 2.5 protein (gp2.5) functions as a ssDNA-binding protein (8). Although the size of the T7 replisome is modest, the multiple interactions of the four proteins give rise to functions provided by additional proteins in other systems. For example, a unique insert in the thumb of gp5, the thioredoxin-binding domain (TBD), provides a binding site for the processivity factor (9–11) and for the binding of gp4 and gp2.5 (11). Furthermore, the covalent attachment of the primase to the helicase in gp4 provides for the requisite interaction of these two components of the replisome. Accessory proteins such as clamp and helicase loaders are not required in the T7 system. Nonetheless, leading- and lagging-strand syntheses are processive and coordinated (12). The lagging strand is synthesized in a discontinuous manner with nascent Okazaki fragments located in replication loops (12, 13).
Fig. 1.
Model of the bacteriophage T7 replication fork. The T7 replisome consists of the DNA polymerase (gp5), the processivity factor E. coli thioredoxin (trx), the primase/helicase (gp4), and the ssDNA-binding protein (gp2.5). The gp5/trx complex synthesizes the leading strand continuously as the helicase unwinds the duplex. The lagging strand is synthesized as short Okazaki fragments. Synthesis of the lagging strand is initiated from RNA primers (green) catalyzed by the primase domain of gp4. A loop is formed on the lagging strand to align both gp5/trx complexes. Gp2.5 coats the ssDNA regions of the lagging strand generated as the helicase unwinds the DNA.
A mechanism to coordinate leading- and lagging-strand syntheses is dictated by the inherently more complex events that occur on the lagging strand. Whereas the leading strand can be synthesized in an uninterrupted fashion, lagging-strand synthesis must occur through the synthesis of short Okazaki fragments (Fig. 1), an event that necessitates the synthesis of an RNA primer, its extension, the formation of a replication loop, and its resolution upon completion of an Okazaki fragment. Using a minicircle DNA, we have shown previously that the rates of synthesis of the two strands are equal, and both strands are synthesized processively (12).
What molecular events account for this remarkable coordination? In lieu of the multiple subunits of the replicative DNA polymerase III of E. coli that provide for the assembly of a dimeric DNA polymerase (14, 15), the T7 replisome must rely on interactions with gp4 and gp2.5. We have proposed that the helicase domain of gp4 binds to both the leading- and lagging-strand DNA polymerases and thus assures that they progress at the same rate (Fig. 1). Gp4 oligomerizes as a hexamer around ssDNA (16) and thus provides multiple subunits to which the two polymerases may bind independently. Gp4 has two modes of binding to gp5/trx (11). One mode occurs in the absence of DNA and involves an interaction of the acidic C terminus of gp4 with the TBD of the polymerase. However, when gp5/trx is bound to a DNA in a polymerization mode, the binding of gp4 to gp5/trx now occurs by a second mode that is considerably tighter than that which occurs in the absence of DNA. Consequently, during coordinated DNA synthesis, the two polymerizing enzymes can each bind to individual subunits of gp4 by this tight-binding mode.
An intriguing problem is the mechanism by which a polymerase is recruited to initiate synthesis on a newly synthesized RNA primer upon completion of an Okazaki fragment. In one model, the polymerase remains within the replisome through contacts with the helicase and ssDNA-binding proteins and subsequently transfers to a new RNA primer without entering into the pool of free enzyme. Our earlier studies showing that both DNA strands are synthesized processively led us to favor this model (12). Recently, Yang et al. (17) reported that in the phage T4 replication system the gene 43 DNA polymerase can exchange with an exogenously added genetically altered inactive gene 43 DNA polymerase. Nonetheless, their results indicate that the polymerases remain highly processive. Such exchange without affecting processivity provides a mechanism by which a polymerase is immediately available for extension of a newly synthesized primer.
In view of these findings, we have reexamined the ability of the DNA polymerases in the T7 replication system to exchange with free DNA polymerase. We find that although DNA synthesis is processive, the leading-strand DNA polymerases can freely exchange with exogenously added DNA polymerase. These results clearly differ from those reported previously. We address these differences in the Discussion.
Results
T7 Wild-Type (WT) Gp5/trx and Gp5-Y526F/trx.
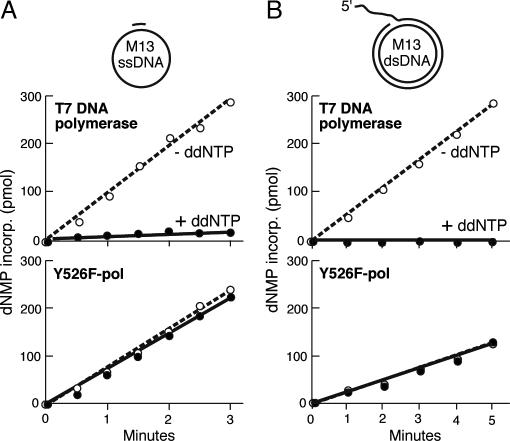
T7 gp5, unlike most other members of the polymerase I family, incorporates chain-terminating ddNTP as well as it does dNTPs (18–20). The lack of discrimination against ddNTPs results from the presence of a tyrosine at position 526 in the nucleotide-binding pocket of the polymerase (20). Replacement of this tyrosine with phenylalanine (gp5-Y526F), a residue found in the comparative position in E. coli DNA polymerase I and Taq polymerase, results in a several hundred fold discrimination against ddNTPs. Gp5-Y526F binds to thioredoxin normally (20), functions in a reconstituted replisome (12), and supports T7 growth (data not shown). The marked differences in the ability to incorporate ddNTPs can be used in assays to monitor reactions at the replication fork (12). In Fig. 2A we show that DNA synthesis catalyzed by gp5/trx is sensitive to the presence of ddNTPs; at a 1:10 ratio of ddGTP to dGTP, essentially no DNA synthesis is observed on primed M13 ssDNA, whereas extensive synthesis is observed in the absence of this analog. However, at this same ratio of ddGTP to dGTP, DNA synthesis by gp5-Y526F proceeds normally.
Fig. 2.
Polymerase activity of gp5/trx and gp5-Y526F/trx on M13 ss- and dsDNA. DNA synthesis by gp5/trx (Upper) and gp5-Y526F/trx (Lower) was monitored by the amount of [3H]dTMP incorporated into DNA over time as described in Materials and Methods. (A) DNA synthesis on primed M13 ssDNA was carried out in the presence and absence of ddGTP. (B) Strand displacement DNA synthesis catalyzed by the T7 DNA polymerase in the presence of gene 4 helicase on M13 dsDNA was carried out in the presence (filled circles) or absence (open circles) of ddGTP.
For gp5/trx to copy duplex DNA, T7 gene 4 helicase must be present to unwind the DNA to provide a ssDNA template (6, 21). Such strand displacement synthesis can be measured on a forked M13 dsDNA template depicted in Fig. 2B. The 5′ ssDNA tail is required for the loading of the hexameric gp4 helicase. Again, at a ddGTP:dGTP ratio of 1:10, all activity catalyzed by the WT gp5/trx is eliminated, whereas that catalyzed by gp5-Y526F/trx is unaffected. Gp5-Y526F/trx consistently has 50% less activity than does WT gp5/trx. Using an established single molecule assay for leading-strand synthesis (22), we have confirmed that gp5-Y526/trx synthesizes DNA with processivity similar to that of WT gp5/trx but with rates that are 50% of that of WT gp5/trx (Antoine van Oijen, S.M.H., and C.C.R., unpublished observations).
Exchange of DNA Polymerases During DNA Synthesis on ssDNA Templates.
T7 gp5 alone has low processivity on ssDNA, dissociating from the primer after the addition of from 1 to 15 nucleotides (2). The processivity factor, E. coli trx, binds to an insert in the thumb of gp5 and confers high processivity (2, 9,11). Recent measurements of the processivity of gp5/trx by using single molecule techniques yield values from 700 to 1,000 nucleotides per binding event (22, 23). Earlier ensemble experiments used to measure processivity have mainly relied on dilution or the addition of a DNA “trap” to limit the observed DNA synthesis to a single binding event (24, 25). However, the availability of gp5-Y526F/trx provides an additional method for measuring processivity. As shown above, gp5-Y526F/trx catalyzes extensive DNA synthesis in the presence of amounts of ddGTP that stop synthesis by WT gp5/trx. Therefore, if an excess of gp5/trx is added to a reaction mixture in which gp5-Y526F/trx is catalyzing DNA synthesis in the presence of ddGTP, then it will be available to replace any molecules of gp5-Y526F/trx that dissociate. The resulting incorporation of ddGMP by WT gp5/trx will result in cessation of DNA synthesis.
By using such a polymerase challenge experiment the processivity of gp5-Y526F/trx is relatively low as shown in Fig. 3A. Shortly after the addition of gp5/trx, all synthesis ceases. Such a result is not surprising because the processivity of gp5/trx is <1,000 nucleotides per binding event, values that would be exceeded in this assay. A significant contribution to the low processivity is the presence of secondary structure within the M13 DNA, structures that impede the polymerase, allowing it to idle by its exonuclease and polymerase modes with eventual dissociation (2, 26). Such a secondary structure is removed by the binding of E. coli ssDNA-binding (SSB) protein giving rise to a marked increase in processivity (26). The addition of SSB protein to the DNA polymerase challenge assay used here dramatically increases processivity as measured by the inability of WT gp5/trx to replace actively polymerizing gp5-Y526F/trx (Fig. 3B).
Fig. 3.
Exchange of DNA polymerases during DNA synthesis on ssDNA templates. DNA polymerase exchange assays were carried out on primed M13 ssDNA in the absence (A) or presence (B) of E. coli SSB protein (165 μg/40-μl reaction) as described in Materials and Methods. Reactions were initiated by the addition of 10 nM gp5-Y526F/trx at 30°C, and a 10-fold molar excess (100 nM) gp5/trx was added to the reaction 30 s later. Reaction mixtures were performed in the absence (open circles) or presence (filled circles) of 50 μM ddGTP.
Processivity of Gp5/trx and Gp4 Helicase Strand Displacement Synthesis on dsDNA.
Gp5/trx and gp4 helicase carry out leading-strand synthesis in a highly processive manner on either a minicircle or M13 dsDNA (12, 24). When the enzymes were subjected to dilution (24), gp5/trx and gp4 helicase continued to polymerize thousands of nucleotides before dissociating from the DNA. A recent report using single-molecule techniques showed that gp5/trx and gp4 catalyzed the polymerization of 17,000 nucleotides per binding event (22). We have used a DNA trap consisting of (dC) 200 (LC)·oligo(dG) 20 (LC) to examine the processivity of gp5/trx and gp4 during strand displacement synthesis.
In the processivity assay presented in Fig. 4, we monitor processivity on both M13 dsDNA as well as a minicircle. Both DNA templates are preincubated with gp5/trx, gp4 helicase, and gp2.5 in the absence of MgCl2 and dNTPs. DNA synthesis is initiated by the addition of MgCl2 and dNTPs along with the DNA trap. The DNA trap binds any free enzymes in the reaction, including any that dissociate from the replication fork as the reaction proceeds. A gel analysis of the products of DNA synthesis on M13 dsDNA and the minicircle are shown in Fig. 4 A and B, respectively. DNA synthesis proceeds in a processive manner for both templates as demonstrated by the resistance of DNA synthesis to the DNA trap. Gp5/trx and gp4 together catalyze the synthesis of DNA products thousands of nucleotides in length without dissociation. In contrast, in the control reactions, when either of the dsDNA templates and the trap are mixed and preincubated with protein, no DNA synthesis is detected.
Fig. 4.
Processivity of gp5/trx and gp4 on dsDNA. (A) The M13 dsDNA or (B) minicircle DNA template was preincubated with gp5/trx and gp4 as described in Materials and Methods. Reactions were initiated with MgCl2, dNTPs and [α-33P]dATP in the absence or presence of (dC)200·oligo(dG)20 to trap any free enzyme that dissociates from the circular DNA. Aliquots were removed from the reaction at the indicated times, and DNA synthesis was stopped by the addition of EDTA before separation of the products on a 0.8% agarose gel. In the control experiment, the trap was added during the preincubation step.
Exchange of DNA Polymerases During Strand Displacement Synthesis.
In principle, it should be possible to confirm the high processivity of DNA synthesis mediated by gp5/trx and gp4 helicase on M13 dsDNA by using a challenge with excess DNA polymerase. Such an experiment is shown in Fig. 5A, where DNA synthesis on M13 dsDNA is initiated with gp5-Y526F/trx and gp4. One minute after the initiation of DNA synthesis, a 10-fold excess of wild-type gp5/trx is added, followed by the addition of ddGTP 1 min later. The addition of WT gp5/trx leads to a 2-fold stimulation of DNA synthesis (blue curve), but all synthesis ceases upon the addition of ddGTP (red curve). If WT gp5/trx is not added, then synthesis continues even when ddGTP is added (black curve). If ddGTP is not added, synthesis continues at the increased rate observed for the WT enzyme relative to gp5-Y526F/trx (see Fig. 2).
Fig. 5.
Exchange of DNA polymerases during strand displacement synthesis. (A) Exchange reactions were performed as described in Materials and Methods in the presence of 2 nM M13 dsDNA. Reactions were initiated by the addition of 16 nM gp4, 4 μM gp2.5, and 16 nM gp5-Y526F/trx. At 1 min, a 10-fold excess of WT gp5/trx was added to the reaction followed by an addition of 50 μM ddGTP at 2 min (red). At the indicated times, aliquots were removed from the assay to determine the amount of [3H]dTMP incorporated into the DNA. In the gp5-Y526F/trx control reactions (black), DNA synthesis was monitored in the absence of excess WT gp5/trx but in the presence and absence of ddGTP. In a WT gp5/trx control reaction (blue), a 10-fold excess of WT gp5/trx was added to the reaction without the addition of ddGTP. (B) In the reverse scheme, 16 nM gp5/trx was used to initiate the reaction, and a 10-fold excess of gp5-Y526F/trx was then added at 1 min and ddGTP at 2 min.
In a reversed reaction scheme from above, we have also initiated DNA synthesis with WT gp5/trx followed by the addition of gp5-Y526F/trx and then ddGTP (Fig. 5B). Now the addition of gp5-Y526F/trx results in a slight slowing of DNA synthesis, but the reaction is not curtailed by the addition of ddGTP (black curve). However, the addition of ddGTP in the absence of added gp5-Y526F/trx terminates DNA synthesis (red curve). We conclude that exogenously added DNA polymerase can exchange rapidly with the actively synthesizing polymerase at the replication fork by a mechanism that does not affect the processivity observed with a DNA trap.
Polymerase Exchange During Coordinated Leading- and Lagging-Strand DNA Synthesis.
We have also examined polymerase exchange where leading and lagging strands are synthesized in a coordinated manner. We have previously used a duplex minicircle consisting of 70 bp to reconstitute the T7 replisome (12, 13). The minicircle has been designed such that one strand contains almost exclusively cytosine and the other guanosine, thus facilitating the measurement of leading- and lagging-strand synthesis. On this minicircle, leading- and lagging-strand syntheses are coordinated, and a replication loop of lagging-strand DNA contains a nascent Okazaki fragment. In addition, gp2.5 is required for coordination.
In the absence of rNTPs DNA synthesis is limited to leading-strand synthesis. Therefore, we first examined the ability of exogenous gp5/trx to exchange with the leading-strand DNA polymerase (Fig. 6A). Synthesis was initiated with gp5-Y526F/trx and gp4, and 1 min later a 10-fold excess of WT gp5/trx was added, followed by ddGTP 1 min later. The results are identical to those determined above with M13 dsDNA. Upon the addition of both gp5/trx and ddGTP, synthesis ceases but not when either is added alone. Upon the addition of rATP and rCTP, the gp4 primase catalyzes the synthesis of primers at the primase recognition sites on the minicircle for lagging-strand synthesis. Again, we find that exogenous gp5/trx exchanges with the leading-strand polymerase even when leading- and lagging-strand syntheses are coordinated (Fig. 6B).
Fig. 6.
Polymerase exchanges during coordinated DNA synthesis. (A) Polymerase exchanges during leading-strand synthesis. Reactions using a 70-nucleotide minicircle and primer–template were carried out as described in Materials and Methods. Reactions were initiated on 16 nM minicircle by the addition of 2 nM hexameric gp4, 1 μM gp2.5, and 14 nM gp5-Y526F/trx. At 1 min, a 10-fold excess of WT gp5/trx was added to the reaction followed by an addition of 50 μM ddGTP at 2 min (red). At the indicated times, aliquots were removed from the assay to determine the amount of [3H]dGMP incorporated into the DNA. In gp5-Y526F/trx control reactions (black), DNA synthesis was monitored in the absence of excess WT gp5/trx but with the presence and absence of ddGTP. In a WT gp5/trx control reaction (blue), a 10-fold excess of WT gp5/trx was added to the reaction without adding ddGTP. (B) Exchange reactions during coordinated leading- and lagging-strand syntheses. Reactions were identical to those described above except ATP and CTP were present to allow for lagging-strand synthesis.
In control experiments with WT gp5/trx, leading- and lagging-strand syntheses proceed at equal rates of 20 pmol of nucleotide polymerized per minute (data not shown). Furthermore, we showed above that leading-strand synthesis occurs processively on the minicircle in the presence of a DNA trap (Fig. 4B). These results confirm that syntheses are coordinated and that leading- and lagging-strand syntheses occur in a processive manner. At first glance, the processivity displayed by the DNA polymerase seems to contradict the results demonstrating exchange of the DNA polymerase during ongoing synthesis. In the Discussion we propose a model that allows for exchange of the replicating DNA polymerase with other polymerases already present within the replisome. We reported previously that gp5/trx did not appear to exchange with gp5-Y526F/trx actively polymerizing nucleotides on either the leading or lagging strand (12), results clearly at odds with those obtained in the present work. In the Discussion we provide an explanation for this difference.
Discussion
The replisome of bacteriophage T7, depicted in Fig. 1, accomplishes all of the tasks of DNA replication with only four proteins. In earlier studies we have shown that the four proteins can be reconstituted in a replisome that carries out coordinated leading- and lagging-strand syntheses in a processive manner (12, 13). A loop of lagging-strand DNA containing a nascent Okazaki fragment is present as visualized by electron microscopy of replicating molecules. All of these events have been confirmed by using single-molecule techniques (22).
Processivity of polymerization by DNA polymerase and its accessory proteins is not a simple parameter. In principal, processivity can be defined as the number of nucleotides polymerized in a single binding event. In the case of gp5, the binding of thioredoxin increases the processivity on M13 ssDNA several hundred-fold (2). However, secondary structure in the ssDNA leads to pausing of the complex and eventually dissociation, a phenomenon that can be eliminated by the addition of SSB protein (2, 26). In reactions involving the combination of helicase and gp5/trx, it is unlikely that secondary structure plays a role, and hence the high processivity may only mimic that seen with E. coli SSB protein-coated DNA. As Joyce (27) has pointed out, “different experimental approaches may yield apparently contradictory answers because they interrogate different steps in the process of dissociation.” The observation that polymerization of nucleotides can occur in a processive manner even when the polymerase is exchanging with other polymerases challenges our definition of processivity even further. On the one hand, we have shown that leading-strand DNA synthesis on a dsDNA template is highly processive, either alone or coupled with lagging-strand synthesis. However, the polymerase exchange that we observe during replication of dsDNA templates suggests that the leading-strand polymerase must dissociate from the DNA to complete the exchange.
How can a polymerase be processive and yet undergo frequent dissociations? The answer is suggested by the inability of the polymerase on ssDNA templates coated with SSB protein to exchange with free DNA polymerase. Two major parameters affect the binding of T7 gp5/trx to primed ssDNA. When the active site is occupied by a properly base-paired dNTP, a stable intermediate is formed as evidenced by the half-life of such a complex when the primer is terminated with a dideoxynucleotide (10). After condensation of the nucleotide, the nucleotide-binding site is transiently empty as the template prepares to align with the next incoming nucleotide. At this point, binding of the polymerase depends on contacts with the duplex portion of the DNA passing through the DNA-binding clef. It is here that thioredoxin and the TBD of the polymerase come into play. Most likely, these two modes of binding alternate during a cycle of polymerization and realignment of the template. If one of these parameters fails, then the polymerase is released from the primer–template and can exchange with free DNA polymerase.
Why then is the polymerase able to exchange freely during synthesis on duplex DNA templates? For synthesis on duplex DNA, the concerted action of the T7 gp4 helicase is required, a requirement that also necessitates a physical interaction of the helicase with the polymerase to assure their coordinated movement along the DNA. Thus, during synthesis on dsDNA, the polymerase is now a part of the replisome because of its association with both the helicase and the primer–template. It is now possible for the polymerase to dissociate transiently from the primer–template yet remain in close proximity because of its contacts with the helicase. The polymerase, although not bound to DNA, is nonetheless unavailable for removal by a DNA trap. The observation that free DNA polymerase can exchange with the polymerase actively synthesizing DNA without slowing the reaction further suggests that multiple DNA polymerases can bind to the helicase, with the helicase serving as a repository for these additional enzymes. Such a scenario is not unreasonable given the hexameric structure of the functional helicase (16). It is relevant to note that gp4 can interact with gp5/trx through two modes (11). One mode, in which the polymerizing gp5/trx is tightly bound to the helicase, does not involve the C terminus of gp4. Rather, this subdomain is essential for the second mode of binding, where it interacts with the TBD of gp5. Consequently, additional polymerases could, in principle, bind to subunits of gp4 by this second mode, whereas the polymerizing gp5/trx binds by the alternative mode. Gp2.5 also has an acidic C terminus that binds to the TBD, and consequently it too could stabilize the polymerase.
An attractive feature of this model is its relevance to lagging-stand DNA synthesis. Once the lagging-strand DNA polymerase completes the synthesis of an Okazaki fragment, it must recycle to a new primer without dissociation from the replisome. Clearly, the association of the lagging-strand polymerase with the helicase and/or gp2.5 allows it to dissociate from the completed Okazaki fragment without entering into solution. However, one can now envision, at least on occasion, a new DNA polymerase being recruited from another subunit of the helicase, thus assuring uninterrupted synthesis. Our experiments show that the leading-strand DNA polymerase can still readily exchange with free DNA polymerase during coordinated DNA synthesis. Inasmuch as the lagging-strand DNA polymerase most likely interacts with gp4 helicase in a manner similar, if not identical, to that of the leading-strand DNA polymerase, it is reasonable to postulate that a new DNA polymerase can be recruited from the helicase either to continue synthesis of an Okazaki fragment or to initiate synthesis on a new primer. We have not examined exchange of the lagging-strand DNA polymerase by using gp5-Y526F and the minicircle because of our inability to obtain a satisfactory inhibition of the lagging strand with ddCTP without affecting leading-strand synthesis. The problem arises from the fact that two guanosines reside within the two primase recognition sites on the leading-strand template. We will delay these experiments until we have available a minicircle in which one stand is lacking adenosine and the other thymine.
In an earlier paper describing the use of a minicircle to characterize coordinated DNA synthesis, we used gp5-Y526F/trx to address recycling of the leading- and lagging-strand DNA polymerases (12). We found that wild-type gp5/trx did not exchange with gp5-Y526F/trx that had been used to initiate synthesis on the minicircle. Reexamination of those experiments revealed that the ratio of ddNTP to dNTP was insufficient to inhibit gp5/trx under the conditions used to monitor exchange (data not shown). We found that there was insufficient ddNTP to inhibit significantly any WT polymerase that exchanged with gp5-Y526F/trx, a value based on an inappropriate control.
In a recent paper, Yang et al. (17) used a catalytically inactive T4 DNA polymerase to address the ability of free polymerase to replace actively synthesizing DNA polymerase in the T4 replication system. They found that free polymerase could exchange with polymerase at the replication fork, although both the leading- and lagging-strand polymerases appeared highly processive by kinetic measurements. They propose that the T4 gene 45 sliding clamp serves as the docking site for a DNA polymerase that can then exchange with the active DNA polymerase also associated with the gene 45 protein. E. coli has a number of proteins that recently have been shown to associate physically with the sliding clamp that anchors DNA polymerase to the primer–template (14). For example, several of the E. coli DNA polymerases involved in the repair of damage in DNA as well as MutL and MutS that are involved in mismatch repair bind to the clamp (28, 29). It has been suggested that the sliding clamps function as a platform for the delivery of proteins that act on DNA (30, 31). T7 has no exact equivalent of the sliding clamp, processivity being conferred by trx. We propose that gp5/trx is anchored to the helicase that in turn serves as the platform to deliver additional DNA polymerases or other proteins.
Materials and Methods
Enzymes.
Gp5/trx and gp5-Y526F/trx were overproduced in E. coli A307(DE3) (26) after transformation with plasmids pGP5–3/pTrxA and pGP5(Y526F)/pTrxA, respectively. A 1:1 complex of gp5 and thioredoxin was purified as described previously (2). Gp4 (21) and gp2.5 (8) were overproduced and purified as described. E. coli SSB protein was purchased from USB (Cleveland, OH).
DNA Polymerase Assay on ssDNA.
DNA polymerase activity was measured by using primed M13 ssDNA (2). DNA polymerase assays (100 μl) contained 50 mM Tris·Cl (pH 7.5); 10 mM MgCl2; 1 mM DTT; 50 mM NaCl; 250 μM each dGTP, dATP, dCTP; 10 Ci/mmol [3H]dTTP; and 7 nM M13 ssDNA that was annealed with a 17-nucleotide primer (-40 primer). Reactions were initiated by the addition of either 2 nM T7 gp5/trx or gp5-Y526F at 37°C. Aliquots (10 μl) were removed at the indicated times, and EDTA was added to a final concentration of 25 mM. The amount of incorporated [3H]dTMP was measured by using a filter-binding assay (2). Assays used to study the effects of ddNTPs on polymerase activity contained 25 μM ddGTP.
Polymerase Exchange Assay on ssDNA Using gp5-Y526F.
Polymerase reactions were as described above. Reactions were initiated by adding 10 nM gp5-Y526F/trx at 30°C. Next, a 10-fold molar excess of gp5/trx (100 nM final concentration) was added to the reaction mixture at 30 s. Reactions were performed in the presence or absence of 50 μM ddGTP. Aliquots (6 μl) were removed at the indicated times and analyzed as described above. When indicated, E. coli SSB protein was present at 165 μg in 40 μl of reaction mixture.
Strand Displacement DNA Synthesis on M13 dsDNA.
Strand displacement synthesis was measured by using a circular M13 dsDNA template having a replication fork (32). Strand displacement synthesis was performed on the 2 nM dsDNA construct at 37°C in standard reaction mixture containing 50 mM Tris·HCl (pH 7.5); 10 mM MgCl2; 5 mM DTT; 50 mM potassium glutamate; and 500 μM each dATP, dCTP, dGTP, and dTTP. Reactions were initiated with the addition of 16 nM gp4, 4 μM gp2.5, and either 16 nM gp5/trx or gp5-Y526F/trx. DNA synthesis was monitored by the amount of [3H]dTMP incorporated. At the indicated times, 6-μl aliquots were removed and analyzed as above. When indicated, ddGTP was present at 50 μM.
Processivity Assay on M13 dsDNA Using a DNA Trap.
M13 dsDNA (3.5 nM) was preincubated with 28 nM hexamer gp4 common 7 μM gp2.5 common 28 nM gp5/trx for 5 min at 37°C in the absence of MgCl2 and dNTPs. The reaction was initiated by adding MgCl2 and dNTPs, with (dC)200·oligo(dG)20 to trap any free enzyme. The processivity assay was then performed at 37°C in a standard strand displacement assay (40-μl total volume) containing 2 nM M13 dsDNA, 16 nM hexameric gp4, 4 μM gp2.5, 16 nM gp5/trx, 0.4 μM (dC)200·oligo(dG)20 DNA trap, and 2,500 Ci/mmol [α-33P]dATP. At the indicated times, 6-μl aliquots were removed, and the reaction was stopped by the addition of 25 mM EDTA. The reaction products were subjected to electrophoresis on a 0.8% alkaline-agarose gel. The gels were dried and exposed to a phosphorus imaging plate followed by scanning with a Fuji BAS 1000 bioimaging analyzer (Fuji Photo Co., Tokyo, Japan). Assays for graphical interpretation had 10 Ci/mmol [3H]dTTP substituted for [α-33P]dATP.
The (dC)200·oligo(dG)20 DNA trap was prepared by mixing 40 pmol of (dC)200 with 5-fold molar excess oligo(dG)20 in a 12-μl total volume and incubating the mixture for 5 min at 75°C followed by 30 min at room temperature. In trap control assays, the DNA trap was added to the preincubation mixture.
Exchange Reaction on M13 dsDNA.
Exchange reactions during strand displacement synthesis were performed in a 40-μl standard strand displacement reaction mixture containing 2 nM M13 dsDNA and 10 Ci/mmol [3H]dTTP. Reactions were initiated by the addition of 16 nM gp4 common 4 μM gp2.5 common 16 nM gp5-Y526F/trx. At 1 min, a 10-fold excess of WT gp5/trx was added to the reaction, followed by the addition of 50 μM ddGTP at 2 min. At the indicated times, 6-μl aliquots were removed, and the amount of DNA synthesis was measured as described above. In the reverse scheme, 16 nM gp5-Y526F/trx was replaced by WT gp5/trx to initiate the reaction, and a 10-fold excess of WT gp5/trx was replaced by gp5-Y526F/trx at 2 min into the reaction.
Exchange Reaction on a Minicircle.
The minicircle containing a replication fork was constructed as described previously (12). Strand displacement synthesis was performed in a 60-μl standard strand displacement reaction mixture containing 16 nM minicircle and 15 Ci/mmol [3H]dGTP to monitor DNA synthesis. Reactions were initiated by the addition of 2 nM hexameric gp4, 1 μM gp2.5, and either 14 nM gp5/trx or gp5-Y526F/trx at 25°C. At 1 min, a 10-fold excess of WT gp5/trx was added to the reaction followed by an addition of 50 μM ddGTP at 2 min. Aliquots (10 μl) were removed at the indicated times, and DNA synthesis was measured as described above. For coordinated leading- and lagging-strand syntheses on the minicircle, 500 μM ATP and CTP were included to the reaction. [3H]dGTP (15 Ci/mmol) or 18 Ci/mmol [3H]dCTP was added to the reaction mixture to monitor leading- or lagging-strand synthesis, respectively.
Processivity on a Minicircle.
To measure processivity of DNA synthesis on the minicircle, 3 nM hexamer gp4, 1.5 μM gp2.5, and 20 nM gp5/trx were first mixed with the 22 nM minicircle and preincubated for 5 min at 25°C in the absence of MgCl2 and dNTPs. The reaction was initiated by the addition of MgCl2/dNTPs/(dC)200·oligo(dG)20 to trap any free enzyme. The processivity assay was performed at 25°C in a standard strand displacement synthesis reaction mixture (60-μl total volume) containing 16 nM minicircle, 2 nM hexameric gp4, 1 μM gp2.5, 14 nM gp5/trx, 0.3 μM (dC)200·oligo(dG)20 DNA trap, and 3,000 Ci/mmol [α-(α-33P)]dGTP to monitor DNA synthesis. As indicated, 10-μl aliquots were removed and analyzed by gel electrophoresis as described above.
Acknowledgments
We thank Stan Tabor (Harvard Medical School) for plasmid pGP5-3, Joonsoo Lee (Harvard Medical School) for plasmid pGP5(Y526F), Ngoc Tron (Harvard Medical School) and Sharmistha Ghosh (Harvard Medical School) for ss-M13, and Steve Moskowitz (Advanced Medical Graphics, Boston, MA) for providing illustrations for the manuscript. This work was supported in part by U.S. Public Health Service Grant GM54397 (to C.C.R.).
Abbreviations
- dd
dideoxy
- gp5
gene 5 DNA polymerase
- gp5/trx
gp5/thioredoxin complex
- SSB protein
ssDNA-binding protein
- TBD
thioredoxin-binding domain
- trx
thioredoxin.
Footnotes
Conflict of interest: C.C.R. is a consultant to General Electronics Corp., which has a license from Harvard University to commercialize DNA polymerase for DNA sequencing.
References
- 1.Richardson CC. Cell. 1983;33:315–317. doi: 10.1016/0092-8674(83)90411-7. [DOI] [PubMed] [Google Scholar]
- 2.Tabor S, Huber HE, Richardson CC. J Biol Chem. 1987;262:16212–16223. [PubMed] [Google Scholar]
- 3.Huber HE, Tabor S, Richardson CC. J Biol Chem. 1987;262:16224–16232. [PubMed] [Google Scholar]
- 4.Matson SW, Tabor S, Richardson CC. J Biol Chem. 1983;258:14017–14024. [PubMed] [Google Scholar]
- 5.Lechner RL, Richardson CC. J Biol Chem. 1983;258:11185–11196. [PubMed] [Google Scholar]
- 6.Bernstein JA, Richardson CC. Proc Natl Acad Sci USA. 1988;85:396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendelman LV, Richardson CC. J Biol Chem. 1991;266:23240–23250. [PubMed] [Google Scholar]
- 8.Kim YT, Tabor S, Bortner C, Griffith JD, Richardson CC. J Biol Chem. 1992;267:15022–15031. [PubMed] [Google Scholar]
- 9.Bedford E, Tabor S, Richardson CC. Proc Natl Acad Sci USA. 1997;94:479–484. doi: 10.1073/pnas.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 11.Hamdan SM, Marintcheva B, Cook T, Lee S, Tabor S, Richardson CC. Proc Natl Acad Sci USA. 2005;102:5096–5101. doi: 10.1073/pnas.0501637102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Chastain PD, II, Kuskabe T, Griffith JD, Richardson CC. Mol Cell. 1998;1:1001–1010. doi: 10.1016/s1097-2765(00)80100-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Chastain PD, Griffith JD, Richardson CC. J Mol Biol. 2002;316:19–34. doi: 10.1006/jmbi.2001.5325. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell M. J Biol Chem. 2006;281:10653–10656. doi: 10.1074/jbc.R500028200. [DOI] [PubMed] [Google Scholar]
- 15.McHenry CS. Mol Microbiol. 2003;49:1157–1165. doi: 10.1046/j.1365-2958.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 16.Egelman EH, Yu X, Wild R, Hingorani MM, Patel SS. Proc Natl Acad Sci USA. 1995;92:3869–3873. doi: 10.1073/pnas.92.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Zhuang Z, Roccasecca RM, Trakselis MA, Benkovic SJ. Proc Natl Acad Sci USA. 2004;101:8289–8294. doi: 10.1073/pnas.0402625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabor S, Richardson CC. Proc Natl Acad Sci USA. 1987;84:4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabor S, Richardson CC. Proc Natl Acad Sci USA. 1989;86:4076–4080. doi: 10.1073/pnas.86.11.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabor S, Richardson CC. Proc Natl Acad Sci USA. 1995;92:6339–6343. doi: 10.1073/pnas.92.14.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notarnicola SM, Mulcahy HL, Lee J, Richardson CC. J Biol Chem. 1997;272:18425–18433. doi: 10.1074/jbc.272.29.18425. [DOI] [PubMed] [Google Scholar]
- 22.Lee JB, Hite RK, Hamdan SM, Xie XS, Richardson CC, van Oijen AM. Nature. 2006;439:621–624. doi: 10.1038/nature04317. [DOI] [PubMed] [Google Scholar]
- 23.Wuite GJL, Smith SB, Young M, Keller D, Bustamante C. Nature. 2000;400:103–106. doi: 10.1038/35003614. [DOI] [PubMed] [Google Scholar]
- 24.Debyser Z, Tabor S, Richardson CC. Cell. 1994;77:157–166. doi: 10.1016/0092-8674(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 25.Cannistraro VJ, Taylor JS. J Biol Chem. 2004;279:18288–18295. doi: 10.1074/jbc.M400282200. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DE, Richardson CC. J Biol Chem. 2003;278:23762–23772. doi: 10.1074/jbc.M301366200. [DOI] [PubMed] [Google Scholar]
- 27.Joyce CM. Proc Natl Acad Sci USA. 2004;101:8255–8256. doi: 10.1073/pnas.0402850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez de Saro FJ, Marinus MG, Modrich P, O'Donnell M. J Biol Chem. 2006;281:14340–14349. doi: 10.1074/jbc.M601264200. [DOI] [PubMed] [Google Scholar]
- 29.Lopez de Saro FJ, Georgescu RE, Goodman MF, O'Donnell M. EMBO J. 2003;22:6408–6418. doi: 10.1093/emboj/cdg603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indiani C, McInerney P, Georgescu R, Goodman MF, O'Donnell M. Mol Cell. 2005;19:805–815. doi: 10.1016/j.molcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Fujii S, Fuchs RP. EMBO J. 2004;23:4342–4352. doi: 10.1038/sj.emboj.7600438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delagoutte E, von Hippel PH. Biochemistry. 2001;40:4459–4477. doi: 10.1021/bi001306l. [DOI] [PubMed] [Google Scholar]