Abstract
Recent evidence indicates that growth hormone-releasing hormone (GHRH) functions as an autocrine/paracrine growth factor for various human cancers. A splice variant (SV) of the full-length receptor for GHRH (GHRHR) is widely expressed in various primary human cancers and established cancer cell lines and appears to mediate the proliferative effects of GHRH. To investigate in greater detail the role of SV1 in tumorigenesis, we have expressed the full-length GHRHR and its SV1 in MCF-7 human breast cancer cells that do not possess either GHRHR or SV1. In accordance with previous findings, the expression of both GHRHR and SV1 restored the sensitivity to GHRH-induced stimulation of cell proliferation, with SV1 being more potent than the GHRHR. Furthermore, MCF-7 cells transfected with SV1 proliferated more quickly than the controls, even in the absence of exogenously added GHRH, suggesting the existence of intrinsic, ligand-independent activity of SV1 after its transfection. In agreement with the stimulation of cell proliferation, the levels of proliferation markers cyclin D1, cyclin E, and proliferating cell nuclear antigen were elevated in MCF-7 cells treated with GHRH, cultured in both serum-free and serum-containing media. In addition, SV1 caused a considerable stimulation of the ability of MCF-7 cells to grow in semisolid medium, an assay considered diagnostic for cell transformation. Collectively, our findings show that the expression of SV1 confers oncogenic activity and provide further evidence that GHRH operates as a growth factor in breast cancer and probably other cancers as well.
Keywords: carcinogenesis, intrinsic activity, transfection
Growth hormone-releasing hormone (GHRH), produced by the hypothalamus, regulates the secretion of growth hormone (GH) by binding to specific receptors in the pituitary (1). In addition to this neuroendocrine action, recent studies also indicate that GHRH functions as an autocrine/paracrine growth factor in various cancers (2–5). The evidence for this concept includes the detection of GHRH expression in several extrapituitary tissues, especially primary human cancers and experimental tumors, and the ability of antagonistic analogs of GHRH to inhibit cancer cell growth by mechanisms that do not involve the GH-induced stimulation of production of hepatic insulin-like growth factor I (2–6).
Our understanding of how GHRH acts in extrapituitary tissues, and especially cancers, is restricted by poor functional characterization of the receptor(s) that mediates its mitogenic effects, especially in view of the limited expression of GHRH receptor (GHRHR) by extrapituitary tissues (7–9). Recently, it has been demonstrated that a splice variant (SV) of GHRHR (designated SV1, which differs from GHRHR in a short segment of the extracellular ligand-binding domain of the receptor protein) is widely expressed by different primary human and experimental cancers and can elicit mitogenic responses in the presence of GHRH (10–12). The suppression of the expression of SV1 by antisense RNA-based approaches indicates that SV1 may also possess some ligand-independent activity in addition to its GHRH-dependent activity (13).
In the present study, we have evaluated the consequences of forced expression of GHRHR and SV1 in MCF-7 human breast cancer cells that are both GHRHR- and SV1-negative (14, 15). The results of our work with this model are reported herein.
Results
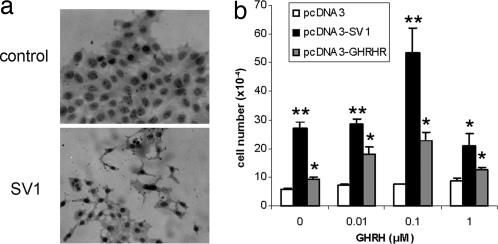
To elucidate the role of the SV1 and GHRHR receptors in cell proliferation, we have stably transfected MCF-7 human breast cancer cells that are GHRHR- and SV1-negative with the constructs pcDNA3-GHRHR and pcDNA3-SV1, which encode for the full-length cDNAs of GHRHR and SV1, respectively. The expression of the corresponding cDNAs was confirmed by immunocytochemistry (Fig. 1a and data not shown). As controls, empty-vector pcDNA3-transfected MCF-7 cells were used. As shown in Fig. 1b, the assessment of the rate of cell proliferation indicated that in the absence of ectopically expressed GHRHR(s), MCF-7 cells do not respond to GHRH, which is consistent with the previously reported lack of endogenous expression of GHRHR and SV1 by these cells. However, when SV1 and GHRHR were expressed in MCF-7 cells, the sensitivity to GHRH was restored, reaching a peak response at 0.1 μM with a reduction in the response at 1 μM GHRH. At a concentration of 0.1 μM GHRH, the number of cells was doubled for both the GHRHR and SV1-transfected cells, compared with the same cells cultured in vitro in the absence of GHRH. Nevertheless, in the absence of exogenously added GHRH in the culture media, the proliferation of SV1-transfected cells was significantly greater, 5-fold compared with the controls, whereas GHRHR-transfected cells exhibited only a minor stimulation of cell proliferation (Fig. 1b). This finding is consistent with a ligand-independent mode of action for SV1 (13).
Fig. 1.
Expression of GHRHR(s) in MCF-7 cells. (a) Immunocytochemical detection of SV1 receptor expression in MCF-7 pcDNA3-SV1 transfected cells. (b) Proliferation rate of MCF-7 cells exposed to different concentrations of GHRH following transfection with pcDNA3 (controls), pcDNA3-SV1, and pcDNA3-GHRHR. Ectopic expression of both SV1 and GHRHR restored sensitivity to GHRH with a peak at 0.1 μM GHRH. In the absence of GHRH, a significant stimulation of cell proliferation was noted in the SV1-transfected cells that is consistent with the ligand-independent activity of this alternatively spliced form of GHRHR. ∗, P < 0.01; ∗∗, P < 0.001.
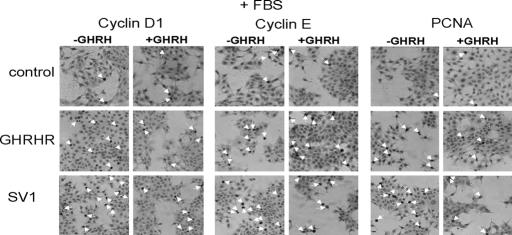
Subsequently, we investigated whether these differences in the rate of cell proliferation, after plasmid-mediated expression of GHRHR and SV1 and exposure to GHRH, were also reflected in the levels of the commonly used proliferation markers cyclin D1, cyclin E, and proliferating cell nuclear antigen (PCNA) (16–18). Because GHRH and other related peptides may be present in the serum and therefore could obscure the effects of the exogenously added GHRH, we decided to perform the analyses with cells cultured in both the presence and absence of serum (FBS). Figs. 2 and 3 show representative results of these immunocytochemical analyses, whereas Figs. 4, 5, and 6 show a graphical presentation of the positivity against each of these markers. The positivity was expressed as the fraction of positive against the total number of cells evaluated.
Fig. 2.
Representative results of the immunocytochemical analysis of MCF-7 cells transfected with pcDNA3, pcDNA3-SV1, and pcDNA3-GHRHR for cyclin D1 (Left), cyclin E (Center), and PCNA (Right), in the presence (+GHRH) or the absence (−GHRH) of exogenously added GHRH. Cells were cultured in media containing 10% FBS. The arrows indicate positive cells.
Fig. 3.
Representative results of the immunocytochemical analysis of MCF-7 cells transfected with pcDNA3, pcDNA3-SV1, and pcDNA3-GHRHR for cyclin D1 (Left), cyclin E (Center), and PCNA (Right) in the presence (+GHRH) or the absence (−GHRH) of exogenously added GHRH. Cells were cultured in serum-free media. The arrows indicate positive cells.
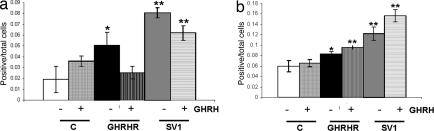
Fig. 4.
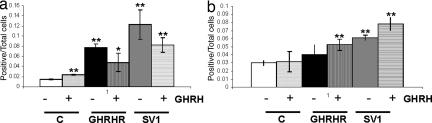
Graphic presentation of the fraction of MCF-7 cells transfected with pcDNA3, pcDNA3-SV1, and pcDNA3-GHRHR that was positive for cyclin D1 expression. The presence or absence of exogenously added GHRH in the culture media is indicated. (a) Cyclin D1 (+FBS). Shows the experiment performed with media-containing serum (FBS). (b) Cyclin D1 (−FBS). Shows the experiment performed with serum-free media. ∗, P < 0.05; ∗∗, P < 0.01.
Fig. 5.
Graphic presentation of the fraction of MCF-7 cells transfected with pcDNA3, pcDNA3-SV1, and pcDNA3-GHRHR that was positive for cyclin E expression. The presence or absence of exogenously added GHRH in the culture media is indicated. (a) Cyclin E (+FBS). Shows the experiment performed with media-containing serum (FBS). (b) Cyclin E (−FBS). Shows the experiment performed with serum-free media. ∗, P < 0.05; ∗∗, P < 0.01.
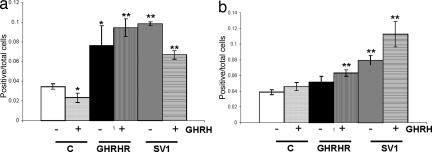
Fig. 6.
Graphic presentation of the fraction of MCF-7 cells transfected with pcDNA3, pcDNA3-SV1, and pcDNA3-GHRHR that was positive for PCNA expression. The presence or absence of exogenously added GHRH in the culture media is indicated. (a) PNCA (+FBS). Shows the experiment performed with media-containing serum (FBS). (b) PCNA (−FBS). Shows the experiment performed with serum-free media. ∗, P < 0.05; ∗∗, P < 0.01.
In general, the results obtained were in agreement with the proliferation results summarized in Fig. 1: Compared with controls, the exposure to GHRH caused a significant stimulation of cell proliferation in the cells expressing either SV1 or GHRHR, but SV1 induced a higher fraction of cells expressing the three markers analyzed. However, contrary to the lack of intrinsic activity in cells expressing GHRHR as assessed by the proliferation rate (Fig. 1b), cyclin D1 and cyclin E proliferation markers were elevated in cells expressing GHRHR even in the absence of GHRH supplementation (Figs. 4a and 5a). This effect was diminished or abolished when the same experiment was performed with cells cultured in the absence of serum (Figs. 4b and 5b), implying that GHRH and/or related peptide in the sera may interact with receptor(s) for GHRH. Furthermore, in the presence of serum, the fraction of cyclin D1 and PCNA-expressing cells was reduced (Figs. 4a and 6a) in both the SV1 and the GHRHR transfectants and in the presence of exogenously added GHRH. This reduction was also noted for cyclin E (Fig. 5a), but only for the SV1-transfectants, and may be because of the saturation of the corresponding signaling pathways by the activation caused by GHRH and/or other related peptides that may be present in the serum of the culture media.
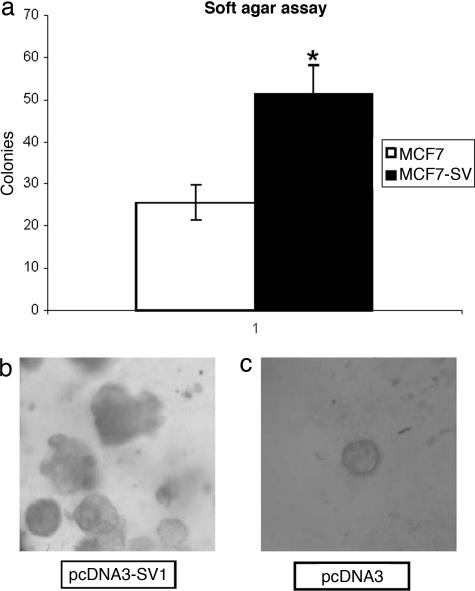
Finally, considering the potency of the mitogenic effects mediated by SV1 in association with its ability to induce cell proliferation without GHRH supplementation, we have evaluated the ability of MCF-7 cells expressing SV1 to grow in semisolid media. As shown in Fig. 7a, ectopic SV1 expression nearly doubled the number of colonies compared with control, empty-vector transfected MCF-7 cells. Furthermore, the colonies were also, in general, larger (Fig. 7 b and c), with noncanonical shape, consistently with the more aggressive phenotype contributed by SV1 expression (Fig. 7b).
Fig. 7.
Growth in soft agar of MCF-7 cells transfected with pcDNA3 or pcDNA3-SV1. (a) Graphic presentation of the results. (b and c) Representative microphotographs. The expression of SV1 stimulated the ability of MCF-7 cells to grow in soft agar. (b) Colonies formed by SV1 expressing cells are larger and noncanonical in shape. ∗, P < 0.05.
Discussion
Besides its neuroendocrine action, GHRH is now recognized as an autocrine/paracrine growth factor for various cancers. However, despite this well established ability to promote cancer growth, the mechanisms that mediate these mitogenic effects of GHRH remain poorly characterized, particularly at the level of the receptor(s) (2–4). Recently, it has been shown that GHRHR mediates mitogenic effects in breast cancer and probably other cancer cells by mechanism(s) that involve signaling pathways of the Ras, Raf, and MAP kinase (15). In contrast to the full-length receptor, SV1 exhibits a relatively restricted pattern of extrapituitary expression and is expressed widely in primary human tumors and established cancer cell lines (12). SV1 represents a product of alternative splicing of GHRHR and differs from the full-length receptor in a small part of the extracellular portion of the receptor protein that results in the alteration of the ligand-binding domain of the receptor. Recently, we have demonstrated that this alteration does not lead to compromised ligand binding because SV1 retains its ability to bind specifically and elicit mitogenic responses in the presence of GHRH (11). Furthermore, experiments involving antisense RNA-mediated inhibition of SV1 expression in HEC1A endometrial carcinoma cells imply that SV1 may also exert ligand-independent activity (13).
In the present study, we have performed a series of experiments to evaluate the biological consequences of expression of SV1 compared with expression of GHRHR in MCF-7 breast cancer cells. MCF-7 cells do not express either SV1 or GHRHR and thus represent a useful system for assessing the effects of the forced expression of these forms of receptors for GHRH. An assessment of the rate of cell proliferation after the treatment with SV1 and GHRHR revealed that GHRH sensitivity in MCF-7 cells was restored, which argues in favor of the specificity of the GHRH-triggered mitogenic responses. According to the typical mode of hormone-mediated action, the stimulation of cell proliferation under our experimental conditions reached a peak at 0.1 μM GHRH, followed by a reduction at higher GHRH concentrations. In the absence of exogenously added ligand, GHRHR-transfected cells exhibited only a minor stimulation of cell proliferation, which was probably due to GHRH or peptides related to GHRH being present in the medium with serum. Cells transfected with SV1 exhibited a strong induction of cell proliferation, which was most likely because of the ligand-independent activity of SV1.
Consistently with the stimulation of the rate of cell proliferation, the levels of markers of cell proliferation cyclin D1, cyclin E, and PCNA were elevated in cells expressing SV1 or GHRHR after the exposure to GHRH. The use of these markers is justified by recent findings. Thus, GHRHR and conceivably SV1, because they share the intracellular part of the receptor, operate by mechanisms that involve activation of Ras signaling. Cyclin D1 and cyclin E represent well established targets of Ras signaling activation (15, 19, 20). Nevertheless, PCNA represents a widely used general marker for cell proliferation (18). In cells growing in the absence of serum, the fraction of these cells expressing these markers consistently increased after the exposure to GHRH. However, when cells were cultured in serum-containing media, GHRH supplementation resulted in the reduction of two proliferation markers (cyclin E and PCNA) in SV1-transfected cells and for cyclin D1 and PCNA for GHRHR-transfected cells. This discrepancy between these experiments performed both in serum-free and serum-containing media may be because of the fact that GHRH and related peptides are probably present in the sera. In fact, the expression of GHRH at relatively high levels has already been reported for MCF-7 cells (14, 15). This may cause some saturation in the corresponding signaling pathways and, after supplementation with exogenous GHRH, results in their down-regulation. Considering the recently reported antiapoptotic effect of GHRH on cancer cells (21), it could be argued that serum deprivation may be sufficient to trigger apoptosis in MCF-7 cells, thus complicating the interpretation of our experimental results. However, apoptosis appears unlikely because, under our experimental conditions, no evidence of cell death was noted after 4 days of culture in the absence of serum, despite the dramatic reduction in the baseline levels of cell proliferation.
Another unexpected finding of our experiments is that both PCNA and cyclin D1 baseline levels were higher in serum-free rather than serum-containing media. The results of these independent sets of experiments are not formally comparable, but it is also conceivable that they reflect a feedback response of the cells under the conditions of serum deprivation.
The assessment of the ability of SV1-expressing MCF-7 cells to grow in semisolid media, an assay that is considered diagnostic for malignant transformation, showed that the expression of SV1 resulted in a statistically significant increase in the number of colonies formed by MCF-7 cells in soft agar. Morphologically, the resulting colonies were also larger than the in controls and exhibited an arbitrary pattern of growth that is also consistent with more aggressive phenotype of SV1.
The ligand-independent activity of SV1, in association with recent findings on the identification of another SV of GHRHR that differs from the full-length receptor at the 3′ end (3-end SV) (22) and operates as a dominant-negative form of the receptor, imply that alternative SVs of the GHRHR form a complex regulatory network. Thus, it is conceivable that GHRHR activity is determined not only by the abundance of the GHRH but also by the relative levels of these SVs that produce either positive (SV1) or negative (3-end SV) effects on GHRHR signaling.
Collectively, the results of this study, together with previous finding on the expression of SV1 in a wide range of primary human tumors (11, 13, 23), indicate a mechanistic association between SV1 expression and carcinogenesis. The latter should be viewed in the context of both the paracrine/autocrine stimulation by GHRH as well as the ligand-independent intrinsic activity of SV1. Consistent with this notion, we have previously reported that SV1 and GHRH do not exhibit an overlapping pattern of expression in primary human breast cancers and probably in other cancers. In addition, the expression of SV1 by normal human (12) and mouse (24) tissues implies that its mitogenic activity is not only limited to neoplasia but also may play a role under physiological conditions. Finally, the apparent association between SV1 and carcinogenesis implies that specific inhibition of SV1 activity, both its GHRH-dependent and especially its ligand-independent activity, may provide means for the development of anticancer therapies.
Materials and Methods
Plasmids.
The construction of plasmids pcDNA3-SV1 and pcDNA3-GHRHR has been reported in refs. 11 and 13.
Cell Culture.
MCF-7 human breast cancer cells were originally obtained from American Type Culture Collection (Manassas, VA) and cultured in DMEM supplemented with antibiotics/antimycotics, and 10% FBS unless otherwise described, at 37°C in a humidified 95% air/5% CO2 incubator. Transfections were performed by seeding 5 × 104 cells in six-well tissue culture plates with 1 μg of the appropriate plasmid by using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Stable lines were generated by selection of the resistant colonies with 500 μg/ml G418 (Invitrogen) for 2 weeks. Culture media were changed daily during selection. The rate of cell proliferation was calculated by seeding 5 × 104 (unless otherwise stated) cells in six-well plates and subsequently counting them after 4 days under a light microscope by using the Trypan-Blue exclusion assay. Three different concentrations (1, 0.1, and 0.01 μM) of hGHRH (1–29)NH2 were used. hGHRH (1–29)NH2 was obtained from A. F. Parlow (National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD).
Soft Agar Growth Assay.
Anchorage-independent growth as a characteristic of in vitro tumorigenicity was assessed by soft agar clonogenic assay. Cells were detached and plated in 0.4% agarose with a 1% agarose underlay (4 × 104 cells per well in six-well plates). The number of foci was counted after 23 days.
Immunocytochemistry.
Exponentially growing MCF-7 cells, after the treatment indicated, were fixed onto poly(l-lysine)-coated slides by exposure to 4% formalin and subjected to immunocytochemistry. The immunohistochemical detection of SV1 was performed with the rabbit anti-SV1 polyclonal antibody 2317/5 (25) diluted with 1× PBS at a ratio of 1:500. Cyclin D1 was detected by using a monoclonal mouse antibody (Oncogene Science, Cambridge, MA) and diluted with 1× PBS at a ratio of 1:1000. Cyclin E was detected by using a rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted with 1× PBS at a ratio of 1:1,000, and PCNA was detected by using a monoclonal mouse antibody (Oncogene Science) diluted with 1× PBS at a ratio of 1:1,000.
Subsequently, all slides were processed by using the Kwik-DAB kit (ThermoShandon, Pittsburgh, PA) according to the manufacturer's instructions. Specimens were evaluated for positive staining by counting an average of 365 cells in at least three optic fields. The results were expressed as positive per total cells assessed.
Statistical Analysis.
The data are expressed as the mean ± SEM. Statistical evaluation of the results was performed by the Student's t test (two-tailed). P values shown are against the control group unless otherwise stated.
Acknowledgments
We thank Dr. A. F. Parlow for providing us with hGHRH (1–29)NH2. This study was supported by grants Kapodistrias (06) from Special Account for Research Grants of the University of Athens (to H.K.) and Pythagoras II from the Ministry of Education (to S.K). A.V.S. is Distinguished Medical Research Scientist of the Veterans Affairs (VA) Department, and his work was supported by the medical research service of the VA. N.B. is a recipient of a fellowship from the Alexander S. Onassis Public Benefit Foundation.
Abbreviations
- GH
growth hormone
- GHRH
GH-releasing hormone
- GHRHR
GHRH receptor
- SV
splice variant
- PCNA
proliferating cell nuclear antigen.
Footnotes
The authors declare no conflict of interest.
References
- 1.Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga JL, Halmos G. Front Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 2.Schally AV, Varga JL. Comb Chem High Throughput Screen. 2006;9:163–170. doi: 10.2174/138620706776055449. [DOI] [PubMed] [Google Scholar]
- 3.Kiaris H, Schally AV, Kalofoutis A. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 4.Kiaris H, Koutsilieris M, Kalofoutis A, Schally AV. Expert Opin Investig Drugs. 2003;12:1385–1394. doi: 10.1517/13543784.12.8.1385. [DOI] [PubMed] [Google Scholar]
- 5.Kineman RD. Proc Natl Acad Sci USA. 2000;97:532–534. doi: 10.1073/pnas.97.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiaris H, Schally AV, Varga J, Armatis P, Groot K. Proc Natl Acad of Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J. Endocrinology. 1995;136:4147–4150. doi: 10.1210/endo.136.9.7649123. [DOI] [PubMed] [Google Scholar]
- 8.Mayo KE. Mol Endocrinol. 1992;6:1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- 9.Gaylinn BD, Harrison JK, Zysk JR, Lyons CE, Lynch KR, Thorner MO. Mol Endocrinol. 1993;7:77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- 10.Rekasi Z, Czompoly T, Schally AV, Halmos G. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiaris H, Schally AV, Busto R, Halmos G, Artavanis-Tsakonas S, Varga JL. Proc Natl Acad of Sci USA. 2002;99:196–200. doi: 10.1073/pnas.012590999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, Szepeshazi K, Koster F, Kovitz K, Groot K, et al. Proc Natl Acad of Sci USA. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiaris H, Chatzistamou I, Schally AV, Halmos G, Varga JL, Koutselini H, Kalofoutis A. Proc Natl Acad Sci USA. 2003;100:9512–9517. doi: 10.1073/pnas.1533185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Fernandez MO, Schally AV, Varga JL, Groot K, Busto R. Breast Cancer Res Treat. 2003;77:15–26. doi: 10.1023/a:1021196504944. [DOI] [PubMed] [Google Scholar]
- 15.Siriwardana G, Bradford A, Coy D, Zeitler P. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0001. [DOI] [PubMed] [Google Scholar]
- 16.Barnes DM, Gillett CE. Breast Cancer Res Treat. 1998;52:1–3. doi: 10.1023/a:1006103831990. [DOI] [PubMed] [Google Scholar]
- 17.Hunt KK, Keyomarsi K. Semin Cancer Biol. 2005;15:319–326. doi: 10.1016/j.semcancer.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Munster PN, Norton L. Breast Cancer Res. 2001;3:361–364. doi: 10.1186/bcr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musgrove EA. Growth Factors. 2006;24:13–19. doi: 10.1080/08977190500361812. [DOI] [PubMed] [Google Scholar]
- 20.Yu Q, Geng Y, Sicinski P. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 21.Rekasi Z, Czompoly T, Schally AV, Boldizsar F, Varga J, Zarandi M, Berki T, Horvath R, Nemeth P. Proc Natl Acad Sci USA. 2005;102:3435–3440. doi: 10.1073/pnas.0410006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElvaine AT, Mayo KE. Endocrinology. 2006;147:1884–1894. doi: 10.1210/en.2005-1488. [DOI] [PubMed] [Google Scholar]
- 23.Halmos G, Schally AV, Czompoly T, Krupa M, Varga JL, Rekasi Z. J Clin Endocrinol Metab. 2002;87:4707–4714. doi: 10.1210/jc.2002-020347. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou C, Schally AV, Chatzistamou I, Kondi-Pafiti A, Lamnissou K, Kouloheri S, Kalofoutis A, Kiaris H. Regul Pept. 2006;136:105–108. doi: 10.1016/j.regpep.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Toller GL, Horvath JE, Schally AV, Halmos G, Varga JL, Groot K, Chism D, Zarandi M. Proc Natl Acad Sci USA. 2004;101:15160–15165. doi: 10.1073/pnas.0406348101. [DOI] [PMC free article] [PubMed] [Google Scholar]