Abstract
Most mammary gland development occurs after birth under the control of systemic hormones. Estrogens induce mammary epithelial cell proliferation during puberty via epithelial estrogen receptor α (ERα) by a paracrine mechanism. Epidermal growth factor receptor (EGFR) signaling has long been implicated downstream of ERα signaling, and several EGFR ligands have been described as estrogen-target genes in tumor cell lines. Here, we show that amphiregulin is the unique EGF family member to be transcriptionally induced by estrogen in the mammary glands of puberal mice at a time of exponential expansion of the ductal system. In fact, we find that estrogens induce amphiregulin through the ERα and require amphiregulin to induce proliferation of the mammary epithelium. Like ERα, amphiregulin is required in the epithelium of puberal mice for epithelial proliferation, terminal end buds formation, and ductal elongation. Subsequent stages, such as side-branching and alveologenesis, are not affected. When amphiregulin−/− mammary epithelial cells are in close vicinity to wild-type cells, they proliferate and contribute to all cell compartments of the ductal outgrowth. Thus, amphiregulin is an important paracrine mediator of estrogen function specifically required for puberty-induced ductal elongation, but not for any earlier or later developmental stages.
Keywords: ductal morphogenesis, epithelial-stromal cross-talk, paracrine
The mammary gland is the only organ that undergoes most of its development after birth, with the female reproductive hormones estrogen, progesterone, and prolactin acting as master regulators (1, 2). During embryogenesis, a rudimentary ductal system develops that grows isometrically with the rest of the body during the first weeks of life. At the onset of puberty, when the ovaries start to secrete estrogens, the ducts extend from the nipple area into a pad of fatty connective tissue that lies under the skin. The tips of the ducts enlarge to form club-shaped structures called terminal end buds (TEBs), which contain highly proliferative cells (3). The ducts penetrate the fat pad by branching dichotomously. Subsequently, the complexity of the milk duct system increases with repeated estrous cycles through the growth of lateral branches. Side-branching is controlled by progesterone and intensifies during pregnancy (4). Subsequently, alveoli bud off the ducts and differentiate to become sites of milk production, a process controlled by prolactin receptor signaling (5).
The epidermal growth factor receptor (EGFR) signaling pathway has long been implicated in mammary gland development and human breast cancer (6). EGFR, a member of the ErbB receptor tyrosine kinase family (7), is activated by members of the EGF-like family of ligands, including EGF, transforming growth factor α (TGF-α), amphiregulin, heparin binding-EGF (HB-EGF), betacellulin (BTC), and epiregulin (EPR). These ligands are produced as transmembrane precursors that are proteolytically cleaved and shed from the cell surface (8).
A model was long held whereby estrogens acting on ERα in the stroma induce EGF ligands, which in turn stimulate proliferation of neighboring epithelial cells during puberty (9, 10). This model was inspired by early observations that arrest of ductal outgrowth and disappearance of TEBs seen in mice ovariectomized during puberty were rescued when 17-β-estradiol was administered locally by means of slow-release pellets (11, 12). Similarly, pellets releasing EGF, TGF-α, or amphiregulin were able to induce cell proliferation, TEB formation, and ductal elongation (13–15). However, tissue recombination experiments with EGFR-deficient mammary glands revealed that EGFR is required in the mammary stroma for ductal morphogenesis rather than in the epithelium (16–18). Furthermore, we recently demonstrated that estrogens drive ductal elongation via the epithelial estrogen receptor α (ERα) and that they act by paracrine mechanism mediated by an unknown factor (19). Here, we identify amphiregulin as the key mediator of ERα signaling essential for the massive epithelial cell proliferation characteristic of pubertal ductal elongation.
Results
Transcriptional Regulation of EGF Family Members by Estrogens in the Peripuberal Mammary Gland.
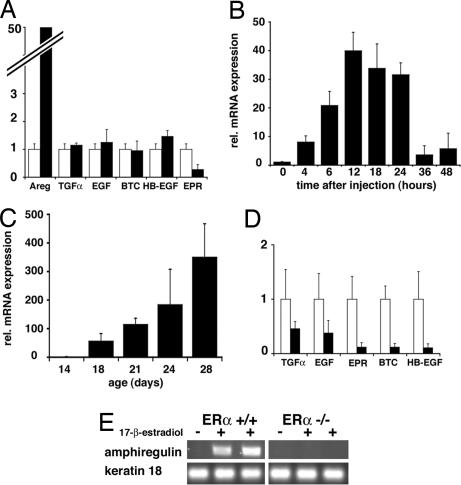
We have recently shown that estrogens induce ductal elongation during puberty acting through the ERα in the mammary epithelium by a paracrine mechanism. EGFR signaling has been implicated downstream of estrogen signaling in the mammary gland (6), and several EGF family members, such as EGF, TGFα, and amphiregulin, are induced by estrogens in different breast cancer cell lines (20–22). To test whether EGF family members are regulated by estrogen in vivo in the puberal mammary gland at a time of exponential cell expansion, we mimicked the beginning of puberty in a controlled fashion. Briefly, mice ovariectomized at 21 days received a single injection of 17-β-estradiol sufficient to induce TEB formation within 3–4 days (data not shown). Eight hours after injection, mammary glands were harvested, and mRNA expression levels of different EGFR ligands were measured. Strikingly, expression of EGF, TGF-α, HB-EGF, BTC, or EPR was not significantly modulated by 17-β-estradiol administration. Amphiregulin mRNA levels, however, were induced ≈50-fold (Fig. 1A); they were up-regulated within 4 h of injection, and expression peaked at 12 h and remained high until it decreased at 24 h and thereafter (Fig. 1B).
Fig. 1.
Regulation of EGFR ligands' expression by estrogens. Quantitative RT-PCR analysis of mammary gland mRNA for amphiregulin and other EGFR ligands normalized to keratin 18. (A and B) Mice ovariectomized before puberty were injected with either vehicle (open bars) or 17-β-estradiol (filled bars) and analyzed either for EGFR ligand expression 8 h later (A) or for amphiregulin over 48 h (B). (C and D) Mammary glands of 14- to 28-day-old mice were analyzed for amphiregulin expression (C) or for EGFR ligand expression (14- and 21-day-old females, open and filled bars, respectively) (D). Bars report the mean values obtained from three different mice. Error bars indicate standard deviation. Relative increase refers to control treated (A and B) or to the 14-day-old mice (C and D). (E) RT-PCR analysis of amphiregulin and keratin 18 expression in glands from ovariectomized WT and ERα−/− mice 6 h after administration of 17-β-estradiol (+) or vehicle (−).
To assess whether this specific regulation by 17-β-estradiol is physiologically relevant, we compared mRNA expression of EGFR ligands in mammary glands of 14-day-old prepuberal and 28-day-old puberal mice; the latter have ovaries that are actively secreting estrogens, whereas the former produce trace amounts of the steroid. Again, amphiregulin mRNA is strongly induced, paralleling increased ovarian estrogen production (Fig. 1C), whereas various other family members are not induced (Fig. 1D).
To test whether 17-β-estradiol-induced expression of amphiregulin is mediated by ERα, we stimulated prepuberal ERα−/− mice by using the same protocol as above. At this stage, ERα−/− and wild-type (WT) glands are phenotypically undistinguishable (19). In the absence of ERα, amphiregulin mRNA did not increase (Fig. 1E). Thus, amphiregulin expression is strongly regulated by 17-β-estradiol in the puberal mammary gland by ERα-dependent transcriptional activation.
Mammary Gland Development in Amphiregulin-Deficient Mice.
Our finding that estrogens specifically control expression of amphiregulin and not of other EGF family members could provide an explanation as to why deletion of amphiregulin, but not of TGFα or EGF, impairs mammary gland development (23). To assess whether the phenotype is specifically linked to estrogen action, we analyzed mammary glands of amphiregulin−/− and their WT littermates at critical developmental stages by whole-mount microscopy.
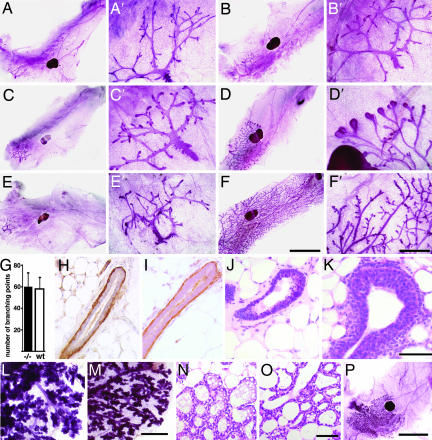
At birth, mutant and WT mammary glands were indistinguishable (data not shown). Similarly, in both genotypes, the rudimentary ductal systems grew isometrically until puberty (Fig. 2 A and B) with comparable numbers of branching points (Fig. 2G). TEBs developed in glands of 6-week-old WT females, whereas the ductal tips did not enlarge in amphiregulin−/− glands (Fig. 2 C and D). At 3 months of age, the WT glands were fully arborized and side-branching occurred, whereas amphiregulin−/− glands still displayed a rudimental ductal tree (Fig. 2 E and F). These observations were consistent with amphiregulin acting downstream of estrogen signaling during puberal development. However, it was conceivable that the inability to grow at puberty was secondary to a structural defect acquired before this stage. To address this concern, we analyzed prepuberal glands histologically and performed immunostainings for smooth muscle actin α (SMAα), a marker of myoepithelial cells. At 24 days, in both WT and amphiregulin−/− mice, the luminal and myoepithelial layers were intact (Fig. 2 H and I). Thus, at least histologically, hormone-independent mammary development is normal. Histological analysis of mammary glands from 26-day-old mice revealed that in amphiregulin−/− females, ductal tips failed to enlarge, whereas TEBs formed in the WT littermates (Fig. 2 J and K).
Fig. 2.
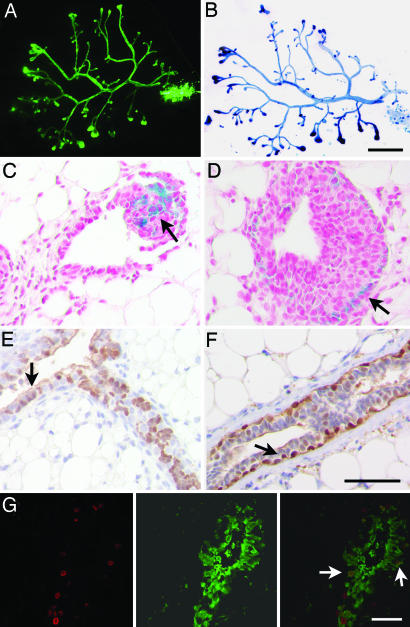
Developmental analysis of amphiregulin−/− mammary glands. (A–F) Whole-mount micrographs of inguinal glands from amphiregulin−/− (A, C, and E) and WT (B, D, and F) females were analyzed at the following developmental stages: day 24 (A and B), 6 weeks (C and D), 3 months (E and F). (Scale bars: A–F, 5 mm; A′–F′, 1 mm.) Micrographs are representative of glands from 12 mice analyzed per time point. (G) Number of branching points in inguinal mammary glands of 24-day-old amphiregulin−/− (filled bar, n = 6) and WT (open bar, n = 7) females. (H and I) Histological sections of mammary glands from 24-day-old amphiregulin−/− and WT mice stained by immunohistochemistry with an anti-SMAα antibody to highlight the myoepithelial cells (brown) counterstained with hematoxylin. H&E (J and K) staining of paraffin sections from 6-week-old amphiregulin−/− (J) and WT (K) glands. (Scale bar: 50 μm.) (L and M) Whole-mount micrographs of amphiregulin−/− (L) and WT (M) glands at 16.5 days of pregnancy. (N and O) Histological sections of amphiregulin−/− (N) and WT (O) mammary glands at 18.5 days of pregnancy. (P) Whole-mount micrograph of gland from an amphiregulin−/− female after 12 pregnancies.
During pregnancy, the rudimentary ductal system of the amphiregulin−/− mice, notwithstanding its limited expansion, still underwent side-branching and alveologenesis to an extent comparable to WT littermates (Fig. 2 L and M). Histological analysis revealed distended alveoli containing lipid droplets as seen in WT glands at this stage (Fig. 2 N and O). Strikingly, we never observed a completely filled fat pad, even after 12 pregnancies (Fig. 2P). Thus, amphiregulin is specifically required for estrogen-induced ductal elongation during puberty, but not for the preceding or later stages of mammary gland development, including side-branching, alveologenesis, or milk production.
The Role of Amphiregulin in Mediating Estrogen-Induced Proliferation and TEB Formation.
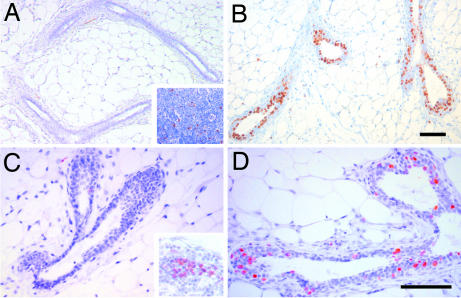
The observation that puberal outgrowth is completely blocked in the absence of amphiregulin, together with the finding that this growth factor is transcriptionally regulated by estrogen in the mammary gland, suggested that amphiregulin mediates estrogen function during puberty. To test this directly, we stimulated ovariectomized amphiregulin−/− and WT females with 17-β-estradiol and monitored proliferation and TEB formation over 4 days. In WT glands, proliferation assessed by BrdU incorporation was detected 24 h after 17-β-estradiol stimulation (Table 1) and peaked at 48 h, with 31 ± 6.7% of the epithelial cells incorporating BrdU (Fig. 3B). Amphiregulin−/− glands analyzed at 48 h did not show any increase in BrdU incorporation more than unstimulated glands (Fig. 3A, Table 1), but cells in the inguinal lymph nodes showed BrdU incorporation (Fig. 3A Inset), validating the BrdU administration. Similarly, 26-day-old amphiregulin−/− mice did not display more proliferation than 14-day-old mice (Fig. 3C), whereas WT glands displayed 18.78 ± 4.6% of BrdU-positive cells (Fig. 3D). Four days after 17-β-estradiol injection, numerous TEBs developed in the WT glands but not in amphiregulin−/− glands (data not shown). Thus, amphiregulin is required for estrogen-induced epithelial proliferation in peripuberal mice, and in the absence of amphiregulin estrogen-induced proliferation and TEB formation are completely abolished.
Table 1.
Epithelial cell proliferation in amphiregulin−/− mammary glands
| Control, % | E |
14 d, % | 26 d, % | ||
|---|---|---|---|---|---|
| 24 h, % | 48 h, % | ||||
| Amphiregulin−/− | 0.4 ± 0.12 | / | 0.2 ± 0.14 | 0.2 ± 0.15 | 0.4 ± 0.13 |
| WT | 0.36 ± 0.17 | 3.5 ± 1.7 | 31 ± 6.7 | 0.3 ± 0.12 | 18.78 ± 4.60 |
The percentage of cells staining positive for BrdU in mammary glands from amphiregulin−/− and WT mice that were ovariectomized at puberty and treated with 17-β-estradiol (E) or vehicle (control) for 24 or 48 h, and intact mice analyzed when they were 14 or 26 days old. More than 1,000 cells were counted on at least one section from three different mice. /, not analyzed.
Fig. 3.
Estrogen-induced proliferation in amphiregulin−/− and WT mammary glands. Immunohistochemistry was performed on sections of amphiregulin−/− (A and C) or WT (B and D) glands either from 3-week-old ovariectomized females 48 h after 17-β-estradiol stimulation (A and B) or 26-day-old mice (C and D) with an anti-BrdU antibody. Positively stained lymph nodes from the same glands are shown as positive control for BrdU incorporation (A and C Insets). (Scale bars: 50 μm.)
To exclude the possibility that estrogen-dependent proliferation is impaired in amphiregulin−/− glands because of altered ERα expression and/or downstream signaling, we determined the receptor status in the mutant glands by immunohistochemistry. Amphiregulin−/− and WT glands displayed comparable ERα expression in 24-day-old mice (data not shown). Moreover, glands of both genotypes showed similar inductions of the estrogen-target progesterone receptor in response to 17-β-estradiol stimulation (data not shown), indicating that ERα signaling remains intact in amphiregulin−/− mammary glands.
The Role of Amphiregulin in the Mammary Epithelium.
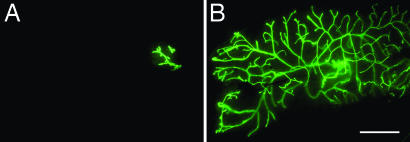
To determine to what extent the mammary phenotype of the amphiregulin−/− mice is attributable to mammary epithelial amphiregulin, we performed mammary gland recombination experiments by using tissue from amphiregulin−/− and WT littermates. In 3-week-old mice, the inguinal glands can be cleared of endogenous epithelium by surgically removing the nipple-near half that contains the rudimentary ductal system. Mammary epithelial cells that are introduced into the remaining “cleared” fat pad will give rise to a new ductal system. They can grow out from a piece of breast tissue that is implanted (24) or from single-cell suspensions injected into the fat pad (25). A caveat of this experimental approach lies in the possibility that endogenous epithelium can be inadvertently left behind and compete with the grafted epithelium for fat pad reconstitution. To circumvent this problem, we crossed the mutant amphiregulin allele into a transgenic strain that ubiquitously expresses GFP. By grafting GFP-positive donor tissue into GFP-negative hosts, we can readily distinguish the grafted from endogenous epithelium. Furthermore, this approach ensured that we engrafted comparable amounts of epithelium on both sides; we prepared pieces of donor tissue under UV illumination before implanting. The engrafted glands were analyzed 2–4 months later by whole-mount microscopy. Amphiregulin−/− epithelial grafts exhibited no outgrowth (Fig. 4A), whereas the WT counterpart filled the fat pad (Fig. 4B). Importantly, in contrast to ERα−/− epithelial grafts that remained rudimentary during pregnancy, amphiregulin−/− epithelia underwent side-branching and alveologenesis (data not shown), resulting in increased fat pad filling (Table 2). Thus, amphiregulin, like ERα, is required for ductal outgrowth during puberty in the mammary epithelium. However, amphiregulin−/− epithelial cells can still proliferate and differentiate in response to progesterone and prolactin.
Fig. 4.
Amphiregulin−/− epithelial transplants. Representative outgrowth of amphiregulin−/− (A) or WT (B) GFP+ mammary epithelium transplanted into WT cleared fat pads. The glands were derived from virgin recipients and observed directly under the fluorescence stereomicroscope (B). (Scale bar: 5 mm.)
Table 2.
Development of amphiregulin−/− mammary epithelium
| Mice | No. with no outgrowth | No. with <20% outgrowth | No. with 20–50% outgrowth | Total no. of mice |
|---|---|---|---|---|
| Virgin | 11 | 2 | 0 | 13 |
| Pregnant and postpartum | 3 | 2 | 4 | 9 |
Results of mammary gland reconstitution experiments with amphiregulin−/− mammary epithelium. Contralateral control grafts showed complete outgrowth. Different extent of outgrowth was observed depending on the developmental stage of the host.
Generation of Amphiregulin−/− and WT Chimeric Epithelia.
To assess whether amphiregulin mediates estrogen-induced proliferation in an autocrine/cell autonomous or paracrine fashion, we created mosaic mammary epithelia containing both WT and amphiregulin−/− cells. The cells of the two distinct genotypes were derived from mice carrying additionally either a GFP or LacZ transgene, allowing us to discriminate between mutant and WT cells. Of 68 successfully engrafted glands with a 1:1 mixture, 31 were composed of both cell populations (Fig. 5 A and B), whereas 37 were composed of WT cells only. As expected, no outgrowth was found to contain only amphiregulin−/− cells. Histological analysis of glands engrafted with amphiregulin−/− LacZ+ and WT cells revealed that amphiregulin−/− cells, identified by X-gal staining, are present among both cap and body cells of the TEBs (Fig. 5 C and D, arrows). Immunostainings for GFP on glands reconstituted with amphiregulin−/− GFP+ and WT cells showed that amphiregulin−/− cells, visualized by immunohistochemistry with an antibody against GFP, are found in both luminal and myoepithelial compartments in the mature ducts (Fig. 5 E and F, arrows).
Fig. 5.
Amphiregulin−/− and WT chimeric mammary epithelia. Whole-mount preparations of a cleared fat pad showing representative mammary reconstitution on injection with a mixture of amphiregulin−/− GFP+ (green), and WT ROSA26+ (blue) epithelial cells in a 1:1 ratio. (A and B) GFP was visualized directly under the fluorescence stereomicroscope (A); β-galactosidase activity revealed after X-Gal staining (B). (C and D) Histological sections of glands reconstituted with amphiregulin−/− LacZ+ and WT cells. Amphiregulin−/− LacZ+ cells (blue) were found both among the body (C, arrow) and the cap cells (D, arrow). (E and F) Glands reconstituted with amphiregulin−/− GFP+ and WT cells were sectioned and stained with anti-GFP antibody. Both luminal (F, arrow) and myoepithelial cells (E, arrow) show GFP staining (brown). (G) Glands reconstituted with amphiregulin−/− GFP+ and WT cells were sectioned and costained with anti-BrdU (Left), and anti-GFP antibodies (Center) merge (Right) reveals double-positive cells. (Scale bars: A and B, 500 μm; C–G, 50 μm.)
The extensive contribution of amphiregulin−/− cells to the reconstitution suggested to us that the mutant cells actively proliferate. To assess whether this is indeed the case, we performed double immunofluorescence for GFP, thereby labeling amphiregulin−/− cells and BrdU to mark the proliferating cells. GFP-positive cells were found to incorporate BrdU (Fig. 5G Right, arrows), indicating that, indeed, amphiregulin−/− mammary epithelial cells proliferate actively. Interestingly, when chimeric epithelia are generated from differentially marked WT cells, two distinct types of chimerism are observed. Either entire ductal segments are constituted by one type of cell or the ducts show a patchy distribution of the two groups of cells (L.C. and C.B., unpublished observations). Glands containing amphiregulin−/− cells showed only the latter type of chimerism (Fig. 5 E and F), indicating that close proximity to nearby WT cells is required to receive, directly or indirectly, the proliferative signal. We conclude that amphiregulin is an important paracrine mediator of estrogen-induced proliferation during ductal morphogenesis.
Discussion
Most mammary gland development occurs after birth under the control of female reproductive hormones. The advent of gene-targeting combined with powerful tissue recombination techniques allows dissecting the mechanisms by which systemic hormones elicit proliferation and morphogenesis. Immunohistochemical studies on human breast tissue (26) and rodent mammary glands (27, 28) revealed that in normal tissue steroid receptor expression and proliferation are dissociated. We recently provided genetic evidence that ERa−/− mammary epithelial cells completely fail to proliferate in vivo (19). However, when the mutant cells were grafted together with WT cells, they proliferated and contributed to all aspects of ductal morphogenesis, indicating that estrogens act by a paracrine mechanism in vivo (19). The nature of the downstream signals that ERα-positive cells send to ERa−/− cells in response to estrogens remained elusive. Here, we identify amphiregulin as a key mediator of paracrine estrogen action required for the massive mammary epithelial cell proliferation that results in ductal outgrowth during puberty.
Our findings support a model in which hormones acting on the mammary epithelium recruit a series of local factors that act by paracrine mechanisms to trigger proliferation of nearby cells (29, 30). More specifically, amphiregulin emerges as a central mediator of estrogen function, while we have previously shown that Wnt-4 is an important mediator of paracrine progesterone-induced side-branching (31), and that prolactin requires IGF-2 to induce alveolar proliferation (32). These indirect signaling mechanisms ensure that the systemic stimulus is amplified within the target organ over several cell diameters and over time, and that the behavior of different cells and cell types participating in the morphogenic event are coordinated and fine-tuned with local requirements.
The downstream events of amphiregulin action remain to be explored. The only known receptor for amphiregulin, EGFR, is expressed in both stromal and epithelial compartments (23, 33), but is required for ductal elongation in the stroma and not in the epithelium (16–18). Although this does not rule out that EGFR signaling also has a role in the epithelium, the prime targets of amphiregulin are stromal cells. In the simplest scenario, the stromal fibroblasts stimulated by amphiregulin could send back a mitogenic signal to the neighboring epithelial cells. Several growth factors, such as HGF, IGF1, and FGF10, are good candidates because they are expressed in the mammary stroma at the time of ductal elongation, whereas the respective receptors are found in the epithelium (34). Alternatively, down-modulation of inhibitory pathways, such as TGFβ signaling, may be involved (35). Yet more complex interactions might be required; thus, macrophages and eosinophils present in the mammary stroma have a role in ductal elongation (36) and could be attracted and activated by signals downstream of EGFR activation.
It is also unclear how amphiregulin, secreted by epithelial cells most likely of the ERα-positive luminal subtype, can reach the stromal target cells through a layer of myoepithelial cells and the basal lamina. Deletion of the disintegrin and metalloproteinase (ADAM) 17 in the mammary epithelium blocks ductal outgrowth similarly to amphiregulin (18). Analysis of tissue recombinants showed that ADAM17 is also required for EGFR activation, although to what extent this normally occurs in the stroma versus the epithelium is unclear (18). Whether the cleavage suffices to activate amphiregulin or whether the protein needs to be further modified and/or actively transported to the stromal target cells remains to be addressed. ADAM17 may also be required more indirectly for processing extracellular matrix and/or the basal lamina to enable amphiregulin to reach the stroma.
We found that the deletion of amphiregulin strictly and specifically affects ductal elongation, not preceding or subsequent developmental stages. At variance with our results, previous reports suggested that some TEBs can develop and ductal outgrowth can occur in the absence of amphiregulin (18, 23), whereas alveologenesis was impaired (23). The discrepancies may be because of the mixed genetic background used in previous studies, as opposed to the pure C57BL/6 used here. Moreover, some of the conclusions were reached based on recombinant glands grafted under the kidney capsule, an environment that is less physiological than the mammary fat pad (18).
Our finding that the ductal outgrowth defect in amphiregulin−/− mice is not overcome by multiple pregnancies is surprising given that other EGFR ligands, in particular EGF, are highly expressed in the glands during pregnancy (37). This suggests that either amphiregulin activates a unique signaling response downstream of EGFR or that EGF and/or other ligands are not expressed in the right cell types and/or not processed in the same ways as amphiregulin. We further speculate that the ability to bind heparin (38) that distinguishes amphiregulin from other family members may bestow this protein with unique functions in the reciprocal interactions with extracellular matrix and/or stromal components.
How does estrogen-induced ductal elongation relate to human breast cancer? When human breast tissue transplanted in mice was stimulated with estradiol under conditions that induce proliferation (39), amphiregulin was among the most highly induced genes (40), strongly suggesting that amphiregulin may similarly be involved in mediating estrogen-induced proliferation in the human breast epithelium. Estrogens and ERα signaling have an important role in the pathogenesis of breast cancer, with >60% of carcinomas expressing ERα and relying on estrogens for growth (41). In normal breast tissue, both human and rodent, steroid receptor expression and proliferation are mostly dissociated (26–28), whereas in tumor samples, most proliferative cells express ERα (26). Our experiments do not address whether amphiregulin can act in an autocrine and/or paracrine fashion on ERα-positive cells. Interestingly, TGF-β actively represses proliferation of ERα-positive cells (42), which are probably exposed to the same growth factors as their ERα-negative neighbors. This suggests that ERα-positive cells might escape the inhibitory influence of TGF-β during tumorigenesis and thereby become responsive to the auto/paracrine effects of amphiregulin resulting in proliferation. To what extent direct versus indirect stimuli account for the growth of ERα-positive breast carcinomas remains to be addressed.
Intriguingly, expression profiles of mammary glands from virgin and parous mice as well as distantly related rat strains revealed that amphiregulin is one of the genes most strongly down-modulated after the first pregnancy (43, 44). This down-modulation correlates with a decrease in susceptibility to tumorigenesis, suggesting this factor is linked to breast carcinogenesis.
Methods
Mice.
ROSA26 mice (45) were purchased from The Jackson Laboratory (Bar Harbor, ME). GFP transgenic, amphiregulin, and ERα-deficient mice were described (see refs. 23, 46, and 47). ROSA26 mice were bred in 129SV/C57BL/6, and ERα, amphiregulin, and GFP mice were bred in C57BL/6 genetic background. The presence of the β-galactosidase transgene was assessed by subjecting a piece of tail to the X-Gal staining procedure described below.
Transplantation of Mammary Epithelium.
The fat pads of 3-week-old C57BL/6 or 129SV/C57BL/6 females were cleared. Pieces of mammary tissue of 1 mm in diameter were removed from the nipple region (24). Alternatively, the cleared fat pads were injected with mixtures of primary mammary epithelial cells as described (see ref. 25).
Hormone Treatment.
17-β-Estradiol (Sigma–Aldrich, St. Louis, MO) 5 mg/ml 100% ethanol stock was diluted in PBS. Three-week-old female mice were ovariectomized and 10 days later were injected s.c. with 17-β-estradiol (5 ng/g of body weight) or vehicle.
Mammary Gland Whole Mounts.
Mammary glands were dissected, spread onto a glass slide, fixed in a 1:3 mixture of glacial acetic acid/100% ethanol, hydrated, stained overnight in 0.2% carmine (Sigma) and 0.5% AlK(SO4)2, dehydrated in graded solutions of ethanol, and cleared in 1:2 benzyl alcohol/benzyl benzoate (Sigma) as described (see ref. 48). Digital pictures were taken on a Leica (Wetzlar, Germany) MZFLIII stereoscope or Leica DM2000 microscope with Leica DC300F and Pixelink (Ottawa, ON, Canada) PL-A622C, respectively.
X-Gal Staining.
The transplanted mammary glands were dissected, fixed for 1 h in 1.5% formaldehyde in PBS, washed three times over 3 h with rinse buffer (2 mM MgCl2/0.1% sodium deoxycholate/0.2% Nonidet P-40 in PBS) and rotated in X-Gal staining solution (1 mg/ml X-Gal, 5 mM potassium ferricyanide, and 5 mM potassium ferrocyanide in rinse buffer) at 37°C for 18 h, washed in PBS, and processed for whole mounting as described above.
Histological Examination and Immunohistochemistry.
For histological examination, whole-mounted mammary glands were washed in 100% ethanol before paraffin embeddment or freshly isolated glands were fixed with 4% paraformaldehyde. Sections were cut at 4 μm. Mice were injected with BrdU (Sigma) 2 h before death. The following antibodies were used: rat monoclonal anti-BrdU (1:300; OBT0030; Oxford Biotechnology, Oxfordshire, U.K.), mouse monoclonal anti-α-SMA (1:400; Ab-1; NeoMarkers, Fremont, CA), rabbit polyclonal anti-GFP (1:5,000; A6455; Molecular Probes, Eugene, OR), rabbit monoclonal anti-PR (1:500; SP2; NeoMarkers), and rabbit polyclonal anti-ERα (1:10,000; sc-542; Santa Cruz Biotechnology, Santa Cruz, CA). The antibodies were applied overnight at 4°C after antigen retrieval in citrate buffer. Biotinylated secondary antibodies were detected with Vectastain Elite kit (Vector Laboratories, Burlingame, CA). For immunofluorescence, the anti-rat Alexa Fluor 568- and anti-rabbit Alexa Fluor 488-conjugated secondary antibodies were used. Pictures were acquired with a Leica DM2000 microscope and Pixelink PL-A622C camera and Zeiss Axioplan 2-imaging fluorescence microscope with Axiocam MRm camera (Zeiss, Thornwood, NY).
Quantitative Real-Time PCR (QRT-PCR).
Glands were homogenized in TRIzol (Invitrogen, Carlsbad, CA), and RNA was extracted with chloroform and processed with the RNeasy Kit (Qiagen, Valencia, CA). Total RNA was reverse transcribed by using reverse transcriptase (GIBCO BRL/ Invitrogen) and random hexamers (Roche Diagnostics, Indianapolis, IN). The resulting cDNAs were used for QRT-PCR analysis by using the iCycler apparatus (Bio-Rad, Hercules, CA) and SYBR Green PCR Core Reagents system (Qiagen). Results were evaluated with iCycler iQ real-time detection system software (Bio-Rad). The following forward and reverse primers were used: amphiregulin, GCCATTATGCAGCTGCTTTGGAGC and TGTTTTTCTTGGGCTTAATCACCT; EGF, GCAACTCCGTCCGGGCGAGGA and GAAGATGACTGTGGTCCCGGG; TGF-α, GTGGCTGCAGCACCCTGCGCT and GATCAGCACACAGGTGATAATGAGG; HB-EGF, CTCCCACTGGATCCACAAAC and GGCAT G G G T C T CTCTTCTTC; EPR, CACCGAGAAAGAAGGATGGA and GGGATCGTCTTCCATCTGAA; BTC, CCCCAAGCAGTACAAGCATT and TGAACACCACCATGACCACT; and keratin 18 (36).
Acknowledgments
We thank G. P. Dotto for carefully reading the manuscript; N. Mueller and C. Morel for technical assistance; and Drs. D. Lee (University of Georgia, Athens, GA), P. Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire and Collège de France, Illkirch Cedex, France), and M. Okabe (Osaka University, Suita, Japan) for generously providing the amphiregulin, ERα mutant, and the C57BL/6-Tg(Act-EGFP) strain, respectively. This work was supported by National Center of Competence in Research, Molecular Oncology, Grant NRP50/SNF.
Abbreviations
- ER
estrogen receptor
- TEBs
terminal end buds
- TGF-α
transforming growth factor α
- HB-EGF
heparin binding-EGF
- BTC
betacellulin
- EPR
epiregulin
- EGFR
epidermal growth factor receptor
- SMAα
smooth muscle actin α.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lyons WR, Li CH, Johnson RE. Recent Prog Horm Res. 1958;14:219–248. [PubMed] [Google Scholar]
- 2.Nandi S. J Natl Cancer Inst. 1958;21:1039–1063. [PubMed] [Google Scholar]
- 3.Hinck L, Silberstein GB. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shyamala G. J Mammary Gland Biol Neoplasia. 1999;4:89–104. doi: 10.1023/a:1018760721173. [DOI] [PubMed] [Google Scholar]
- 5.Brisken C. J Mammary Gland Biol Neoplasia. 2002;7:39–48. doi: 10.1023/a:1015718406329. [DOI] [PubMed] [Google Scholar]
- 6.Troyer KL, Lee DC. J Mammary Gland Biol Neoplasia. 2001;6:7–21. doi: 10.1023/a:1009560330359. [DOI] [PubMed] [Google Scholar]
- 7.Stern DF. Exp Cell Res. 2003;284:89–98. doi: 10.1016/s0014-4827(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 8.Harris RC, Chung E, Coffey RJ. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 9.Cunha GR, Young P, Hom YK, Cooke PS, Taylor JA, Lubahn DB. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 10.Woodward TL, Xie JW, Haslam SZ. J Mammary Gland Biol Neoplasia. 1998;3:117–131. doi: 10.1023/a:1018738721656. [DOI] [PubMed] [Google Scholar]
- 11.Daniel CW, Silberstein GB, Strickland P. Cancer Res. 1987;47:6052–6057. [PubMed] [Google Scholar]
- 12.Silberstein GB, Van Horn K, Shyamala G, Daniel CW. Endocrinology. 1994;134:84–90. doi: 10.1210/endo.134.1.8275973. [DOI] [PubMed] [Google Scholar]
- 13.Coleman S, Silberstein GB, Daniel CW. Dev Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- 14.Snedeker SM, Brown CF, DiAugustine RP. Proc Natl Acad Sci USA. 1991;88:276–280. doi: 10.1073/pnas.88.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenney NJ, Smith GH, Rosenberg K, Cutler ML, Dickson RB. Cell Growth Differ. 1996;7:1769–1781. [PubMed] [Google Scholar]
- 16.Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, Hom YK, Cunha GR, DiAugustine RP. Cell Growth Differ. 1998;9:777–785. [PubMed] [Google Scholar]
- 17.Wiesen JF, Young P, Werb Z, Cunha GR. Development (Cambridge, UK) 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- 18.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Development (Cambridge, UK) 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallepell S, Krust A, Chambon P, Brisken C. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates SE, Davidson NE, Valverius EM, Freter CE, Dickson RB, Tam JP, Kudlow JE, Lippman ME, Salomon DS. Mol Endocrinol. 1988;2:543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- 21.Dickson RB, Huff KK, Spencer EM, Lippman ME. Endocrinology. 1986;118:138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- 22.Kenney NJ, Saeki T, Gottardis M, Kim N, Garcia-Morales P, Martin MB, Normanno N, Ciardiello F, Day A, Cutler ML. J Cell Physiol. 1993;156:497–514. doi: 10.1002/jcp.1041560309. [DOI] [PubMed] [Google Scholar]
- 23.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Development (Cambridge, UK) 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 24.Deome KB, Faulkin LJ, Jr, Bern HA, Blair PB. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 25.Daniel CW, Deome KB. Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- 26.Clarke RB, Howell A, Potten CS, Anderson E. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 27.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. Mol Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 28.Russo J, Ao X, Grill C, Russo IH. Breast Cancer Res Treat. 1999;53:217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 29.Silberstein GB. Microsc Res Tech. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Clarke RB. Steroids. 2003;68:789–794. doi: 10.1016/s0039-128x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 31.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 32.Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. Dev Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- 33.DiAugustine RP, Richards RG, Sebastian J. J Mammary Gland Biol Neoplasia. 1997;2:109–117. doi: 10.1023/a:1026395513038. [DOI] [PubMed] [Google Scholar]
- 34.Imagawa W, Pedchenko VK, Helber J, Zhang H. J Steroid Biochem Mol Biol. 2002;80:213–230. doi: 10.1016/s0960-0760(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 35.Silberstein GB, Daniel CW. Science. 1987;237:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- 36.Gouon-Evans V, Lin EY, Pollard JW. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder JA, Lee DC. Cell Growth Differ. 1998;9:451–464. [PubMed] [Google Scholar]
- 38.Schuger L, Johnson GR, Gilbride K, Plowman GD, Mandel R. Development (Cambridge, UK) 1996;122:1759–1767. doi: 10.1242/dev.122.6.1759. [DOI] [PubMed] [Google Scholar]
- 39.Clarke RB, Howell A, Anderson E. Breast Cancer Res Treat. 1997;45:121–133. doi: 10.1023/a:1005805831460. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CL, Sims AH, Howell A, Miller CJ, Clarke RB. Endocr Relat Cancer. 2006;13:617–628. doi: 10.1677/erc.1.01165. [DOI] [PubMed] [Google Scholar]
- 41.Platet N, Cathiard AM, Gleizes M, Garcia M. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Ewan KB, Oketch-Rabah HA, Ravani SA, Shyamala G, Moses HL, Barcellos-Hoff MH. Am J Pathol. 2005;167:409–417. doi: 10.1016/s0002-9440(10)62985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Cruz CM, Moody SE, Master SR, Hartman JL, Keiper EA, Imielinski MB, Cox JD, Wang JY, Ha SI, Keister BA, Chodosh LA. Mol Endocrinol. 2002;16:2034–2051. doi: 10.1210/me.2002-0073. [DOI] [PubMed] [Google Scholar]
- 44.Blakely CM, Stoddard AJ, Belka GK, Dugan KD, Notarfrancesco KL, Moody SE, D'Cruz CM, Chodosh LA. Cancer Res. 2006;66:6421–6431. doi: 10.1158/0008-5472.CAN-05-4235. [DOI] [PubMed] [Google Scholar]
- 45.Friedrich G, Soriano P. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 46.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 47.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Development (Cambridge UK) 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 48.Mueller SO, Clark JA, Myers PH, Korach KS. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]