Abstract
The introduction of genes that impair Plasmodium development into mosquito populations is a strategy being considered for malaria control. The effect of the transgene on mosquito fitness is a crucial parameter influencing the success of this approach. We have previously shown that anopheline mosquitoes expressing the SM1 peptide in the midgut lumen are impaired for transmission of Plasmodium berghei. Moreover, the transgenic mosquitoes had no noticeable fitness load compared with nontransgenic mosquitoes when fed on noninfected mice. Here we show that when fed on mice infected with P. berghei, these transgenic mosquitoes are more fit (higher fecundity and lower mortality) than sibling nontransgenic mosquitoes. In cage experiments, transgenic mosquitoes gradually replaced nontransgenics when mosquitoes were maintained on mice infected with gametocyte-producing parasites (strain ANKA 2.34) but not when maintained on mice infected with gametocyte-deficient parasites (strain ANKA 2.33). These findings suggest that when feeding on Plasmodium-infected blood, transgenic malaria-resistant mosquitoes have a selective advantage over nontransgenic mosquitoes. This fitness advantage has important implications for devising malaria control strategies by means of genetic modification of mosquitoes.
Keywords: malaria control, genetic modification, gene drive
The use of genetically modified mosquitoes refractory to pathogen transmission is a potential strategy for controlling vector-borne diseases (1–4). In the past few years, important technical advances, including germ-line transformation of mosquitoes, the characterization of tissue-specific promoters, and the identification of molecules that interfere with parasite development (effector molecules), have led to the generation of transgenic mosquitoes that are impaired in their capacity to transmit the malaria parasite (1, 5). However, although the feasibility of genetically modifying mosquito vector competence has been demonstrated in the laboratory, we do not yet know how transgenes can be introgressed into mosquito populations in the field. Several possible approaches have been proposed (transposable elements, Wolbachia, and meiotic drive), but success of any mechanism will depend in part on the fitness load that is imposed by the presence of the transgene.
Previous studies suggested that transgenic mosquitoes are less fit than wild type (WT) (6, 7). Catteruccia et al. (6) found that Anopheles stephensi homozygous transgenic lines have lower fitness compared with WT, and Irvin et al. (7) reached similar conclusions with transgenic lines of Aedes aegypti. However, these studies could not distinguish fitness effects due to the transgene itself versus insertion-site effects and/or inbreeding depression (8). In a separate study, Moreira et al. (2) demonstrated that hemizygous transgenic mosquitoes expressing the SM1 dodecapeptide under the control of the blood-inducible carboxypeptidase promoter were as fit as WT controls when fed on blood of noninfected mice.
Plasmodium and other pathogens, even in low numbers, are known to decrease the fertility of their insect hosts [reviewed by Hurd (9)]. Accordingly, we hypothesized that when fed on Plasmodium-infected blood, transgenic mosquitoes expressing antiparasite genes would be more fit than WT on account of their reduced parasite prevalence. To investigate the validity of this premise, cage-invasion experiments were performed in which a starting population of equal numbers of WT and SM1-transgenic A. stephensi mosquitoes were maintained on P. berghei-infected blood. We found that when fed on Plasmodium-infected blood, the transgenic malaria-resistant mosquitoes had a significant fitness advantage over WT.
Results and Discussion
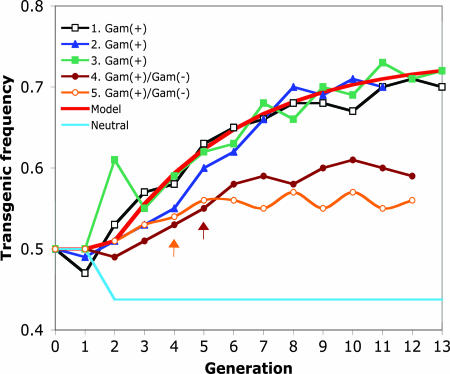
Previous experiments had shown that when transgenic and nontransgenic mosquitoes are fed noninfected blood, Hardy–Weinberg equilibrium (44% transgenic/56% WT mosquitoes; Fig. 1, neutral line) is reached and maintained after the second generation (2). In contrast, in the present experiments, when mosquitoes were fed Plasmodium-infected blood, transgenic mosquito frequency reached ≈70% by the ninth generation and remained relatively constant thereafter (Fig. 1, experiments 1–3). One possible explanation for this plateau is that the transgene exhibits overdominance; i.e., there is a cost (discussed below) associated with being homozygous for the transgene, and thus the hemizygote has higher fitness than either the homozygote or the WT. This hypothesis is supported by the observation that in the last generation of the cage-invasion experiments, a significantly higher frequency of transgenic hemizygotes (and a corresponding lower frequency of homozygotes) was observed than would be expected by chance (χ2 = 7.57, df = 2, P = 0.023; data not shown).
Fig. 1.
Changes in the prevalence of transgenic mosquitoes in populations maintained with Plasmodium-infected blood. Experiment 1 consisted of a cage started with 250 transgenic virgin females (CCBF6 line) and 250 WT males. Experiment 2 (reverse) was 250 WT virgin females and 250 transgenic males (CCBF6). Experiment 3 consisted of 250 transgenic virgin females (CCBF3 line) with 250 WT males. Experiment 4 was 250 transgenic virgin females (CCBF3) and 250 WT males. Mosquitoes fed on ANKA 2.34-infected mice up to generation 4 and on ANKA 2.33 from generation 5 onward (arrow). Experiment 5 (reverse) consisted of 250 WT females and 250 transgenic males (CCBF3). Mosquitoes fed on ANKA 2.34-infected mice up to generation 3 and on ANKA 2.33 from generation 4 onward (arrow). Neutral indicates expected outcome if there were no cost or benefit to being transgenic (Hardy–Weinberg equilibrium). Model indicates the predicted outcome if there were a 50% benefit to being transgenic and a 35% cost to being homozygous for the transgene (see text). Significant deviation from the neutral frequency of 56% WT and 44% transgenic (Hardy–Weinberg equilibrium) was observed after the second generation in experiment 1 and after the third generation in experiments 2 and 3 (χ2 test, P < 0.05).
Additional cage-invasion experiments were conducted in which mosquito feeding was switched in generation 4 or 5 from blood containing gametocyte-producing parasites (ANKA 2.34) to blood containing a gametocyte-deficient parasite strain (ANKA 2.33). The latter parasites multiply normally in the mammalian host but do not develop in the mosquito. Transgene frequency did not increase after the switch (Fig. 1, experiments 4 and 5), indicating that it was parasite development in the mosquito and not the presence of asexually dividing parasites or factors produced by the mammalian host that affected mosquito fitness.
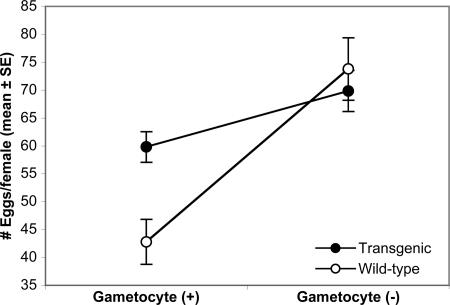
To further examine the basis for the competitive advantage of transgenic mosquitoes, mosquitoes were fed on a mouse infected with either P. berghei ANKA 2.34 (gametocyte-producing) or ANKA 2.33 (gametocyte-deficient) and kept individually for measurements of egg production and mortality. Transgenic mosquitoes had significantly higher fecundity (Fig. 2) and lower mortality (Table 1) when fed on blood containing gametocyte-producing parasites. It should be noted that these experiments measured differences in early mortality and fecundity (during the initial 6 days of life) and thus may not capture detailed differences in lifetime fitness characteristics between strains. However, because of the cumulative nature of life-table parameters, life-table trajectories are most sensitive to occurrences in early life (10). Thus, our experiments measured fitness effects during the period of mosquito life that has the most significant effect on total lifetime fitness. Conversely, no significant differences were observed when mosquitoes were fed on gametocyte-deficient parasites, indicating that progression of the Plasmodium infection in the mosquito was responsible for the observed differences. Furthermore, no differences in egg hatching, larval development time, and adult male survivorship were observed between transgenic and nontransgenic mosquitoes when they were fed either of the two parasite strains (data not shown). Our overall results suggest that the fitness advantage observed in SM1-transgenic mosquitoes when fed on infectious P. berghei is due to blockage of ookinete invasion of the mosquito midgut.
Fig. 2.
Fecundity of transgenic and WT mosquitoes fed on a parasite-containing blood meal. Hemizygous transgenic males were crossed with virgin nontransgenic females to yield a population of 50% hemizygous transgenic and 50% nontransgenic sibling mosquitoes originating from the same rearing pans. Mosquitoes were fed on mice infected with gametocyte-producing (ANKA 2.34) or gametocyte-deficient (ANKA 2.33) parasites after which single engorged females were kept in separate containers for egg collection. The interaction line chart shows eggs produced by transgenic and WT females. ANOVA indicated a significant effect of parasite type on number of eggs oviposited per female and a significant interaction between parasite type and transgenic type (Parasite: F = 25.1, d.f. = 1, P < 0.0001; Transgenic: F = 2.54, d.f. = 1, P = 0.113; Parasite × Transgenic: F = 6.56, d.f. = 1, P = 0.011).
Table 1.
Mortality of transgenic mosquitoes maintained on Plasmodium-infected blood
| Mosquito | Gametocyte (ANKA 2.34) | Gametocyte-deficient (ANKA 2.33) |
|---|---|---|
| Transgenic | 38.5 (161) | 24.4 (45) |
| WT | 51.4 (138) | 22.2 (45) |
| P | 0.027 | 0.99 |
Data were collected from the experiment described in Fig. 2. Mortality was measured four times per day for the first 6 days. Percent cumulative mortality at day 6 after the blood meal is reported. The number of mosquitoes examined is given in parentheses. Significance (P) was calculated by using the two-tailed Fisher's exact test. The relatively high mortality of control mosquitoes fed on ANKA 2.33 may have been caused by increased manipulation for sorting mosquitoes or by conditions in the small individual containers. However, note that in every experiment control and experimental mosquitoes were treated identically.
These findings offer an interesting contrast to the results of Hurd and colleagues (11, 12) who reported that the parasite imposes a fitness cost on the mosquito (as we found) but that resistant mosquitoes that up-regulate natural immune responses to Plasmodium infection are not more fit than nonresistant mosquitoes, presumably because the fitness costs of being hyperimmune negate the fitness advantage of not being infected. A potential explanation for this discrepancy is that the transgenic mosquitoes used in our study are Plasmodium-refractory because of expression of a presumably harmless (2) gene (SM1) rather than activation of the endogenous innate immune cascade. Importantly, SM1 inhibits development at a very early stage of Plasmodium development in the mosquito, preventing midgut invasion and activation of the mosquito immune system. When developing future transgenes for vector-borne disease control, we suggest that the mode of action and timing of transgene expression be taken into consideration. The gene product should have minimal or no effect on mosquito fitness and should act early enough to avoid generalized up-regulation of the mosquito immune system.
We used simple population genetics modeling (13) to investigate the dynamics of transgene spread in the mosquito cage populations. When compared with our empirical observations, best-fit model simulations indicated a 50% benefit over WT mosquitoes to being transgenic (hemizygous or homozygous) when feeding on parasitemic blood, and an independent 35% cost to being homozygous for the transgene (Fig. 1, Model). There are three nonexclusive hypotheses for this observation. Fitness costs observed in mosquitoes homozygous for the transgene could be due to hitchhiking of recessive deleterious genes residing near the point of transgene insertion (8). This would imply that such recessive deleterious genes occur fairly commonly in the mosquito genome, because a similar homozygous cost was observed with two independently derived transgenic lines. Alternatively, a negative effect of the transgene product could be directly responsible for the cost. This would require that the effect is highly dose-sensitive because no effect was observed with one dose of the gene in hemizygous mosquitoes (2). A third alternative is that the cost was due to insertional mutagenesis during transgene integration into the chromosome. This possibility is less likely because two independent transgenic strains behaved similarly in the cage experiments (Fig. 1). Moreover, at least in Drosophila where this possibility was examined in detail, loss of fitness due to insertional mutagenesis is a rare event (8).
To our knowledge, no one has previously reported a demonstration that transgenic mosquitoes can exhibit a fitness advantage over nontransgenics. Although these conclusions were drawn from a model system, they suggest that transgenic advantage might be an important phenomenon in epidemiologically significant mosquito–parasite systems and should be studied in experiments using nonmodel organisms that more closely emulate field conditions. The results have important implications for implementation of malaria control by means of genetic modification of mosquitoes. Because even Plasmodium burden of less than five oocysts can significantly reduce the fecundity of anophelines in experimental or natural systems (9), it can be predicted that the transgene may confer a significant advantage and promote its spread into field populations. In the field, where infection prevalence is lower than in laboratory systems and only a relatively small proportion of mosquitoes become infected (14), gene introgression is predicted to be considerably slower and possibly not of sufficient magnitude to establish the transgene in the population. However, once established, transgenic mosquitoes that interfere with parasite development should make more difficult the reintroduction of the parasite after eradication of malaria from the target area.
Materials and Methods
Mosquitoes.
Two independently derived A. stephensi transgenic lines (CCBF3 and CCBF6) of the same construct expressing the SM1 peptide in the midgut (1) and nontransgenic mosquitoes were used to perform the experiments. The A. stephensi mosquitoes were received from the National Institutes of Health, where they have been kept since 1970, probably originating from mosquitoes from India. The transgenic stocks had been kept in hemizygous conditions by crossing transgenic males with virgin WT females from laboratory population cages for at least 25 generations, assuring that the transgenic mosquitoes were of similar genetic constitution as their sibling WT counterparts. Mosquitoes were maintained in a rearing room at 27 ± 0.2°C and 80 ± 2% humidity. Larvae were reared in sparse density (≈250 larvae per ≈30 × 34-cm tray) to avoid production of runt/weak adults. Adults were kept in metal cages of ≈50 × 50 × 50 cm at a population level of ≈1,200 mosquitoes per cage. P. berghei-infected mosquitoes were kept in a room at 19.5°C.
Transgene Frequency Changes in Cage-Invasion Experiments.
The cage-invasion experiments were initiated by placing 250 hemizygous transgenic A. stephensi from one sex and WT mosquitoes of the opposite sex in a cage (50 × 50 × 50 cm). To produce eggs for the next generation, mosquitoes were fed on mice infected with the gametocyte-producing ANKA 2.34 parasite (Experiments 1–3) or mice infected with either ANKA 2.34 or with the gametocyte-deficient ANKA 2.33 parasite (Experiments 4 and 5) in a 19.5°C chamber. Four to 6 days later, they were allowed to lay eggs. Filter papers containing at least 2,000 eggs were hatched in plastic trays with water and reared under standard laboratory conditions. The larvae were reared at 27°C in trays set up to not be overpopulated, with ≈250 larvae per 30 × 34 cm tray. Pupae were then transferred to a mosquito cage containing a 10% sugar solution to yield cages with ≈1,200 mosquitoes. At each generation, transgenic frequency was assessed by counting the number of GFP-positive (transgenic) and GFP-negative (nontransgenic) mosquitoes in random samples of ≈100 individuals. A total of 250 mated adult females were randomly picked from the cage to start the next generation. In the last two generations of experiments 3, 4, and 5, homozygous and hemizygous transgenic mosquitoes were separately counted by using green fluorescence intensity as described (6). The adult mosquitoes were kept at 19°C, during and after feeding on infected mice with both P. berghei strains (ANKA 2.33 or ANKA 2.34).
Fecundity and Mortality Analysis.
A mixture of hemizygous transgenic (CCBF6 line) and sibling WT mosquitoes reared in the same container were fed on the same mouse (infected with either ANKA 2.33 or ANKA 2.34). Engorged females were separated into one female per container. Eggs collected from individual females were counted with the aid of a dissecting microscope as described (2). Significant difference in the number of eggs produced by transgenic and WT mosquitoes fed on gametocyte-producing or nonproducing parasites was determined by two-factor ANOVA, where the factors were transgenic state and parasite type. Viability was checked four times a day over 6 days, and dead mosquitoes were examined for the presence of the transgene (EGFP expression). Three independent experiments were performed with ANKA 2.34 and two with ANKA 2.33.
Modeling Dynamics of Transgene Spread in Mosquito Populations.
The model is a simple extension of the standard general selection model (12). There are two alleles (A, a), where A indicates transgene presence, and a indicates transgene absence. There are three possible genotypes: AA, Aa, and aa. b indicates the fitness advantage to being transgenic. Both AA and Aa genotypes have the same value for b. c indicates fitness disadvantage to being homozygous for the transgene. This only affects AA genotypes. We assume that b and c act independently of one another.
The frequencies of each genotype at generation t (before selection) are as follows:
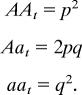 |
The fitness (W) of each genotype can be calculated as
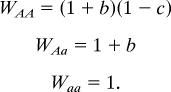 |
Thus, the proportional contribution of each genotype can be calculated as
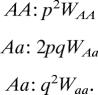 |
The average fitness of the population (Wave) is calculated as the sum of these three terms:
The frequency of each genotype at generation t + 1 (after selection) is calculated as
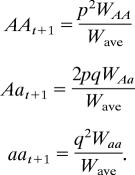 |
To match the empirical experimental design, simulated cage invasions were initiated with a starting population of an equal number of hemizygous transgenic mosquitoes from one sex and WT mosquitoes of the opposite sex, and thus mating between transgenic and WT is not random in the first generation. Because the GFP marker is dominant, 50% of the first-generation progeny are expected to be GFP-positive. Mating in subsequent generations was random.
Acknowledgments
We thank Neil Cheddie for expert technical assistance. This work was supported by grants from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 2.Moreira LA, Wang J, Collins FH, Jacobs-Lorena M. Genetics. 2004;166:1337–1341. doi: 10.1534/genetics.166.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James AA. Trends Parasitol. 2005;21:64–67. doi: 10.1016/j.pt.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Christophides GK. Cell Microbiol. 2005;7:325–333. doi: 10.1111/j.1462-5822.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 5.Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- 6.Catteruccia F, Godfray HC, Crisanti A. Science. 2003;299:1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- 7.Irvin N, Hoddle MS, O'Brochta DA, Carey B, Atkinson PW. Proc Natl Acad Sci USA. 2004;101:891–896. doi: 10.1073/pnas.0305511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrelli MT, Moreira CK, Kelly D, Alphey L, Jacobs-Lorena M. Trends Parasitol. 2006;22:197–202. doi: 10.1016/j.pt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Hurd H. Annu Rev Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. [DOI] [PubMed] [Google Scholar]
- 10.Carey JR. Applied Demography for Biologist with Special Emphasis on Insects. New York: Oxford Univ Press; 1993. [Google Scholar]
- 11.Hurd H, Taylor PJ, Adams D, Underhill A, Eggleston P. Evol Int J Org Evol. 2005;59:2560–2572. [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed AM, Hurd H. Microbes Infect. 2006;8:308–315. doi: 10.1016/j.micinf.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Mettler LE, Gregg TG, Schaffer HE. Population Genetics and Evolution. Englewood Cliffs, NJ: Prentice–Hall; 1998. pp. 129–156. [Google Scholar]
- 14.Pringle G. Trans R Soc Trop Med Hyg. 1966;60:626–632. doi: 10.1016/0035-9203(66)90009-5. [DOI] [PubMed] [Google Scholar]