Abstract
Mitochondria play a central role in the regulation of programmed cell death signaling. Here, we report the finding of a mitochondrial matrix-targeted protein phosphatase 2C family member (PP2Cm) that regulates mitochondrial membrane permeability transition pore (MPTP) opening and is essential for cell survival, embryonic development, and cardiac function. PP2Cm is highly conserved among vertebrates, with the highest expression levels detected in the heart and brain. Small hairpin RNA (shRNA)-mediated knockdown of PP2Cm resulted in cell death associated with loss of mitochondrial membrane potential in cultured cardiac mycoytes and an induction of hepatocyte apoptosis in vivo. PP2Cm-deficient mitochondria showed elevated susceptibility to calcium-induced MPTP opening, whereas mitochondrial oxidative phosphorylation activities were not affected. Finally, inactivation of PP2Cm in developing zebrafish embryos caused abnormal cardiac and neural development as well as heart failure associated with induced apoptosis. These data suggest that PP2Cm is a novel mitochondrial protein phosphatase that has a critical function in cell death and survival, and may play a role in regulating the MPTP opening.
Keywords: Mitochondrial permeability transition pore, protein phosphatase, cell death, heart failure, developmental defects, zebrafish
It is well established that mitochondria play a central role in the intrinsic apoptosis pathway where internal and external death signals lead to the release of cytochrome c and apoptosis-inducing factor (AIF), as well as other proapoptotic molecules through Bcl-2 family members (Bax and Bak) located on the mitochondria outer membrane. In addition, mitochondria-mediated apoptosis can also be triggered by the opening of the mitochondria permeability transition pore (MPTP). Complexes of the voltage-dependent anion channel (VDAC), the adenine nucleotide translocator (ANT), and cyclophilin D (CypD) are already implicated to have cyclosporine A (CsA)-dependent MPTP activity in the mitochondrial inner membrane. The loss of inner membrane potential due to the MPTP opening triggers mitochondrial swelling and outer membrane rupture, which in turn leads to the release of proapoptotic factors from the intermembrane space (IMS). However, the underlying molecular mechanisms and the signaling molecules involved in the regulation of MPTP opening under stress conditions are largely unknown.
In addition to being an essential organelle for cellular metabolism and survival, the mitochondrion has been recognized as a site where diverse signaling pathways converge and integrate (Ravagnan et al. 2002; Newmeyer and Ferguson-Miller 2003; Horbinski and Chu 2005). Indeed, a number of Ser/Thr protein kinases, including PKA, PKB/AKT, PKC, and JNK, have been found to be located in mitochondria. In addition, a number of anchoring proteins for various protein kinases are also identified in mitochondria, including AKAP, PICK, Grb10, and Sab (Huang et al. 1999; Nantel et al. 1999; Alto et al. 2002; Wiltshire et al. 2002; Wang et al. 2003). PKA activity targeted in the mitochondrial inner membrane and matrix is shown to activate Complex I of the respiratory chain in the bovine heart (Scacco et al. 2000; Technikova-Dobrova et al. 2001; Chen et al. 2004). In addition, PKB/AKT activity leads to phosphorylation of the mitochondrial ATP synthase β subunit and glycogen synthase kinase 3β (Gsk3β), which in turn, inhibits pyruvate dehydrogenase activity (Bijur and Jope 2003). Likewise, several PKC isoforms are detected in mitochondria with different target proteins. For instance, PKCε promotes cardioprotection against ischemia/reperfusion injury through phosphorylation of a VDAC, which prevents the opening of the MPTP (Baines et al. 2003). Further, both PKCε and PKCδ have been implicated in mitochondrial KATP channel regulation in ischemic preconditioning (Cohen et al. 2000; Chen et al. 2001). Finally, ERK and JNK kinases are targeted to mitochondria to phosphorylate apoptotic proteins, including Bcl-xl, Bcl-2, and BAD, and regulate apoptosis (Deng et al. 2000; Kang et al. 2003; Schroeter et al. 2003; Brichese et al. 2004). In short, Ser/Thr protein kinase targeting to mitochondria has significant impact on pro- or anti-apoptotic factors, respiration, and ionic channel activity, illustrating the important role of mitochondria protein phosphorylation in cellular signaling. Protein phosphorylation is a dynamic process involving a balancing act of kinases and phosphatases (Shenolikar 1994). Therefore, mitochondria protein phosphorylation will also most likely be regulated by mitochondria-targeted protein phosphatases. Although Protein Phosphatase 1(PP1) and PP2A have been found in mitochondria (Ruvolo et al. 2002; Dagda et al. 2003; Brichese et al. 2004; Tamura et al. 2004), their specific contribution to the mitochondrial function remains unclear. Recently, a novel tyrosine protein phosphatase, PTPMT1, was reported to be located specifically in the mitochondria matrix and to play an important role in both ATP production and insulin secretion (Pagliarini et al. 2005), further supporting the notion that protein phosphorylation and dephosphorylation in mitochondria are an important mechanism of cell signaling. However, the molecular mechanism regulating protein phosphorylation in mitochondria, particularly in the mitochondria matrix, has not been investigated.
In this report, we identified a novel Ser/Thr protein phosphatase, named PP2Cm, that is targeted exclusively to the mitochondria matrix. PP2Cm is highly conserved from fish to mammals and contains a Ser/Thr phosphatase domain commonly shared by all PP2C family members. It is targeted to the mitochondria matrix via a mitochondrial targeting sequence at its N-terminal end, making it the first Ser/Thr protein phosphatase so far identified in this mitochondrial compartment. From both in vitro and in vivo studies, we demonstrate that PP2Cm is an essential protein for cellular survival and MPTP regulation. Loss of PP2Cm expression leads to cell death and MPTP opening in response to calcium overload without measurable impact on mitochondrial respiration. PP2Cm expression is diminished in hypertrophic and failing hearts, and suppression of PP2Cm expression in zebrafish causes developmental defects in the central nerves system and heart. All of this evidence suggests that PP2Cm is a modulator of MPTP function and plays a critical role in cell death regulation and normal development and physiology in heart and other systems. This finding reveals a new signaling component in MPTP regulation, and suggests a previously uncharacterized mechanism in apoptosis regulation involving the mitochondrial matrix protein phosphorylation/dephosphorylation.
Results
PP2Cm is an exclusively mitochondria targeted Ser/Thr phosphatase
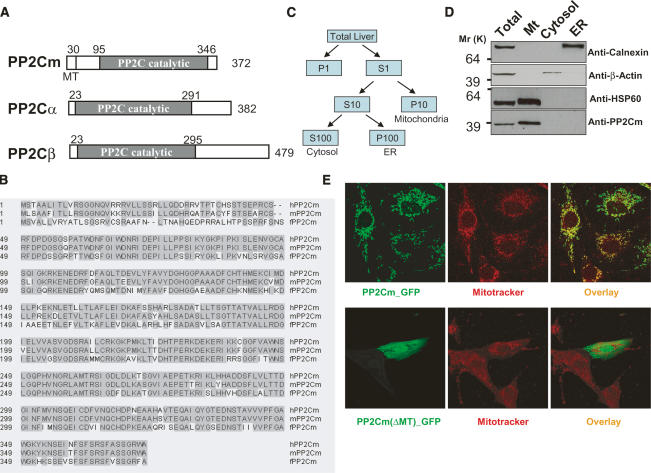
Through genome mining, we identified a new member of the PP2C gene family from the human, mouse, and zebrafish genome based on its highly conserved sequences with known PP2C family members in the catalytic domain. In addition, a putative mitochondria targeting sequence at the N-terminal of the protein was predicted using two different programs: Mitoprot (http://ihg.gsf.de/ihg/mitoprot.html) and iPSORT (http://hc.ims.u-tokyo.ac.jp/iPSORT) (Fig. 1A,B). Therefore, we name the gene PP2C in Mitochondria (PP2Cm). The full-length cDNAs for the human, mouse, and zebrafish PP2Cm gene were subsequently cloned from a human EST clone, a mouse heart cDNA library, and a fish embryo cDNA library, respectively, by PCR based on predicted sequences (see Materials and Methods for details). The human and mouse PP2Cm genes are predicted to encode a 372-amino-acid protein. We raised a polyclonal antibody specifically against the PP2Cm protein that has no cross-activity with other known PP2C family members (Supplementary Fig. 1). By Western blotting using this antibody, PP2Cm protein was detected only in the mitochondrial subfraction of mouse liver extracts but not in the cytosolic or endoplasmic reticulum (ER) fractions (Fig. 1C,D), suggesting that PP2Cm is indeed a resident mitochondrial protein. When the PP2Cm coding sequence was fused to either GFP or Flag tag at its C-terminal, the fusion protein was detected by immunofluorescent staining for GFP or Flag tag with a complete overlapping pattern with mitotracker signals in cultured myocytes (Fig. 1E; Supplementary Figs. 2, 3). Similar mitochondrial localization was also observed when PP2Cm fusion protein was expressed in COS-1 cells and HEK 293 cells (data not shown). Deletion of the first 30 amino acid residues at its N-terminal abolished its mitochondrial localization pattern (Fig. 1E; Supplementary Fig. 2). These data lead to the conclusion that PP2Cm is indeed a mitochondrial protein that requires its mitochondria targeting sequence at its N terminus for proper intracellular targeting.
Figure 1.
PP2Cm is a new PP2C family member encoding a mitochondrial Ser/Thr phosphatase. (A) Schematic diagram of human PP2C isoforms. Domain analysis reveals that PP2Cm contains a unique mitochondria targeting sequence at the N terminus using Mitoprot and iPSORT in addition to a highly conserved PP2C catalytic domain. (MT) Mitochondria targeting sequence. (B) Amino acids alignment of the human PP2Cm and its mouse and zebrafish orthologs using the Clustal W program. The shaded region indicates the consensus amino acids. The asterisk (*) marks the residues that were highly conserved catalytic sites and that mutated in subsequent studies. The predicted mitochondria targeting sequence is underlined. (C) Diagram of a subcellular fractionation scheme with details described in Materials and Methods. (D) Immunoblotting analysis of subcellular fractions from mouse liver with antibodies as indicated. (E) Neonatal cardiomyocytes transfected with either wild-type hPP2Cm-GFP or hPP2Cm(ΔMT)-GFP were stained with Mitotracker Red and visualized under confocal microscopy.
PP2Cm exists as a soluble protein in the mitochondria matrix
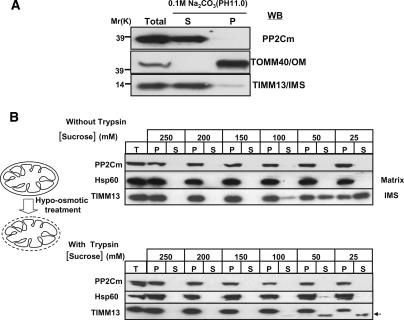
Because the suborganellar localization of PP2Cm may be important for its function within the mitochondrion, we utilized hypotonic lysis (Koehler et al. 1998) and carbonate extraction (Fujiki et al. 1982) techniques to determine if PP2Cm was localized to the mitochondrial matrix, inner membrane, or IMS. Purified mouse liver mitochondria were treated with 0.1 M Na2CO3 (pH 11), and membrane proteins were recovered in the pellet after centrifugation. PP2Cm was recovered in the supernatant, indicating it is a soluble protein like TIMM13 and not an integral membrane protein like TOMM40 (Fig. 2A). Mitochondria were also subjected to hypotonic lysis in buffers containing decreased concentration of the osmoticum sucrose (Fig. 2B). At 200 mM sucrose, the outer membrane begins to rupture, but the inner membrane remains intact, even when the sucrose concentration is decreased to 25 mM. Based on protease protection, PP2Cm is localized in the matrix like Hsp60 and not in the IMS like TIMM13. Thus, PP2Cm is a soluble protein localized in the mitochondrial matrix.
Figure 2.
PP2Cm is a soluble mitochondria matrix protein. (A) Isolated mouse liver mitochondria fraction was subjected to sodium carbonate extraction. After centrifugation, pellet (P) and supernatant (S) are analyzed by immunoblotting using anti-TOMM40, an outer membrane protein (OM), and anti-TIMM13, a mitochondria IMS protein, as indicated. (B) Isolated mouse liver mitochondria were incubated in hypotonic sucrose buffers as indicated to disrupt the outer membrane in the absence (top panel) and presence (bottom panel) of soybean trypsin. After centrifugation, total (T), pellet (P), and supernatant (S) fractions were analyzed by immunoblotting with anti-Hsp60, a matrix protein, or anti-TIMM13 antibodies as indicated.
PP2Cm is a bona fide Ser/Thr phosphatase with enriched expression in heart and brain, but reduced expression in failing heart
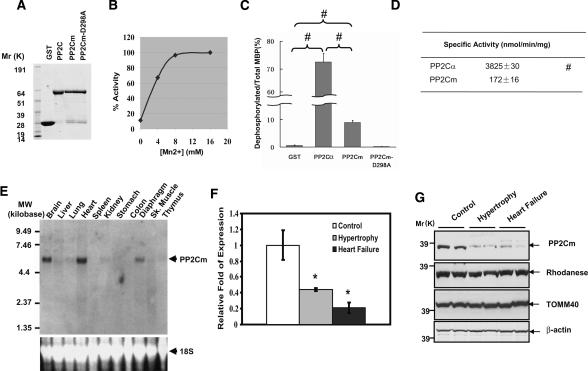
To demonstrate that PP2Cm has active protein phosphatase activity, we measured the phosphatase activity of recombinant PP2Cm proteins using 32P-labeled myelin basic protein (MBP) as a generic substrate and recombinant PP2Cα as a reference enzyme (Fig. 3). As shown in Figure 3B, wild-type PP2Cm recombinant protein showed significant protein phosphatase activity in an expected Mn2+-dependent manner, whereas proteins with a specific mutation in highly conserved aspartate at position 298 (D298A), known to be essential for catalytic activity of other PP2C isoforms, showed no detectable phosphatase activity (Fig. 3D). However, PP2Cm only dephosphorylated <10% of the total phosphorylated sites in MBP substrate in contrast to >70% efficiency carried out by PP2Cα (Fig. 3C). The specific activity of PP2Cm was also significantly lower than PP2Cα under the same assay conditions (Fig. 3D). All these data indicate that PP2Cm is a bona fide Ser/Thr phosphatase with different substrate selectivity and specific activity compared with PP2Cα. By Northern blot, mouse PP2Cm transcript was detected as a single species at ∼5.9 kb in size, with the highest level detected in the adult heart, brain, and diaphragm, and lower levels in the liver, lung, kidney, skeletal muscle, and thymus (Fig. 3E). Interestingly, these tissues all have high metabolic activities and extensive mitochondrial contents. Western blot analysis using PP2Cm-specific antibody showed that the PP2Cm protein is also enriched in the heart, brain, liver, and thymus, with lower expression detected in other tissues (Supplementary Fig. 5). To determine the expression profile of PP2Cm in the diseased heart, we created pressure overload by trans-aortic constriction (TAC) in adult mouse hearts, which has been described to induce cardiac hypertrophy and a transition to heart failure (Takimoto et al. 2005). Cardiac hypertrophy with preserved function was observed within 1 wk post-TAC, while decompensated heart failure was apparent after 3 wk post-TAC (Takimoto et al. 2005; S. Ren and Y. Wang, unpubl.). The expression of PP2Cm at both the mRNA and protein levels was significantly reduced in hypertrophied hearts, and was further reduced in the decompensated failing heart (Fig. 3F,G). In contrast, other mitochondria proteins, including Rhodanease and TOMM40, were not changed compared with the control. These data suggest that PP2Cm expression is highly associated with mitochondria content and metabolic status, and its selective loss of expression correlates with the progression of pathological remodeling in diseased hearts.
Figure 3.
PP2Cm is bona fide Ser/Thr protein phosphatase with enriched expression in the heart and brain. (A) Coomassie-stained SDS-PAGE gel of GST recombinant proteins as indicated at the top. (B) Phosphatase activity measured from purified recombinant human wild-type PP2Cm-GST fusion protein for its Mn2+ dependency using 32P-labeled MBP as substrate. (C) One microgram of 32P-labeled MBP was incubated overnight with 0.5 μg of purified wild-type PP2Cα, PP2Cm, and PP2Cm-D298A mutant as labeled. The dephosphorylation efficiency of the recombinant phosphatases was expressed as the ratio of dephosphorylated versus total 32P-labeled MBP at the end of the reaction. Values are mean ± SD of three independent experiments. (#) p < 0.01. (D) Specific phosphatase activities of PP2Ca and PP2Cm toward MBP were calculated based on V0 measured from the linear phase of the dephosphorylation reaction. The results were mean ± SD of three independent experiments. (#) p < 0.01 versus PP2Cm. (E) Radiograph of Northern blot analysis for the PP2Cm mRNA level in different tissues of the adult mouse (top panel) and a fluorescent signal of ethidium bromide-stained 18S ribosomal RNA used as an equal loading control (bottom panel). (F) PP2Cm mRNA levels in hypertrophy or failing mouse hearts were quantified by real-time quantitative RT–PCR after normalization against GAPDH. At least three animals of each group were examined. Shown are mean ± SD. The cardiac hypertrophic or heart failure was established by transaortic constriction for 1 or 3 wk, respectively, as described in Materials and Methods. (G) PP2Cm and other mitochondria protein levels in hypertrophy or failing hearts were determined by immunostaining using the anti-PP2Cm antibody, anti-Rhodanese, and anti-TOMM40, as indicated. The β-actin level was used for equal loading.
PP2Cm is essential for myocyte survival and maintaining mitochondria membrane potential in vitro
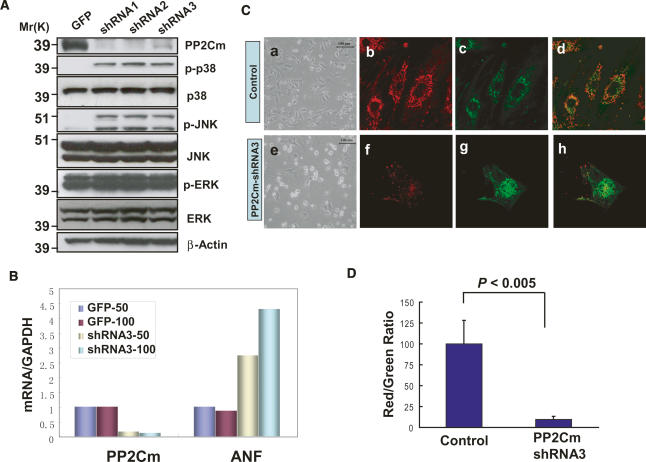
To investigate the functional significance of PP2Cm, endogenous PP2Cm was inhibited in cultured neonatal rat ventricular myocytes using small hairpin RNA (shRNA) delivered via adenovirus vectors. As shown in Figure 4A, all three shRNAs targeting different segments of rat PP2Cm mRNA effectively reduced endogenous PP2Cm protein expression 3 d post-transfection. PP2Cm inactivation led to significant and selective induction of stress-activated mitogen-acitvated protein (MAP) kinases, c-Jun N-terminal Kinase (JNK), and p38 kinase, while MAP kinase ERK was not affected (Fig. 4A). In addition, inactivation of PP2Cm also resulted in marked induction of the atrial natriuretic factor (ANF), a well-established marker gene for myocyte stress response (Fig. 4B). Seven days post-shRNA-mediated knockdown of PP2Cm, a significant induction of cell death was observed compared with control cells (Fig. 4C). Induction of cell death was associated with dissipated mitochondrial membrane potential (ΔΨ) as measured by a significant reduction in a red/green fluorescence ratio of the JC-1 signal in PP2Cm-deficient myocytes (Reers et al. 1995) (Fig. 4C,D). Inactivation of PP2Cm in HeLa cells resulted in loss of cell proliferation and induced cell death similar to that observed in cardiomyocytes (data not shown). These in vitro analyses suggest that PP2Cm is essential for cell survival, and plays an important role in maintaining mitochondrial membrane potential.
Figure 4.
Loss of PP2Cm in vitro promotes cell death and mitochondria membrane potential dissipation. (A) Neonatal cardiomyocytes were transfected with Adv-GFP or Adv-shRNAs targeting three different regions of PP2Cm coding sequence for 48 h at a multiplicity of infection (MOI) of 50. The silencing efficiency was confirmed by immunoblotting with a PP2Cm polyclonal antibody. The MAP kinases activation was examined with total and anti-phospho-JNK, anti-phospho-p38, and anti-phospho-ERK antibodies as indicated. (B) mRNA levels of PP2Cm and ANF by real-time quantitative RT–PCR relative to GAPDH. Neonatal rat ventricular myocytes were transfected with either Adv-GFP or Adv-PP2Cm-shRNA3 using either 50 or 100 MOI as labeled. Total RNAs were isolated 3 d post-transfection. (C, panels a,e) Representative images of cells observed in cardiomyocytes 7 d post-Adv-PP2Cm-shRNA3 transfection compared with controls. Cells were stained with JC-1 fluorescent dye and imaged under identical exposure parameters using Rhodamin (red; panels b,f) and FITC (green; panels c,g) filters on a Zeiss confocal microscope as described in Materials and Methods. The merged images are shown in panels d and h. (D) The average ratios of red versus green fluorescent signal intensities (shown as relative pixel intensity) were measured from eight control and eight PP2C-shRNA3-treated cells from digitally recorded images using the Metamorph program.
Loss of PP2Cm leads to hepatic injury and hepatocyte apoptosis in vivo
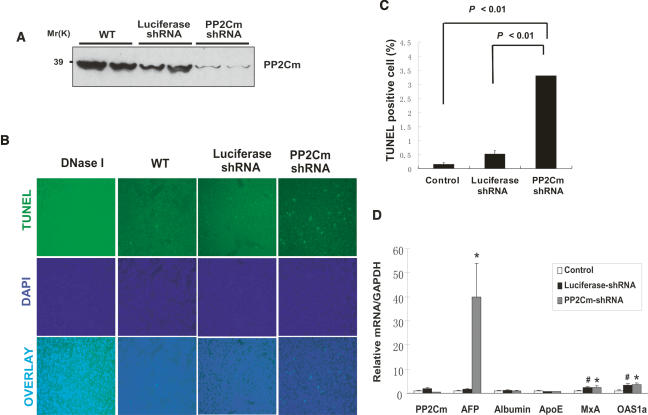
To further investigate the in vivo function of PP2Cm, we employed shRNA to specifically inactivate the endogenous PP2Cm in mouse liver using the recombinant adenovirus vector administered through intravenous injection. It has already been established that systemic delivery of adenovirus vectors in mice leads to effective targeting to the liver (Wang et al. 1996). Indeed, application of Adv-PP2Cm shRNA significantly reduced endogenous PP2Cm expression at both mRNA and protein levels in the mouse liver while administration of a control Adv-luciferase shRNA vector had no detectable impact on PP2Cm expression (Fig. 5A,C). The mice receiving Adv-PP2Cm shRNA showed significantly elevated hepatocyte apoptosis measured by TUNEL staining (Fig. 5B,C), and a marked induction of α-fetal protein (AFP) gene (Fig. 5D), a marker for hepatic injury and pathology. Two interferon response genes (MxA and OSA1α) were also modestly elevated; however, their inductions were observed in both Luciferase-shRNA and PP2Cm-shRNA-treated tissues. These data indicate that, similar to its role in cultured myocytes, endogenous PP2Cm is essential for normal survival and function of hepatocytes in vivo.
Figure 5.
Loss of PP2Cm in vivo induces hepatocyte apoptosis and liver injury. (A) Western blot analysis of PP2Cm expression in total liver lysates from wild-type mice or mice treated with adenovirus expressing either luciferase control shRNA or PP2Cm-specific shRNA3 for 7 d. (B, top panel) Mouse livers treated as described above were fixed and assessed for apoptisis by TUNEL staining. (Middle panel) The nuclei were counterstained with DAPI. (Bottom panel) Corresponding images were overlaid to distinguish false positive signals. DNase I-treated liver sections served as a positive control. (C) The percentage of apoptotic cells was tallied and presented as mean ± SD (n = 4). (D) Hepatic gene profiles of mouse livers treated as above were examined with quantitative RT–PCR by normalizing against GAPDH. (*) p < 0.05, PP2Cm shRNA versus control; (#) p < 0.05, luciferase shRNA versus control, Student’s t-test.
Loss of PP2Cm sensitizes mitochondria to Ca-induced permeability transition
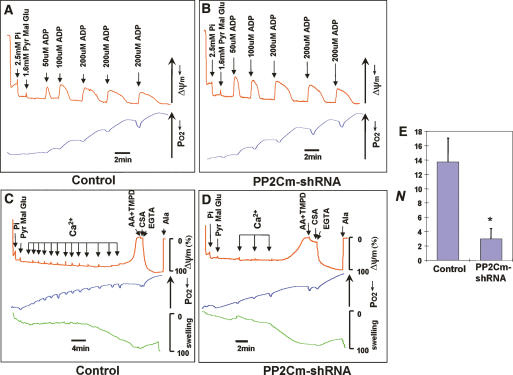
To reveal the underlying mechanisms involved in PP2Cm-mediated mitochondria regulation, we first determined the effect of PP2Cm inactivation on oxidative phosphorylation and ATP production in isolated mitochondria. We measured oxygen consumption, ΔΨ, and the mitochondrial matrix volume simultaneously after energizing the mitochondria with Complex I substrates and adding ADP in multiple pulses (Fig. 6A,B). From this experiment, we found that PP2Cm inactivation did not affect ΔΨ recovery, oxygen consumption, or the mitochondrial matrix volume after repeated ADP challenges, suggesting that PP2Cm deficiency does not have a significant impact on oxidative phosphorylation (Fig. 6A,B). In contrast, in the presence of consecutive 2-μM Ca2+ pulses, PP2Cm-deficient mitochondria quickly lost ΔΨ and displayed matrix swelling, the key features of mitochondrial permeability transition, whereas control mitochondria tolerated challenges with Ca2+ pulses much better (Fig. 6C,D). Importantly, the loss of mitochondrial membrane potential and increased matrix volume were reversed by CsA and EGTA (Fig. 6C), confirming that PP2Cm deficiency sensitized the opening of MPTP. These data suggest that PP2Cm is an important regulator of MPTP opening, and the loss of PP2Cm can promote cell death by triggering the mitochondrial permeability transition.
Figure 6.
The effect of PP2Cm knockdown on mitochondria function. Liver mitochondria were isolated from wild-type mice (A,C) and the mice were treated with adenovirus expressing PP2Cm-specific shRNA3 for 7 d (B,D). Then the mitochondria (0.25 mg/mL) in 100 mM (A,B) or 150 mM (C,D) KCl buffer containing 0.2% BSA and 2.5 mM Pi were energized with Complex I substrates (1.6 mM pyvuvate [Pyv], malate [Mal], and glutamate [Glu]). Subsequently, ADP (A,B) or Ca pulses (C,D) were added with the indicated concentration. Mitochondria membrane potential (ΔΨm, red), oxygen consumption (blue), and matrix volume (green) were monitored by measuring TMRM fluorescence, buffer PO2, and light scattering at 520 nm, respectively. After the Ca load triggered the mitochondria permeability transition, Complex IV substrate (2 mM ascorbic acid [AA] and 0.2 mM N,N,N′,N′-tetramethyl-p-phenylenediamine [TMPD]), 1.5 μM CsA, and 1 mM EGTA were added to recover the mitochondrial membrane potential, and then 10 μg of alamethicin (Ala) were added to induce maximal mitochondrial swelling and mitochondrial membrane potential dissipation. (E) Summary of number of Ca loads (2 μM) required for 95% mitochondrial matrix swelling N` (relative to alamethicin) of control and PP2Cm knockdown mitochondria (0.25 mg). Shown are mean ± SD. (*) p < 0.01, Student’s t-test. The data were representative of four experiments.
PP2Cm is essential to normal development of brain and digestive system of zebrafish
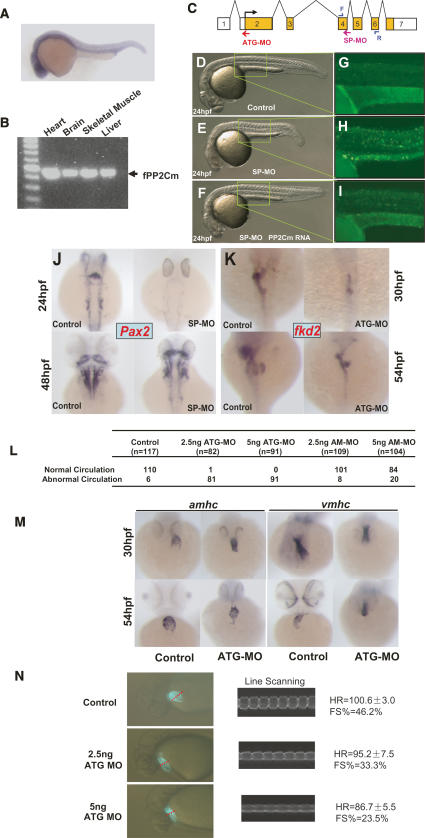
Zebrafish PP2Cm is highly homologous to mammalian counterparts based on sequence alignment (Fig. 1), and shows similar mitochondria-targeted intracellular localization when it was expressed in mammalian cells (Supplementary Fig. 5). In developing zebrafish embryos, PP2Cm expression was highly expressed in the CNS, heart, and other tissues at 24 h post-fertilization (hpf) as revealed by whole-mount in situ hybridization using an antisense PP2Cm cDNA probe (Fig. 7A). In adult zebrafish, PP2Cm transcript was detected in the brain, heart, skeletal muscle, and liver by RT–PCR (Fig. 7B). To investigate whether PP2Cm in zebrafish has a conserved function in regulating cell survival, we employed two types of morpholinos, ATG-MO and SP-MO, to suppress PP2Cm expression in the developing zebrafish embryo (Fig. 7C). The ATG-MO targets the start codon of PP2Cm to inhibit translation initiation. The SP-MO targets the splicing donor of PP2Cm intron 4, which should result in a truncated PP2Cm (1–236) mutant protein due to premature translation termination (Supplementary Fig. 6). To ensure target specificity of PP2Cm morpholinos, we used a modified morpholino (AM-MO) with a 5-base-pair (bp) mismatch to PP2Cm ATG-MO as a negative control for the ATG-MO. In addition, we coinjected PP2Cm mRNA with SP-MO to demonstrate the specificity of the SP-MO effects. Suppression of PP2Cm expression by SP-MO in the developing embryos resulted in a marked induction of apoptosis and developmental defect that was significantly mitigated by coinjection of wild-type zebrafish PP2Cm mRNA (Fig. 7D–I; Supplementary Fig. 8). Based on embryo size and the presence of brain cell death, 58.4% (59/101) of the embryos coinjected with SP-MO and PP2Cm mRNA were completely rescued, 22.3% (23/101) were partially rescued, while the remaining 18.8% (19/101) showed no obvious differences from SP-MO morphants without coinjection of PP2Cm mRNA. Similarly, ATG-MO resulted in abnormal development associated with significant induction in apoptosis as measured from acridine orange (Supplementary Fig. 7) staining and TUNEL staining (data not shown) compared with untreated embryos (data not shown) or embryos injected with the control AM-MO. Suppression of PP2Cm expression by either SP-MO or ATG-MO reduced brain size (Fig. 7J,K,M), and reduced and delayed a CNS marker gene Pax2 expression in the mid/hindbrain boundary, otic vesicle, and spinal cord in 24 and 48 hpf embryos as determined by in situ hybridization (Fig. 7J; Supplementary Fig. 8). Suppression of PP2Cm expression also resulted in defective development of the liver, pancreas, and gut as demonstrated from the reduced expression of an endoderm marker gene fkd2 in 30 and 54 hpf embryos (Fig. 7K). In short, PP2Cm has a widespread expression pattern in developing zebrafish with particular abundance in the heart and CNS. Reduced PP2Cm expression led to induced apoptosis and broad range defects in the CNS and digestive system.
Figure 7.
PP2Cm regulates zebrafish cell apoptosis, development, and cardiac function. (A) Lateral view at 24 hpf, anterior to the left. In situ hybridization analysis with a PP2Cm probe showed that PP2Cm was strongly expressed in the brain and the neural tube at 24 hpf. (B) RT–PCR analysis of total RNA isolated from indicated adult zefrafish tissues revealed that PP2Cm mRNA expressed in all tissues examined. (C) Genomic structure of PP2Cm. The PP2Cm translation morpholino (ATG-MO, red arrow) binds to the start codon to block translation. The PP2Cm splicing morpholino (SP-MO, pink arrow) targets the exon 4 splicing donor to inhibit splicing out intron 4–5. The positions of PCR primers used for splicing blockage analysis are marked by blue arrows. (D–F) Overall morphology of control (D), 6 ng of SP-MO-injected (E), or 6 ng of SP-MO and 500 pg of zPP2Cm (F) coinjected embryos by 24 hpf. (G–I). An acridine orange-stained embryo was visualized under UV illumination to access apoptotic cell death in the trunk regions as highlighted in D, E, and F. (J) Dorsal view, anterior to the top, of embryos not injected (control) or injected with 6 ng of SP-MO at 24 and 48 hpf using in situ hybridization with Pax2 as the probe. (K) Dorsal view, anterior to the top. In situ hybridization of embryos not injected (control) or injected with 5 ng of ATG-MO at 30 and 54 hpf, probed with fdk2. (L) Noninjected and ATG morpholino-injected embryos with a dose of 2.5 ng or 5 ng. As a control, a 5-bp mismatch ATG morpholino (AM-MO) was administered in the same manner. At 30 hpf, incidence of absent blood circulation was identified and tallied among the total number of embryos examined. (M) In situ hybridization of noninjected and 5-ng ATG-MO-injected embryos using atria-specific probe amhc and ventricle-specific probe vmhc. (Top panel) By 30 hpf, the atria and ventricle were significantly smaller in ATG-MO-injected embryos, in which ventricle leftward jogging was also not observed. (Bottom panel) By 54 hpf, the heart of the ATG-MO-injected embryos failed to loop. (N) Heart rate and contractility measured from digitally recorded images of cmlc2∷GFP fish injected with control or ATG-MO morpholino as indicated at 30 hpf using a custom-made line-scanning program (LQ-1). No fibrillation or arrhythmia was observed. Left panels illustrate the digital images of the fish, with dotted red lines marking the scanning line position. The right panels illustrate M-mode atrial chamber contraction similar to images obtained from echocardiograph. The heart rate (HR) and fractional shorterning (FS%) were calculated based on an M-mode graph as described in Materials and Methods.
Suppression of PP2Cm expression leads to abnormal cardiac development and heart failure
As summarized in Figure 7L and Supplementary Table 1, circulatory defects were also observed in ATG-MO-treated embryos, although the vasculature structure showed no significant difference between ATG-MO-injected embryos vs. untreated or AM-MO-treated embryos based on the in situ hybridization pattern of a marker gene flick (data not shown). As revealed by amhc and vmhc staining, normal cardiac jogging of the ventricle at 30 hpf was not detected in ATG-MO-treated embryos, and heart looping was also impaired at 54 hpf (Fig. 7M). Moreover, suppression of PP2Cm resulted in the loss of the atria–ventricular boundary staining pattern of Bmp4 or Notch1B at the myocardium or endocardium, respectively (Supplementary Fig. 9). These results suggest PP2Cm has an essential role in normal cardiac development. From direct measurement of digital images of atrial contraction in the background of cmlc2∷GFP zebrafish (Huang et al. 2003) using a custom-made line-scanning analysis tool (Q. Luo and Y. Wang, unpubl.), we detected a marked reduction of ejection fraction and slower heart rates in PP2Cm morphant hearts compared with controls (Fig. 7N). The severity of the contractile defect correlated with the dosage of injected PP2Cm ATG-MO. Therefore, the loss of PP2Cm caused significant development defects and contractile dysfunction in the developing fish heart. These observations again support the general conclusion that protein phosphorylation in the mitochondrial matrix regulated by PP2Cm is a conserved mechanism that regulates cell survival. PP2Cm-mediated mitochondrial signaling plays an important role in normal CNS and cardiac development and function.
Discussion
In this report, we identified and characterized a new member of the Ser/Thr protein phosphatase from the PP2C family, PP2Cm. From both biochemical and cellular studies, we demonstrate that PP2Cm is a mitochondria-targeted protein phosphatase exclusively located in the mitochondrial matrix soluble fraction. To our knowledge, this is the first ser/thr protein phosphatase identified that displays such an exclusive intracellular localization. Specific down-regulation of PP2Cm in vitro and in vivo promotes cell death and stress-signaling activation associated with loss of the ΔΨ and MPTP opening. In contrast, mitochondrial respiration and oxidative phosphorylation remain unaffected based on the in vitro assay. In developing zebrafish, suppressed expression of the PP2Cm gene leads to brain and cardiac defects associated with impaired cardiac function and elevated apoptosis. From all these data, we conclude that PP2Cm is a mitochondrially targeted protein phosphatase that plays an essential function in MPTP regulation and serves as an indispensable component of a conserved regulatory mechanism for cellular survival.
It is well established that mitochondria are not only important for energy metabolism but also for intracellular signal integration regulating cell survival and programmed cell death (Crow et al. 2004; Kim et al. 2006). One critical step in the mitochondrially mediated intrinsic programmed cell death pathway involves the release of proapoptotic factors (cytochrome c and AIFs) from the IMS. The release of these proapoptotic proteins can be caused either by the formation of nonselective megachannels in the outer membrane or from outer membrane rupture due to mitochondrial matrix swelling triggered by the MPTP opening (Lemasters et al. 2002). It is well established that outer membrane megachannels involving Bid and Bak proteins are regulated by Bcl-2 family members in response to a variety of pro- or anti-apoptotic signaling. Although calcium, inorganic phosphate, reactive oxygen species (ROS), and other stimuli are known to induce MPT opening, the molecular components of the MPTP and their regulatory mechanisms are still unclear (Lemasters et al. 2002). It has been speculated that the MPTP can be formed by protein complexes involving ANT in the inner membrane, VDAC in the outer membrane, and the CsA-binding protein CypD in the matrix (Honda et al. 2005). However, it is not clear how these triggers can induce MPTP opening and cell death under stress conditions. Recent evidence suggest that PKCε promotes cardioprotection against ischemia/reperfusion injury through phosphorylation of VDAC and prevents the opening of the MPTP (Baines et al. 2003). However, specific post-translational modification of the mitochondrial matrix proteins involved in the MPTP regulation and cell death has not yet been reported. Our report provides the first molecular evidence to suggest that protein phosphorylation/dephosphorylation in the mitochondria matrix can have a major impact on MPTP regulation and cell death. However, it is not clear which of the known components of the MPTP are subjected to protein phosphorylation and targeted by PP2Cm. It is also not entirely clear whether PP2Cm exerts its effect on MPTP opening by direct dephosphorylation of any of the MPTP protein components or by indirect modulation of other triggers, such as calcium homeostasis, pH, ROS production, etc. The fact that respiration and oxidative phosphorylation in PP2Cm-deficient mitochondria is not impaired would suggest that the effect of PP2Cm on the MPT opening is not secondary to the loss of ATP production. Although our accumulated data are consistent with an effect on MPTP regulation, other effects on energy production or other essential processes cannot be excluded and need further investigation. Therefore, detailed analysis of PP2Cm regulation on the coupling efficiency of each respiration complex, matrix calcium regulation, and matrix protein phosphorylation in general will be important in future studies. Our data from in vitro assays using recombinant proteins indicate that PP2Cm has a relatively high selectivity and low specific activity for generic substrates compared with PP2Cα. However, our resulst could be misleading as PP2Cm may require specific pH/salt conditions for optimal enzymatic activity. Clearly, identification of the molecular targets of PP2Cm in the mitochondrial matrix is critical for achieving a better understanding of this novel member of the PP2C family at molecular, biochemical, and functional levels. In this regard, current efforts leading to the identification of PP2Cm substrates and their dynamic phosphorylation pattern under physiological/pathological conditions should bring important new insights to the regulatory mechanism in mitochondria function and cell death.
Mitochondrial function, including MPTP regulation, is important to normal cellular function and development. Consistent with this notion and our observation in cultured cells, loss of PP2Cm expression in the liver and developing fish embryos led to increased apoptosis. Conversely, PP2C expression was diminished in the mouse model of cardiac hypertrophy and heart failure. Indeed, loss of PP2Cm expression in the mouse liver resulted in hepatic injury, while knockdown of PP2Cm in zebrafish lead to abnormal development in the heart, CNS, and liver. These results provide clear evidence that mitochondrial matrix phosphorylation is a critical signaling mechanism for cell death regulation during normal development. It is not clear whether abnormal development in the heart, brain, and liver are the consequences of induced cell death or induced stress signaling or both. In addition, it is not clear whether PP2Cm expression is affected in neuronal or hepatic diseases such as observed in the heart. Nevertheless, we can speculate that PP2Cm may also play an important role in CNS and other organ function development. More rigorous investigation with targeted genetic manipulation in model systems will be needed to fully reveal the functional role of this molecule in development and diseases.
Materials and methods
Molecular cloning of PP2Cm cDNAs
Human PP2Cm(NM_152542) were identified by a BLAST search with the catalytic domain of human PP2Cα (AF070670). Then, full-length human PP2Cm was amplified from a human EST clone (BG713950) by PCR using the following primers: sense, 5′-TAAAAGATCTGCCACCATGTCAACAGCTGCC TTA-3′; antisense, 5′-TAAACTCGAGTCAGGCCCATCGTC CACTGGAGGC-3′. The full-length coding sequence of mouse PP2Cm was amplified from a mouse heart cDNA library by PCR using the following primers: sense, 5′-TAAAGCGGCC GCCACCATGTTATCAGCGGCCTT-3′; antisense, 5′-TAAA CTCGAGTCAGGCCCATCTCCCACTGGA-3′, based on the GeneBank nucleotide sequence NM_175523. A full-length zebrafish PP2Cm sequence was amplified from an embryonic cDNA library by PCR using the following primers: sense, 5′-TAAAGAATTCGCCACCATGTCAGTGGCTCTCCTGGT-3′; antisense, 5′-TAAAGCGGCCGCTTGTTTGTTCAGGCGAAG CG-3′, based on the zebrafish ENSEMBL gene sequence ENSDARG 00000010655. Details of the cloning procedure are described in the Supplemental Material.
RNA interference (RNAi)
To achieve efficient knockdown of endogenous PP2Cm, synthetic 64-nucleotide (nt) oligonucleotide pairs targeting mouse and rat PP2Cm were annealed and ligated into a modified version of pSUPER vector (Oligoengine), pShuttle-pSUPER, which was constructed by subcloning the H1-RNA expression cassette into pShuttle (Adeasy, Stratagene) (a kind gift from J. Han, Scripps, La Jolla, CA). Then a recombinant adenovirus expressing PP2Cm shRNA was generated according to the manufacturer’s instructions. As a control, a recombinant adenoviral shRNA vector targeting firefly luciferase was constructed as described above. The targeting sequences were listed as follows: PP2Cm shRNA1, 5′-TCTGGGATAACCGCATTGA-3′; PP2Cm shRNA2, 5′-GAAGCTGACCACTGACCAT-3′; PP2Cm shRNA3, 5′-GA AGCTGACCACTGACCAT-3′; Luciferase shRNA, 5′-CTGAC GCGGAATACTTCGA-3′.
Antibodies
Polyclonal antibody (Abgent) against mouse PP2Cm were generated by immunizing rabbits with synthetic peptide corresponding to PP2Cm (47–65). The antibody was further affinity-purified using Protein G column. A polyclonal antibody against β-actin (Sc-1616) and Calnexin (SC-11397), and anti-Flag M2 (Sigma) were purchased from the indicated suppliers. A polyclonal antibody against HSP60, TOMM40, TIMM13, and Rhodanese were kind gifts from Carla Koehler.
Protein phosphatase assays
Protein phosphatase activity was determined by 32P release from the MBP, phosphorylated by the catalytic subunit of cyclic AMP-dependent protein kinase (PKA) with details described in the Supplemental Material.
RNA analysis
Total RNA was extracted from multiple mouse tissues using Trizol (Invitrogen) according to the manufacturer’s protocols. Northern blot analysis was performed with the use of a 32P-labeled full-length PP2Cm cDNA probe as described previously (Petrich et al. 2002). Quantitative real-time RT–PCR was performed as described in detail in the Supplemental Material.
Zebrafish strains and functional analysis
Zebrafish colonies were maintained as previously described (Westerfield 1995). The developmental stages of wild-type fish strain AB and cmlc2∷GFP transgenic fishes were determined by morphological features of fish raised at 28.5°C (Kimmel et al. 1995). Digital images of fish were recorded at 30 frames per second under UV illumination and analyzed using the LQ-1 program, a custom-made software program to perform line-scanning analysis of the fish atrial contraction. The heart rate was calculated from cycle numbers per minute. Fractional shortening (FS%) was calculated from Dd − Ds/Dd × 100%, where Dd is the diastolic chamber diameter and Ds is the systolic chamber diameter.
Morpholino injections
Morpholino antisense oligonucleotides (Gene Tools) PP2CmATG (5′-ACATGCTCAGTGGCAGAAAACCACA-3′, complementary to the translation initiation region), PP2CmAM (5′-ACATcCTCAcTGcCAcAAAAcCACA-3′, a 5-bp mismatch to PP2CmATG as a control), and PP2CmSP (5′-CGCTGGAAAT TGCTCACCTCTCTTT-3′, complementary to the exon 4 splicing donor region) were synthesized. Embryos were injected with Morpholino oligos at the one- to four-cell stage, followed by digital imaging analysis and whole-mount in situ hybridization at 30 hpf and 54 hpf. At 24 hpf, total RNA isolated from embryos injected with PP2CmSP MO was subjected to RT–PCR analysis to determine the splicing inhibition effect using two primers flanking the exon 4 splicing donor (For, 5′-CCTCTGT GTTGAGTGCAGGA-3′; Rev, 5′-AGCCTGCTCAGATATGC GTT-3′).
In situ hybridization
Embryos for in situ hybridization were raised in embryo medium supplemented with 0.2 mM 1-phenyl-2-thiourea to maintain optical transparency (Westerfield 1995). Whole-mount in situ hybridization was performed as described previously (Chen and Fishman 1996). The antisense RNA probes used in this study were PP2Cm, Pax2, vmhc, amhc, Bmp4, Notch1B, fkd2, and flick.
Acridine orange staining
Live embryos were stained with 5 μg/mL acridine orange in E3 embryo buffer for 20 min, washed with E3 three times, and then observed by a Zeiss fluorescence microscope.
TUNEL staining
Mouse liver fixed with 10% formalin was embedded in O.C.T. tissue freezing medium and then snap-frozen in 2-methylbutane bath cooled with liquid nitrogen. Eight-micron liver cryosections were examined to determine the apoptosis index using ApopTag TUNEL staining kit (Chemicon) according to the manufacturer’s instructions. Sections incubated with 10 U/mL deoxyribonuclease I (Sigma) for 10 min served as positive controls.
Mitochondrial assays
All mitochondrial assays were carried out as described previously (Korge et al. 2005) with details provided in the Supplemental Material. In brief, mitochondrial ΔΨm was estimated from Tetramethylrhodamine methyl ester (TMRM) fluorescence at 580 nm. Mitochondrial matrix volume change was monitored by recording 90° light scattering at 520 nm. Oxygen consumption was recorded by measuring PO2 in the buffer via a fiberoptic oxygen sensor. For ΔΨm measurement in cultured cardiomyocytes, cells were stained with cationic fluorescent dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Molecular Probes), and examined under a laser-scanning confocal microscope (Olympus Fluoview). The green fluorescence emission of JC-1 monomers and the red fluorescence of JC-1 aggregates were recorded and quantified by MetaMorph (Universal Imaging Corp.), and the relative ratio of aggregates/monomers (red/green) fluorescence was taken as a measurement of mitochondrial membrane potential (ΔΨm).
Acknowledgments
We thank Haiying Pu and Jing Gao for their excellent technical assistance, and Mr. Qing Luo for developing the LQ-1 program. We also thank Dr. Peipei Ping and Dr. Enrico Stefani for insightful discussions. This work is supported in part by Laubisch Foundation at UCLA, grants from the National Institutes of Health (HL062311 and HL080111), and an Established Investigator Award (to Y.W.), and a Predoctoral Fellowship (to G.L.) from the American Heart Association.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1499107
References
- Alto N.M., Soderling J., Scott J.D., Soderling J., Scott J.D., Scott J.D. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C.P., Song C.X., Zheng Y.T., Wang G.W., Zhang J., Wang O.L., Guo Y., Bolli R., Cardwell E.M., Ping P., Song C.X., Zheng Y.T., Wang G.W., Zhang J., Wang O.L., Guo Y., Bolli R., Cardwell E.M., Ping P., Zheng Y.T., Wang G.W., Zhang J., Wang O.L., Guo Y., Bolli R., Cardwell E.M., Ping P., Wang G.W., Zhang J., Wang O.L., Guo Y., Bolli R., Cardwell E.M., Ping P., Zhang J., Wang O.L., Guo Y., Bolli R., Cardwell E.M., Ping P., Wang O.L., Guo Y., Bolli R., Cardwell E.M., Ping P., Guo Y., Bolli R., Cardwell E.M., Ping P., Bolli R., Cardwell E.M., Ping P., Cardwell E.M., Ping P., Ping P. Protein kinase Cε interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur G.N., Jope R.S., Jope R.S. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichese L., Cazettes G., Valette A., Cazettes G., Valette A., Valette A. JNK is associated with Bcl-2 and PP1 in mitochondria: Paclitaxel induces its activation and its association with the phosphorylated form of Bcl-2. Cell Cycle. 2004;3:1312–1319. doi: 10.4161/cc.3.10.1166. [DOI] [PubMed] [Google Scholar]
- Chen J.N., Fishman M.C., Fishman M.C. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122:3809–3816. doi: 10.1242/dev.122.12.3809. [DOI] [PubMed] [Google Scholar]
- Chen L., Hahn H., Wu G., Chen C.H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Hahn H., Wu G., Chen C.H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Wu G., Chen C.H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Chen C.H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Cavallaro G., Banci L., Guo Y., Bolli R., Banci L., Guo Y., Bolli R., Guo Y., Bolli R., Bolli R., et al. Opposing cardioprotective actions and parallel hypertrophic effects of δ PKC and ε PKC. Proc. Natl. Acad. Sci. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Fearnley I.M., Peak-Chew S.Y., Walker J.E., Fearnley I.M., Peak-Chew S.Y., Walker J.E., Peak-Chew S.Y., Walker J.E., Walker J.E. The phosphorylation of subunits of complex I from bovine heart mitochondria. J. Biol. Chem. 2004;279:26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- Cohen M.V., Baines C.P., Downey J.M., Baines C.P., Downey J.M., Downey J.M. Ischemic preconditioning: From adenosine receptor to KATP channel. Annu. Rev. Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- Crow M.T., Mani K., Nam Y.J., Kitsis R.N., Mani K., Nam Y.J., Kitsis R.N., Nam Y.J., Kitsis R.N., Kitsis R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- Dagda R.K., Zaucha J.A., Wadzinski B.E., Strack S., Zaucha J.A., Wadzinski B.E., Strack S., Wadzinski B.E., Strack S., Strack S. A developmentally regulated, neuron-specific splice variant of the variable subunit Bβ targets protein phosphatase 2A to mitochondria and modulates apoptosis. J. Biol. Chem. 2003;278:24976–24985. doi: 10.1074/jbc.M302832200. [DOI] [PubMed] [Google Scholar]
- Deng X., Ruvolo P., Carr B., May W.S., Jr., Ruvolo P., Carr B., May W.S., Jr., Carr B., May W.S., Jr., May W.S., Jr. Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc. Natl. Acad. Sci. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A.L., Fowler S., Lazarow P.B., Hubbard A.L., Fowler S., Lazarow P.B., Fowler S., Lazarow P.B., Lazarow P.B. Isolation of intracellular membranes by means of sodium carbonate treatment: Application to endoplasmic reticulum. J. Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H.M., Korge P., Weiss J.N., Korge P., Weiss J.N., Weiss J.N. Mitochondria and ischemia/reperfusion injury. Ann. N. Y. Acad. Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- Horbinski C., Chu C.T., Chu C.T. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic. Biol. Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Huang L.J., Wang L., Ma Y., Durick K., Perkins G., Deerinck T.J., Ellisman M.H., Taylor S.S., Wang L., Ma Y., Durick K., Perkins G., Deerinck T.J., Ellisman M.H., Taylor S.S., Ma Y., Durick K., Perkins G., Deerinck T.J., Ellisman M.H., Taylor S.S., Durick K., Perkins G., Deerinck T.J., Ellisman M.H., Taylor S.S., Perkins G., Deerinck T.J., Ellisman M.H., Taylor S.S., Deerinck T.J., Ellisman M.H., Taylor S.S., Ellisman M.H., Taylor S.S., Taylor S.S. NH2-terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J. Cell Biol. 1999;145:951–959. doi: 10.1083/jcb.145.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.J., Tu C.T., Hsiao C.D., Hsieh F.J., Tsai H.J., Tu C.T., Hsiao C.D., Hsieh F.J., Tsai H.J., Hsiao C.D., Hsieh F.J., Tsai H.J., Hsieh F.J., Tsai H.J., Tsai H.J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Kang B.P., Urbonas A., Baddoo A., Baskin S., Malhotra A., Meggs L.G., Urbonas A., Baddoo A., Baskin S., Malhotra A., Meggs L.G., Baddoo A., Baskin S., Malhotra A., Meggs L.G., Baskin S., Malhotra A., Meggs L.G., Malhotra A., Meggs L.G., Meggs L.G. IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. Am. J. Physiol. Renal Physiol. 2003;285:F1013–F1024. doi: 10.1152/ajprenal.00209.2003. [DOI] [PubMed] [Google Scholar]
- Kim R., Emi M., Tanabe K., Emi M., Tanabe K., Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother. Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F., Kimmel S.R., Ullmann B., Schilling T.F., Ullmann B., Schilling T.F., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Koehler C.M., Merchant S., Oppliger W., Schmid K., Jarosch E., Dolfini L., Junne T., Schatz G., Tokatlidis K., Merchant S., Oppliger W., Schmid K., Jarosch E., Dolfini L., Junne T., Schatz G., Tokatlidis K., Oppliger W., Schmid K., Jarosch E., Dolfini L., Junne T., Schatz G., Tokatlidis K., Schmid K., Jarosch E., Dolfini L., Junne T., Schatz G., Tokatlidis K., Jarosch E., Dolfini L., Junne T., Schatz G., Tokatlidis K., Dolfini L., Junne T., Schatz G., Tokatlidis K., Junne T., Schatz G., Tokatlidis K., Schatz G., Tokatlidis K., Tokatlidis K. Tim9p, an essential partner subunit of Tim10p for the import of mitochondrial carrier proteins. EMBO J. 1998;17:6477–6486. doi: 10.1093/emboj/17.22.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge P., Honda H.M., Weiss J.N., Honda H.M., Weiss J.N., Weiss J.N. K+-dependent regulation of matrix volume improves mitochondrial function under conditions mimicking ischemia–reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H66–H77. doi: 10.1152/ajpheart.01296.2004. [DOI] [PubMed] [Google Scholar]
- Lemasters J.J., Qian T., He L., Kim J.S., Elmore S.P., Cascio W.E., Brenner D.A., Qian T., He L., Kim J.S., Elmore S.P., Cascio W.E., Brenner D.A., He L., Kim J.S., Elmore S.P., Cascio W.E., Brenner D.A., Kim J.S., Elmore S.P., Cascio W.E., Brenner D.A., Elmore S.P., Cascio W.E., Brenner D.A., Cascio W.E., Brenner D.A., Brenner D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002;4:769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- Nantel A., Huber M., Thomas D.Y., Huber M., Thomas D.Y., Thomas D.Y. Localization of endogenous Grb10 to the mitochondria and its interaction with the mitochondrial-associated Raf-1 pool. J. Biol. Chem. 1999;274:35719–35724. doi: 10.1074/jbc.274.50.35719. [DOI] [PubMed] [Google Scholar]
- Newmeyer D.D., Ferguson-Miller S., Ferguson-Miller S. Mitochondria: Releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Pagliarini D.J., Wiley S.E., Kimple M.E., Dixon J.R., Kelly P., Worby C.A., Casey P.J., Dixon J.E., Wiley S.E., Kimple M.E., Dixon J.R., Kelly P., Worby C.A., Casey P.J., Dixon J.E., Kimple M.E., Dixon J.R., Kelly P., Worby C.A., Casey P.J., Dixon J.E., Dixon J.R., Kelly P., Worby C.A., Casey P.J., Dixon J.E., Kelly P., Worby C.A., Casey P.J., Dixon J.E., Worby C.A., Casey P.J., Dixon J.E., Casey P.J., Dixon J.E., Dixon J.E. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic β cells. Mol. Cell. 2005;19:197–207. doi: 10.1016/j.molcel.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Petrich B.G., Gong X., Lerner D.L., Wang X., Brown J.H., Saffitz J.E., Wang Y., Gong X., Lerner D.L., Wang X., Brown J.H., Saffitz J.E., Wang Y., Lerner D.L., Wang X., Brown J.H., Saffitz J.E., Wang Y., Wang X., Brown J.H., Saffitz J.E., Wang Y., Brown J.H., Saffitz J.E., Wang Y., Saffitz J.E., Wang Y., Wang Y. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ. Res. 2002;91:640–647. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- Ravagnan L., Roumier T., Kroemer G., Roumier T., Kroemer G., Kroemer G. Mitochondria, the killer organelles and their weapons. J. Cell. Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- Reers M., Smiley S.T., Mottola-Hartshorn C., Chen A., Lin M., Chen L.B., Smiley S.T., Mottola-Hartshorn C., Chen A., Lin M., Chen L.B., Mottola-Hartshorn C., Chen A., Lin M., Chen L.B., Chen A., Lin M., Chen L.B., Lin M., Chen L.B., Chen L.B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- Ruvolo P.P., Clark W., Mumby M., Gao F., May W.S., Clark W., Mumby M., Gao F., May W.S., Mumby M., Gao F., May W.S., Gao F., May W.S., May W.S. A functional role for the B56 α-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J. Biol. Chem. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- Scacco S., Vergari R., Scarpulla R.C., Technikova-Dobrova Z., Sardanelli A., Lambo R., Lorusso V., Papa S., Vergari R., Scarpulla R.C., Technikova-Dobrova Z., Sardanelli A., Lambo R., Lorusso V., Papa S., Scarpulla R.C., Technikova-Dobrova Z., Sardanelli A., Lambo R., Lorusso V., Papa S., Technikova-Dobrova Z., Sardanelli A., Lambo R., Lorusso V., Papa S., Sardanelli A., Lambo R., Lorusso V., Papa S., Lambo R., Lorusso V., Papa S., Lorusso V., Papa S., Papa S. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J. Biol. Chem. 2000;275:17578–17582. doi: 10.1074/jbc.M001174200. [DOI] [PubMed] [Google Scholar]
- Schroeter H., Boyd C.S., Ahmed R., Spencer J.P., Duncan R.F., Rice-Evans C., Cadenas E., Boyd C.S., Ahmed R., Spencer J.P., Duncan R.F., Rice-Evans C., Cadenas E., Ahmed R., Spencer J.P., Duncan R.F., Rice-Evans C., Cadenas E., Spencer J.P., Duncan R.F., Rice-Evans C., Cadenas E., Duncan R.F., Rice-Evans C., Cadenas E., Rice-Evans C., Cadenas E., Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: New target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem. J. 2003;372:359–369. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S. Protein serine/threonine phosphatases—New avenues for cell regulation. Annu. Rev. Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- Takimoto E., Champion H.C., Li M., Belardi D., Ren S., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Champion H.C., Li M., Belardi D., Ren S., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Li M., Belardi D., Ren S., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Belardi D., Ren S., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Ren S., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Rodriguez E.R., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Bedja D., Gabrielson K.L., Wang Y., Kass D.A., Gabrielson K.L., Wang Y., Kass D.A., Wang Y., Kass D.A., Kass D.A. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- Tamura Y., Simizu S., Osada H., Simizu S., Osada H., Osada H. The phosphorylation status and anti-apoptotic activity of Bcl-2 are regulated by ERK and protein phosphatase 2A on the mitochondria. FEBS Lett. 2004;569:249–255. doi: 10.1016/j.febslet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Technikova-Dobrova Z., Sardanelli A.M., Speranza F., Scacco S., Signorile A., Lorusso V., Papa S., Sardanelli A.M., Speranza F., Scacco S., Signorile A., Lorusso V., Papa S., Speranza F., Scacco S., Signorile A., Lorusso V., Papa S., Scacco S., Signorile A., Lorusso V., Papa S., Signorile A., Lorusso V., Papa S., Lorusso V., Papa S., Papa S. Cyclic adenosine monophosphate-dependent phosphorylation of mammalian mitochondrial proteins: Enzyme and substrate characterization and functional role. Biochemistry. 2001;40:13941–13947. doi: 10.1021/bi011066p. [DOI] [PubMed] [Google Scholar]
- Wang Y., Krushel L.A., Edelman G.M., Krushel L.A., Edelman G.M., Edelman G.M. Targeted DNA recombination in vivo using an adenovirus carrying the cre recombinase gene. Proc. Natl. Acad. Sci. 1996;93:3932–3936. doi: 10.1073/pnas.93.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.L., Yeh S.F., Chang Y.I., Hsiao S.F., Lian W.N., Lin C.H., Huang C.Y., Lin W.J., Yeh S.F., Chang Y.I., Hsiao S.F., Lian W.N., Lin C.H., Huang C.Y., Lin W.J., Chang Y.I., Hsiao S.F., Lian W.N., Lin C.H., Huang C.Y., Lin W.J., Hsiao S.F., Lian W.N., Lin C.H., Huang C.Y., Lin W.J., Lian W.N., Lin C.H., Huang C.Y., Lin W.J., Lin C.H., Huang C.Y., Lin W.J., Huang C.Y., Lin W.J., Lin W.J. PICK1, an anchoring protein that specifically targets protein kinase Cα to mitochondria selectively upon serum stimulation in NIH 3T3 cells. J. Biol. Chem. 2003;278:37705–37712. doi: 10.1074/jbc.M304619200. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book, a guide for the laboratory use of zebrafish (Danio rerio) University of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Wiltshire C., Matsushita M., Tsukada S., Gillespie D.A., May G.H., Matsushita M., Tsukada S., Gillespie D.A., May G.H., Tsukada S., Gillespie D.A., May G.H., Gillespie D.A., May G.H., May G.H. A new c-Jun N-terminal kinase (JNK)-interacting protein, Sab (SH3BP5), associates with mitochondria. Biochem. J. 2002;367:577–585. doi: 10.1042/BJ20020553. [DOI] [PMC free article] [PubMed] [Google Scholar]