Abstract
The σS subunit of RNA polymerase is a master regulator of Escherichia coli that retards cellular senescence and bestows cells with general stress protective functions during growth arrest. We show that mutations and drugs triggering translational errors elevate σS levels and stability. Furthermore, mutations enhancing translational fidelity attenuate induction of the rpoS regulon and prevent stabilization of σS upon carbon starvation. Destabilization of σS by increased proofreading requires the presence of the σS recognition factor SprE (RssB) and the ClpXP protease. The data further suggest that σS becomes stabilized upon starvation as a result of ClpP sequestration and this sequestration is enhanced by oxidative modifications of aberrant proteins produced by erroneous translation. ClpP overproduction counteracted starvation-induced stabilization of σS, whereas overproduction of a ClpXP substrate (ssrA-tagged GFP) stabilized σS in exponentially growing cells. We present a model for the sequence of events leading to the accumulation and activation of σS upon carbon starvation, which are linked to alterations in both ribosomal fidelity and efficiency.
Keywords: Escherichia coli, stationary phase, RpoS, SprE, rpsL, ClpP, protein oxidation
Unicellular and multicellular organisms harbor genetic networks that sense the quality of the environment and boost the individual’s maintenance functions when growth conditions become suboptimal (Larsen 1993; Dukan and Nyström 1998, 1999; Hekimi and Guarente 2003; Hsu et al. 2003; Libina et al. 2003). Examples of such regulatory networks are the RAS/TOR pathways, the FOXO forkhead transcription factor family working in concert with insulin/insulin like signaling (Rohde et al. 2001; Libina et al. 2003; Hwangbo et al. 2004; Schmelzle et al. 2004; Kloting and Bluher 2005), and, in Gram-negative prokaryotes, the σ factor σS regulon. The σS, FOXO, and RAS/TOR regulatory systems are functionally analogous; they all respond to starvation (e.g., dietary restriction); they are required to mount general stress protection; and they are longevity determinants (Larsen 1993; Marchler et al. 1993; Lange and Hengge-Aronis 1994; Johnson et al. 2000; Weber et al. 2005; Powers et al. 2006). It is not clear which individual genes of these regulons are most important in slowing down senescence, but oxidative stress defense has been argued to be a key feature of all of them (Eisenstark et al. 1996; Dukan and Nyström 1998; Hlavata et al. 2003; Heeren et al. 2004; Kondo et al. 2005). In addition, several virulence traits are regulated by σS, and enteric bacteria lacking this σ factor are less pathogenic (Heiskanen et al. 1994; Webb et al. 1999). In fact, up to 10% of all Escherichia coli genes are directly or indirectly controlled by σS, indicating that this σ factor controls an exceptionally large network of genes (Weber et al. 2005).
An almost baffling number of cis-regulatory determinants and trans-acting regulatory factors involved in σS regulation have been identified (Hengge-Aronis 2002). For example, cAMP-CRP, GlcIIa, BarA, polyphosphate, homoserine lactone, acetate, and the NADH/NAD ratio have been shown to affect rpoS transcription, whereas DnaK, DksA, Hfq, HU, StpA, LeuO, and several small regulatory RNAs control rpoS translation (Hengge-Aronis 2002; Repoila et al. 2003). Yet, the key process responsible for the accumulation of σS during carbon depletion is regulated σS proteolysis. In cells growing exponentially in minimal medium, the half-life of σS is ∼1 min, but the protein is rapidly and drastically stabilized upon carbon starvation of cells (Hengge-Aronis 2002). The protease ClpXP and the two-component orphan response regulator SprE (RssB), a specific σS recognition factor, are essential for the process of σS degradation (Muffler et al. 1996; Pratt and Silhavy 1996; Becker et al. 1999; Moreno et al. 2000; Mandel and Silhavy 2005). The affinity of SprE for σS is modulated, in vitro, by phosphorylation of the SprE receiver domain (Moreno et al. 2000; Klauck et al. 2001; Mandel and Silhavy 2005). Thus, it is tempting to speculate that stabilization of σS during starvation is a result of dephosphorylation of the SprE response regulator, and it has been proposed that the ArcB two-component sensor might be involved in such a mechanism (Mika and Hengge 2005). However, mutations (SprED58A) in the conserved phosphorylation site of SprE only affect basal levels of σS, whereas its accumulation and increased stability upon starvation/stationary phase remain normal (Peterson et al. 2004; Bougdour et al. 2006). Thus, it appears that the sensing/signaling device used by E. coli to stabilize σS upon carbon starvation operates, to a large extent, independently of SprE phosphorylation/dephosphorylation.
Nutrient limitation can be sensed by a variety of mechanisms, the most direct being performed by proteins associated with nutrient uptake systems, such as GlcIIa and PhoU, which sense/measure the presence/concentration of glucose and phosphate, respectively (Wanner 1993; Meadow et al. 2006). Depletion of ammonium or non-PTS carbon sources, on the other hand, are sensed by systems measuring the ratio of key metabolites, such as glutamine/α-ketoglutarate (Senior 1975; Magasanik 1989) and PEP/pyruvate (Hogema et al. 1998). The stringent response to amino acid shift-down relies instead on the ribosomes as sensors of amino acid deficiency (Cashel et al. 1996). Specifically, an uncharged tRNA finding its way into the A-site of the ribosome activates ppGpp production via the ribosome-associated ppGpp synthase I (PSI; RelA) (Cashel et al. 1996). Under a variety of other stress conditions, ppGpp is produced by the ppGpp synthase II (PSII) encoded by spoT (Cashel et al. 1996). The exact sensing–signaling pathway responsible for SpoT activation is not known, but fatty acid metabolism and interactions with the acyl carrier protein appear essential for SpoT activity under some conditions (Battesti and Bouveret 2006). The alarmone ppGpp is the effector molecule of the stringent response, which, in concert with the RNAP-binding protein DksA, affects a plethora of physiological activities, the main target being transcription (Paul et al. 2004b). In addition to its role in repressing superfluous rRNA synthesis during starvation (Cashel et al. 1996; Paul et al. 2004a; Magnusson et al. 2005), ppGpp also acts as a positive effector of gene expression, and σS-dependent genes require this nucleotide for their induction during starvation (Gentry et al. 1993; Kvint et al. 2000). One reason for the ppGpp-dependency of σS-regulated genes is that σS competes more successfully with the housekeeping σ factor σ70 when the RNA polymerase is programmed with ppGpp (Jishage et al. 2002). Thus, the ribosome, via ppGpp, can act as a sensor/signaling component, which regulates a switch toward maintenance functions during nutrient depletion by affecting the activity (competitiveness) of σS.
In this study, we demonstrate that the ribosome also acts as a sensor/signaling device contributing to the accumulation of σS during carbon starvation. This accumulation of σS is linked to the fidelity of the ribosome rather than ppGpp production and acts via production of aberrant and oxidized proteins sequestering the ClpP protease. We present a model for the physiological sequence of events leading to σS accumulation and activation upon carbon starvation.
Results
σS levels are regulated by translational accuracy
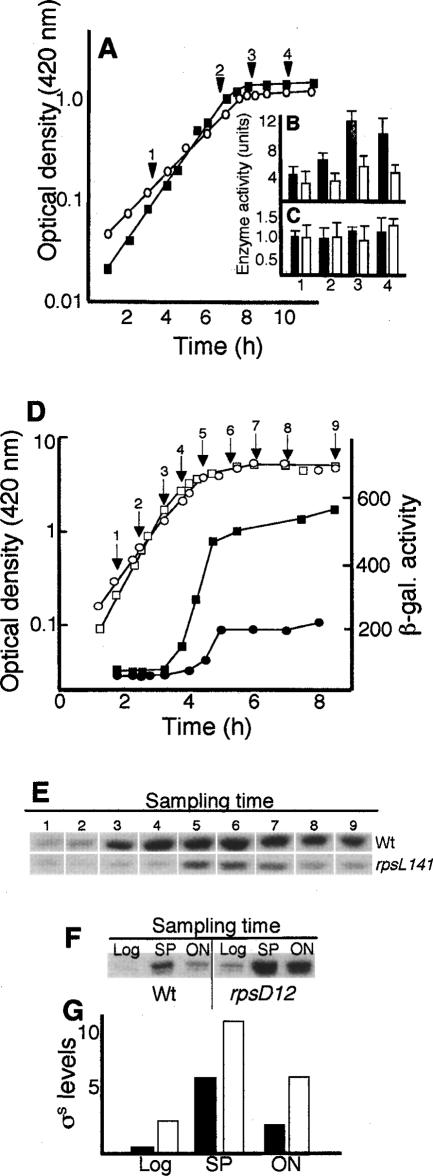
A link between translational fidelity and σS regulation was serendipitously discovered during our analysis of protein oxidation in stationary-phase cells. Specifically, previous experiments have demonstrated that stasis-induced, deleterious oxidative modifications of proteins can be reduced by the rpsL141 allele, which increases translational accuracy (Ballesteros et al. 2001; Fredriksson et al. 2006). This allele, encoding a mutant ribosomal protein S12, has been argued to reduce protein oxidation by mitigating the production of aberrant proteins, since aberrant proteins are intrinsically sensitive targets of oxidative attack (Dukan et al. 2000). Another possibility is that the levels and/or activities of oxidant defense systems are elevated in the rpsL mutant. To analyze this, superoxide dismutase (SOD) and catalase activity (CAT) were determined in wild-type and rpsL141 mutant strains during growth and glucose starvation-induced growth arrest. While SOD activity was similar in both strains (Fig. 1A,C), CAT activity was significantly lower in the rpsL mutant (Fig. 1A,B). Intrigued by the unexpected reduction of CAT activity, specifically during glucose starvation, we wondered whether expression of the katE gene, which encodes the starvation-induced catalase II, was affected by the rpsL141 mutation. As seen in Figure 1D, katE expression was markedly less induced in the rpsL141 mutant than in the wild-type strain upon glucose starvation. Since katE is regulated by σS, carbon-starvation induction of other genes of the σS-dependent regulon (bolA and uspB) was analyzed, demonstrating a poor induction also of these genes during carbon starvation (data not shown). In addition, Western blot analysis revealed that the accumulation of σS upon starvation was less pronounced in the rpsL141 mutant (Fig. 1E). To further test if ribosomal proofreading is a key process regulating the RpoS regulon, σS levels were determined in an rpsD12 mutant. The rpsD12 allele encodes a mutant ribosomal protein, S4, which reduces ribosomal proofreading (Ballesteros et al. 2001; Fredriksson et al. 2006). This allele elevated σS levels both during growth and glucose starvation (Fig. 1F,G). In addition, introduction of a mutated gene for 16S rRNA (on the plasmid pKK726G), which when incorporated into the ribosome renders it prone to errors (Prescott and Dahlberg 1990), elevated expression of the σS regulon (data not shown). The results demonstrate that σS levels correlate directly to changes in the proofreading capacity of the translational apparatus.
Figure 1.
Effect of translational fidelity on σS-dependent gene expression and σS levels during growth and glucose starvation. (A) Growth of the wild-type (Wt/Δ14; closed squares) and the rpsL141 mutant (S12/Δ14; open circles) strains under aerobic conditions. The arrows and numbers indicate the time at which samples were taken for catalase activity (B) and superoxide dismutase activity (C). Catalase and superoxide dismutase activities were determined and expressed as described in Materials and Methods. One unit of catalase is defined as the amount of enzyme per milligram of total protein that degrades 1 μmol of hydrogen peroxide in 1 min at 25°C (Dukan et al. 2000). One unit of superoxide dismutase is defined as the amount of enzyme per milligram of total protein that inhibits the rate of cytochrome c reduction by 50% at 25°C. Filled bars represent the wild type and open bars represent the rpsL141 mutant. (D) Growth (open symbols) and katE promoter activity (closed symbols) in the wild type (MBN7; squares) and rpsL141 mutant (MBN8; circles). (E) Levels of σS in the wild type (DV206) and rpsL141 mutant (ÅF1), as indicated, during growth and during carbon starvation. The sampling times are indicated in D. (F) Levels of σS in the wild type (DV206) and rpsD12 mutant (MBN27) during growth and glucose starvation. Samples were taken in exponential growth (Log), early glucose starvation, depicting the highest σS levels reached (SP), and cultures starved overnight (ON). (G) Quantitative representation, using the Image Gauge software, of the data shown in F. (Filled bars) Wild type; (unfilled bars) rpsD12 mutant. All experiments were repeated at least three times. Representative results are shown.
The heat-shock chaperone DnaK has been implicated as a positive regulator of σS upon entry of cells into stationary phase (Rockabrand et al. 1998). Since the heat-shock regulon is negatively affected by the rpsL141 mutation (Ballesteros et al. 2001; Fredriksson et al. 2006), it is possible that the low levels of σS are a consequence of reduced induction of dnaK in the mutant. However, ectopic overproduction of DnaK failed to counteract the low levels of σS in the rpsL141 mutant (data not shown).
Translational fidelity affects σS stability
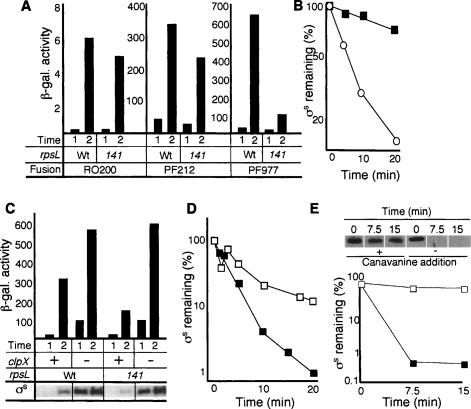
To determine at which level translational proofreading affects σS levels, a series of lacZ fusion constructs was used as reporters of rpoS transcription (RO200), transcription and translation (PF212), and full control; that is, including σS stability-determining elements, including Lys173, of the σS-β-galactosidase fusion protein (PF977). As shown in Figure 2A, the effect of reduced translational errors was most clearly seen when the reporter construct included elements controlling σS stability; the β-galactosidase activity obtained from PF977 were more than sixfold lower in the rpsL141 mutant compared with the wild type (Fig. 2A). To confirm that increased translational accuracy affects σS stability, antibodies against σS were used to measure the half-life of the σ factor after a total block of protein synthesis with spectinomycin or chloramphenicol. Western blot analysis of extracts from glucose-starved cells revealed that the rate of σS degradation is very much increased by the rpsL141 mutation (Fig. 2B). (Note that the rpsl141 mutant was not more sensitive to the protein synthesis inhibitors used, spectinomycin and chloramphenicol, than the wild-type strain.) In addition, the half-life of σS in exponentially growing wild-type and rpsL141 cells, when mistranslation is relatively low in both strains, was similar (between 1 and 2 min) (data not shown). To further ascertain that the poor induction of σS-dependent genes upon carbon starvation in the hyperaccurate mutant is caused by increased σS proteolysis, we analyzed whether mutations in clpX could suppress the effect of rpsL141. ClpXP is an ATP-dependent protease responsible for σS degradation (Schweder et al. 1996). As depicted in Figure 2C, deletion of clpX suppressed the poor induction of katE–lacZ in the rpsL141 mutant strain, confirming that increased proofreading acts on the RpoS regulon by affecting degradation of σS. Note that the clpX mutation elevates σS levels markedly in exponential-phase cells without a concomitant induction of katE (Fig. 2C). This is because σS-dependent genes require elevated levels of ppGpp for their full induction (Kvint et al. 2000; Jishage et al. 2002).
Figure 2.
Effect of increased translational fidelity on σS production and stability. (A) The effects of the rpsL141 allele on rpoS transcription (strains MBN31/32: Wt/rpsL; fusion RO200), rpoS transcription/translation (strains MBN34/35: Wt/rpsL; fusion PF212), and rpoS transcription/translation and σS stability (strains MBN37/38: Wt/rpsL; fusion PF977). Sampling times are for exponentially growing cells (1) and glucose-starved cells depicting the highest β-galactosidase activity reached from the different lacZ fusions (2). (B) Stability of σS in the wild type (DV206; closed squares) and the rpsL141 mutant (ÅF1; open circles) in glucose-starved cells. Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation, and σS levels were determined by Western blot analysis and quantified using Image Gauge software. (C) The effect of a clpX deletion on katE expression and σS levels in the wild-type and rpsL141 strains. The β-galactosidase activity is from the katE–lacZ fusion, and the levels of σS (Western blots) are depicted at the bottom of the graph for the respective mutants; strains: MBN7 (Wt), MBN19 (Wt, clpX1∷kan), MBN8 (rpsL141), MBN20 (rpsL141, clpX1∷kan). Sampling times are for exponentially growing cells (1) and glucose-starved cells depicting the highest σS levels reached (2). (D) Stability of σS in the wild type (DV206; closed squares) and the rpsD12 mutant (MBN27; open squares) in exponentially growing cells. (E) Effect of canavanine on σS levels and stability. A wild-type culture was grown to early exponential phase in LB and divided into two cultures, with one half receiving canavanine (12.8 mg/mL) and the other half receiving only LB. Forty-five minutes later, protein synthesis was inhibited by the addition of chloramphenicol, and the half-life of σS was determined by Western blotting. Above each lane, the time after the addition of chloramphenicol is shown. Below is the quantification of the σS levels, with closed squares representing no canavanine addition and open squares representing canavanine addition. All experiments were repeated at least three times. Representative results are shown.
In support of mistranslation controlling the stability of σS, we found that the elevated levels of σS in exponentially growing rpsD12 mutants (Fig. 1G) were accompanied by increased σS stability (Fig. 2D). Also, the addition of canavanine to an exponentially growing culture stabilized σS (Fig. 2E). Canavanine is an analog of arginine in which the terminal methylene group has been replaced by oxygen. When incorporated into proteins, it causes misfolding (Miersch et al. 2000). Thus both genetically and chemically induced mistranslation enhanced σS stability.
Effects of ribosomal fidelity on σS stability in cells lacking and overproducing SprE
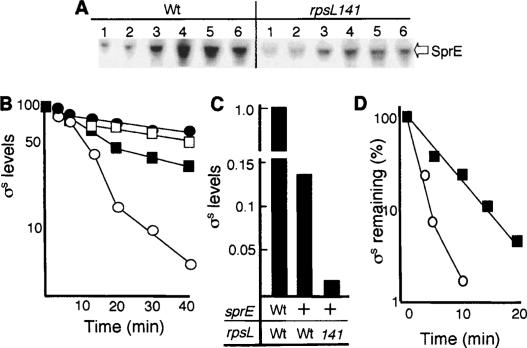
ClpXP-dependent degradation of σS is facilitated by the recognition factor SprE (Muffler et al. 1996; Pratt and Silhavy 1996; Becker et al. 1999; Mandel and Silhavy 2005), and we wondered whether increased translational accuracy might destabilize σS by elevating the levels of this recognition factor. This was not the case. Instead, Western analysis revealed that the levels of SprE were lowered by the rpsL141 mutation (Fig. 3A), which is consistent with lower levels of σS, since σS is a positive feedback regulator of sprE (Ruiz et al. 2001). However, the effect of the rpsL141 allele on σS stability was totally abolished in cells lacking SprE (Fig. 3B). We also tested whether increased accuracy affected σS levels in cells overproducing SprE. For this purpose, the rssA2∷TnCam mutant was used in which sprE transcription is constitutively overexpressed from the cam promoter (Ruiz et al. 2001). This mutant exhibits reduced levels and decreased stability of σS due to the elevated levels of SprE. Still, the rpsL141 mutation was able to further reduce σS levels in the rssA2∷TnCam mutant (Fig. 3C) and rendered the half-life of the protein even shorter (Fig. 3D). Thus, the effects of translational proofreading on σS stability do not act via increased levels of SprE, but increased proofreading requires the presence of SprE to destabilize σS.
Figure 3.
Effect of increased translational fidelity on σS stability in SprE-deficient SprE-overactive cells. (A) Levels of SprE in the wild-type (DV206) and rpsL141 mutant (ÅF1) strains during growth and glucose starvation. Sampling points were exponential growth (1), transition phase (2), glucose starvation-induced stationary phase (30 min to 3 h) (3–5), and overnight glucose-starved cultures (6). (B) Stability of σS in wild-type cells (DV206; closed squares), rpsL141 cells (ÅF1; open circles), sprE∷tet cells (ÅF132; open squares), and rpsL141, sprE∷tet cells (ÅF133; closed circles) during carbon starvation. Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. (C) Levels of σS in wild-type cells (DV206), SprE-overactive cells (ÅF82), and SprE-overactive cells carrying the rpsL141 allele (ÅF84). (D) Stability of σS in SprE-overactive cells (ÅF82; closed squares) and SprE-overactive cells carrying the rpsL141 allele (ÅF84; open circles) in glucose-starved cells. Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. All protein levels were determined by Western blot analysis and quantified using Image Gauge software. All experiments were repeated at least three times. Representative results are shown.
Sequestration of ClpP stabilizes σS upon glucose starvation
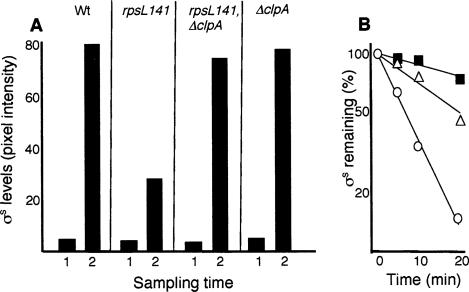
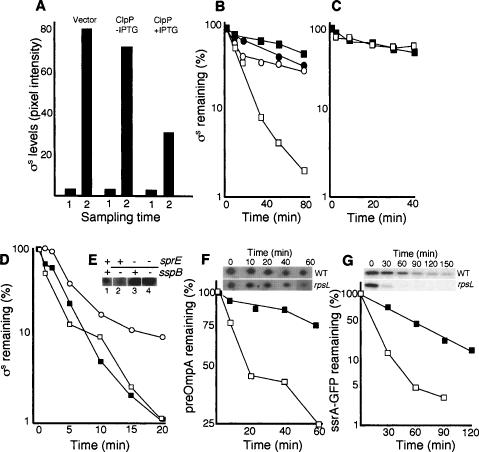
To obtain hints toward a mechanistic explanation for the link between ribosomal proofreading and σS stability, we looked for mutations that could suppress the low levels of σS-dependent gene expression in the rpsL141 mutant. As expected, the clpX mutation restored stationary-phase induction of σS-dependent genes and stabilized σS both in exponential phase and during glucose starvation (see Fig. 2). Unexpectedly, however, a mutation in clpA also restored σS-dependent gene expression during glucose starvation (data not shown), which was the result of elevated σS levels (Fig. 4A) and increased σS stability (Fig. 4B) in the rpsL141 mutant background. Both ClpAP and ClpXP catalyze ATP-dependent unfolding and proteolysis. Their substrates generally contain recognition signals (∼10 amino acids) at the N or C terminus, but σS is a specific substrate for ClpXP (Flynn et al. 2003). Thus, stabilization of σS by a clpA mutation in the rpsL141 background, presumably, cannot be due to the relief of ClpAP degradation of σS itself. Note also that suppression by clpA, in contrast to clpX (Fig. 2), is conditional in the sense that σS levels are only restored in glucose-starved cells (Fig. 4A). We entertained the idea that the effect of rpsL141 on σS stability and its suppression by clpA are both features linked to the pool size of aberrant proteins. The rpsL141 mutation is known to reduce the production of aberrant and oxidatively modified proteins. If such proteins are targets for ClpXP and ClpAP, then more of the ClpXP protease will be available for σS degradation in the rpsL141 mutant, and σS would be destabilized. However, a clpA mutation would increase the pool size of aberrant proteins in the rpsL141 strain, and if ClpAP and ClpXP, to some extent, share aberrant substrates, ClpXP would be increasingly occupied with such substrates, and σS would be stabilized. There are two critical notions included in this reasoning; first, that ClpAP and ClpXP to some degree recognize similar substrates (this has previously been shown) (Flynn et al. 2003), and second, that ClpXP or one of its individual components is limited in the cell. To approach the latter notion, we tested whether ectopic overproduction of ClpX or ClpP counteracted σS accumulation and decreased σS stability in glucose-starved cells. We found that overproduction of ClpP alone was enough to cause such effects (Fig. 5A,B). ClpP overproduction, like the rpsL141 mutation (Fig. 3B), required the presence of SprE (Fig. 5C) to destabilize σS. Thus, the accumulation of σS upon entry of cells into stationary phase must be due, at least in part, to limitation in ClpP availability. It should be noted also that ClpP levels do not change during starvation (Schweder et al. 1996; Mandel and Silhavy 2005).
Figure 4.
Effects of a clpA deletion on σS levels and stability in the rpsL141 mutant. (A) Levels of σS in the wild-type (DV206), rspL141 (ÅF1), rpsL141/clpA∷kan (ÅF81), and clpA∷kan (ÅF80) strains as indicated. Cells were sampled during exponential growth (1) and carbon starvation depicting the highest σS levels reached (2). (B) Stability of σS in glucose-starved wild-type (DV206; closed squares), rpsL141 (ÅF1; open circles), and rpsL141/clpA∷kan (ÅF81; open triangles) cells. Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. All protein levels were determined by Western blot analysis and quantified using Image Gauge software. All experiments were repeated at least three times. Representative results are shown.
Figure 5.
Effect of overproducing ClpP and a ClpPX substrate on σS accumulation and stability. (A) Levels of σS in the cells carrying the vector control (ÅF113), cells (ÅF125) carrying the uninduced (no IPTG) Plac–clpP construct, and cells (ÅF125) induced (+IPTG) for clpP overexpression, as indicated. Sampling times are in exponential growth (1) and glucose starvation depicting the highest σS levels reached (2). (B) Stability of σS in glucose-starved wild-type cells (DV206; closed squares), cells carrying the vector control (ÅF113; closed circles), cells carrying the uninduced (no IPTG) Plac–clpP construct (ÅF125; open circles), and cells induced (+IPTG) for clpP overexpression (ÅF125; open squares). Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. (C) Stability of σS in sprE∷tet mutant cells carrying the uninduced (no IPTG) Plac–clpP construct (ÅF135; open squares), and cells induced (+IPTG) for clpP overexpression (closed squares). Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. (D) Stability of σS in exponentially growing wild-type cells (closed squares), cells carrying the uninduced (no IPTG) Plac–ssrA-gfp construct (ÅF142; open squares), and cells induced (+IPTG) for ssrA-gfp overexpression (open circles). (E) A knockout mutation of sspB lowers σS levels in a SprE-dependent manner. Samples were taken in the exponential growth phase (LB). The presence (+) and absence (−) of the sprE and sspB genes are indicated on top of the Western blot. The strains used are CNP119 (lane 1), CNP217 (lane 2), CNP153 (lane 3), and CNP218 (lane 4). All protein levels were determined by Western blot analysis and quantified using Image Gauge software. (F) Stability of the preOmpA in wild-type (DV206; closed squares) and rpsL141 mutant cells (ÅF1; open squares) during carbon starvation (1 h). Inset shows the preOmpA spots on two-dimensional gels after inhibition of protein synthesis in the wild type and the rpsL141 mutant. (G) Stability of the ssrA-GFP fusion protein in wild type (ÅF142; closed squares) and the rpsL141 mutant (ÅF143; open squares) during carbon starvation (1 h). Inset shows the ssrA-GFP protein on Western blots in the wild type and rpsL141 mutant after inhibition of protein synthesis. All experiments were repeated at least three times. Representative results are shown.
To further approach the possibility of ClpP being limiting for σS degradation, we tested the effects of overproducing a ClpXP substrate on σS stability. Ectopic overproduction of an ssrA-tagged GFP resulted in stabilization of σS in exponentially growing cells (Fig. 5D). We also tested the effects of mutating the sspB gene. SspB is an adaptor protein that facilitates the ClpXP-mediated degradation of ssrA-tagged truncated proteins (Levchenko et al. 2000; Flynn et al. 2004). The C-terminal region of SspB has been shown to be the site of ClpX binding and is very similar to the C-terminal region of SprE (Dougan et al. 2003). We argued that SspB deficiency might lead to more ClpXP being available for degradation of σS, which does not require SspB or ssrA tagging. Indeed, a knockout mutation of sspB markedly lowers σS levels (Fig. 5E). This effect of an sspB mutation was dependent on the presence of SprE; in the sprE∷tet background, the presence of the sspB∷cam allele did not affect σS levels (Fig. 5E), demonstrating that the effect of the sspB∷cam mutation is at the level of σS stability rather than expression.
If more ClpP is available for σS degradation in the rpsL141 mutant because this mutant produces fewer aberrant proteins, then other ClpXP/ClpAP substrates may exhibit a similar decreased stability. We tested the stability of the preOmpA (i.e., OmpA with the signal sequence), reported to be a substrate for both ClpXP and ClpAP (Flynn et al. 2003), in the wild type and rpsL141 mutant upon carbon starvation and found that preOmpA, like σS, is less stable in the rpsL141 background (Fig. 5F). Likewise, the stability of the ssrA-GFP fusion was markedly reduced in the rpsL141 mutant (Fig. 5G).
The effect of ribosomal fidelity on σS accumulation requires oxidative conditions
Translational frameshifting (Barak et al. 1996; Wenthzel et al. 1998; Fredriksson et al. 2006), missense errors (O’Farrell 1978), and stop codon readthrough (Ballesteros et al. 2001) increase immediately upon carbon starvation of E. coli cells. Since aberrant proteins are more susceptible to oxidation than native ones, this sudden increase in mistranslation results in increased levels of oxidatively modified proteins (Ballesteros et al. 2001; Fredriksson et al. 2006). The rpsL141 mutant retains its translational fidelity during stasis, and protein oxidation is drastically attenuated in the early stages of stasis in the cells carrying this allele (Ballesteros et al. 2001). We approached the question of whether such oxidative modification of mistranslated proteins is important for the accumulation of σS in stationary phase and found a reduced expression of σS-dependent genes (e.g., katE) and a reduced accumulation of σS (compared to aerobically starved cells) in cells starved for carbon anaerobically (Fig. 6A,B). In addition, the rpsL141 allele had no effect on σS-dependent gene expression or σS accumulation in anaerobically starved cells (Fig. 6A,B). As shown previously (Fredriksson et al. 2006), we found that mistranslation occurs more frequently in anaerobically cultivated and starved cells and that this mistranslation is almost totally blocked by the rpsl141 allele (Fig. 6C). Yet, the production of aberrant proteins is not “sensed” by the cells, with respect to the σS system, in the absence of oxygen. However, anaerobically cultivated cells would carry a relatively high load of aberrant proteins that could act as potential “inducers” of σS accumulation once they become oxidized. Thus, a shift from anaerobic conditions to aerobic conditions (a true up-shift condition) could cause an instantaneous elevation of σS levels since this shift allows oxidative modification of the accumulated pool of aberrant proteins to occur. Indeed, σS was rapidly, and transiently, accumulated during such a shift within a fraction of the generation time (Fig. 6D). Moreover, the accumulation of σS during such shifts in oxygen availability was reduced in cells carrying the rpsL141 allele (Fig. 6D), demonstrating that the effect observed is intimately coupled to the pool size of aberrant proteins.
Figure 6.
Effects of oxygen on σS activity and levels. (A) Growth (open symbols) and katE expression (closed symbols) in wild-type (Wt/Δ14; ▴◽ and ▴▵) and rpsL141 (S12/Δ14; ●○ and ▾▿) cells grown and carbon-starved aerobically (squares and circles) and anaerobically (triangles). Arrows indicate sampling times for analysis of σS levels. (B) Levels of σS in wild-type (Wt/Δ14) and rpsL141 (S12/Δ14) cells growing aerobically (+) and anaerobically (−) as indicated. The sampling times 1–4 are those indicated in A. (C) Mistranslation, (nonsense suppression) in wild-type (Wt/U4 to Wt/Δ14) and rpsL141 (S12/U4 to S12/Δ14) cells growing and starving aerobically (+) and anaerobically (−) as indicated. Mistranslation was determined as described in Materials and Methods. A value of 0.01 indicates that one out of 100 transcripts generates a full-length protein due to nonsense readthrough. Sampling times are in exponential growth (1) and glucose starvation depicting the highest level of mistranslation reached (2). (D) Growth (open symbols) and levels of σS (closed symbols) in wild-type (Wt/Δ14; squares) and rpsL141 (rpsL141; circles) cells upon a shift from aerobic to anaerobic conditions as indicated. The dotted line denotes the preshift growth rate of the wild-type culture. σS levels were determined by Western blot analysis and quantified using Image Gauge software. All experiments were repeated at least three times. Representative results are shown.
Discussion
The σS regulon is rapidly induced as cells experience starvation and its member genes are required for cells to remain viable under starvation-induced growth arrest. Despite the fact that a large number of trans- and cis-regulatory components have been identified as important in regulating σS levels, the sensing–signaling device used by E. coli to trigger σS accumulation upon starvation has not been fully deciphered (Hengge-Aronis 2002). Based on the result presented here, we present a model for the physiological sequence of events contributing to σS accumulation and activation during carbon starvation.
An immediate consequence of carbon starvation and amino acid shift-downs is a reduction in the pool size of charged tRNAs. This diminished availability of amino acyl-tRNAs leads to increased mistranslation; for example, misincorporation of erroneous amino acids, translational frameshifting, and stop-codon readthrough (O’Farrell 1978; Barak et al. 1996; Wenthzel et al. 1998; Ballesteros et al. 2001). The rapid increase in the levels of oxidatively modified proteins upon starvation is a direct consequence of this reduction in translational fidelity because the aberrant protein isoforms produced exhibit increased susceptibility to oxidative attack (Ballesteros et al. 2001; Fredriksson et al. 2006). We show here that the sudden drop in translational fidelity upon carbon starvation is a key event also in the accumulation of σS. We suggest that such accumulation of σS is the consequence of a protease titration mechanism in which the surge in the pool size of aberrant proteins upon carbon starvation sequesters the ClpP protease. It has been shown previously that ClpP is required for degradation of both misfolded, puromycyl-containing proteins (Thomsen et al. 2002) and proteins damaged by oxidative carbonylation (Nair et al. 2003). It appears that the oxidatively modified species of the aberrant proteins are more efficient in titrating the ClpP protease (Figs. 6, 7). However, it is also possible that increased ribosome stalling and ssrA-tagging of truncated peptides contribute to σS stabilization during carbon starvation (Fig. 7).
Figure 7.
Schematic representation of the model for σS stabilization upon starvation. (1) Translational errors increase as an immediate response to, for example, amino acid and carbon starvation resulting in the production of erroneous and misfolded proteins σS. (2) The aberrant proteins are susceptible to oxidative modifications, which cause further structural deviations of the proteins. (3) The aberrant, especially oxidatively modified, species of the mistranslated proteins are substrates for the ClpAP and ClpXP proteases, which as a consequence of the reduced fidelity of the translational apparatus becomes increasingly occupied in the management of aberrant polypeptides. (4) Thus, less ClpXP is available for SprE-dependent degradation of σS, and even a marginal elevation of σS levels caused by this ClpXP sequestration might titrate out SprE and further stabilize σS upon these conditions. The limiting factor of the proteolytic system may be ClpP, since ClpP overproduction greatly diminished σS accumulation. (5) Elevated levels of SsrA-tagged peptides generated on stalled ribosomes may also sequester ClpXP and stabilize σS. Another consequence of starving ribosomes is the RelA-dependent production of ppGpp (6), which binds to RNA polymerase (7) and enhances the ability of σS to compete for core binding (8). During other starvation and stress conditions, σS competition is favored by ppGpp produced from SpoT.
An important feature of the model is that one or several components of the σS degradation machinery must be limiting, at least during entry of cells into stationary phase. Indeed, overproduction of ClpP alone reduced σS accumulation and partly counteracted σS stabilization in early stationary phase (Fig. 7), suggesting that ClpP denotes such a limiting component. In line with the model, mutations that reduce translational errors, omission of oxygen, and ClpP overproduction are all conditions that reduce the accumulation and stabilization of σS in starved cells. In addition, decreased translational proofreading and overproduction of a ClpXP substrate stabilized σS already in exponential phase. Both elevated proofreading and ClpP overproduction required the presence of SprE to destabilize σS, suggesting that the canonical SprE/ClpXP pathway achieves the degradation of the σ factor under these conditions. In addition, the fact that clpX and also clpA deletions suppressed the instability of σS in glucose-starved rpsL141 mutants suggests that the ClpAP and ClpXP to some extent are occupied with the same aberrant substrates in carbon-starved cells (Fig. 7). It has been proposed that SprE is limiting in vivo and that a marginal increase in the cellular concentration of σS—for example, by elevated translation—will titrate out SprE and cause a drastic stabilization of σS (Pruteanu and Hengge-Aronis 2002). We suggest that sequestration of ClpP upon starvation-induced mistranslation might be an additional physiologically relevant event that titrates the SprE recognition factor during carbon starvation. In this scenario, σS is stabilized by two sequential titration events, titration of ClpP followed by SprE.
Nitrogen starvation has been shown to cause a similar increased mistranslation and elevated levels of protein carbonyls as carbon starvation (Ballesteros et al. 2001), but σS does not reach the same high concentration during nitrogen as carbon starvation (Mandel and Silhavy 2005). Possibly, mistranslation/protein oxidation and ClpP titration may account for most of the stabilization of σS upon nitrogen starvation, whereas another mechanism works in parallel to ClpP titration during carbon starvation, giving rise to even higher levels of the σ factor. This notion is consistent with the fact that there is residual induction of the rpoS regulon and accumulation of the σ factor in the rpsL141 mutant upon carbon starvation (Fig. 1D,E). In contrast to carbon and nitrogen starvation, translational errors and protein oxidation do not increase significantly during phosphate starvation (Ballesteros et al. 2001). Thus, the stabilization of σS upon phosphate depletion is expected to be accomplished by a mechanism other than titration of ClpP by aberrant proteins. Interestingly, it has recently been shown that σS accumulation during phosphate starvation involves a novel protein, IraP, which interferes with SprE-dependent degradation of σS during phosphate, but not carbon, starvation (Bougdour et al. 2006). In addition, increased translation of the rpoS transcript appears to be more important for σS accumulation during phosphate starvation than carbon starvation (Mandel and Silhavy 2005).
Experiments with strains lacking the alarmone ppGpp suggest that there are more components than SprE of importance in regulating σS stability. Overproduction of σS is difficult to achieve in exponentially growing cells (rich media—low levels of ppGpp) and in ΔrelA ΔspoT mutants (deficient in ppGpp), and we have noticed that σS is unstable under such conditions despite the fact that overproduction ought to titrate the SprE factor. In addition, cells lacking ppGpp display increased mistranslation and levels of carbonylated proteins (M. Ballesteros, L. Magnusson, and T. Nyström, in prep.), yet σS is not stabilized in this genetic background. This instability of σS may be due to the fact that ppGpp is required for σS to compete successfully for RNA polymerase (E) binding (Jishage et al. 2002). Thus, binding of σS to E, which would protect the σ factor from degradation, is another important aspect of regulating σS stability and activity, and the involvement of ppGpp in this context provides an important hierarchy of physiological regulation. The requirement of σS for ppGpp suggests that the σS regulon can only be efficiently induced under suboptimal growth conditions, which elevate the production of this nucleotide. In fact, we do not know of any condition that triggers expression of σS regulon genes without a concomitant increase in ppGpp levels. The requirement for ppGpp may thus be an important checkpoint control such that elevated levels of σS will not automatically trigger the regulon if the cell senses that its physiological status (low ppGpp) does not call for the functions encoded by the σS regulon. This may be the case during, for example, a shift from anaerobic to aerobic conditions. As seen in Figure 6, such a shift results in an immediate accumulation of σS. However, we found that the σS regulon genes are not induced during this shift (data not shown). This can be explained by the fact that this is a true up-shift condition that does not elevate ppGpp levels. In fact, under such up-shift conditions, which primarily require housekeeping functions (Eσ70), successful competition of σS for E binding would reduce the fitness of the cells.
In summary, decreased ribosomal fidelity generating aberrant and oxidized proteins that sequester the ClpP protease are key events contributing to the stabilization of the σS transcription factor upon carbon starvation. Future analysis may clarify whether specific aberrant substrates may act as specific carbon starvation “sensors” in the sense that they sequester the ClpP protease upon entry of cells into stationary phase or if σS stabilization is due to a more general and nonspecific effect of mistranslation of bulk proteins.
Materials and methods
Chemicals and reagents
Anti-DnaK mouse monoclonal antibodies were from Stressgen Bioreagents (Biosite), anti-σS mouse monoclonal antibodies were from Neoclone, and SprE antibodies were a gift from N. Ruiz. GFP antibodies were purchased from Roche. Anti-mouse IgG peroxidase conjugates, 2-nitrophenyl β-D-thiogalactoside (ONPG), and isopropyl β-thiogalactopyranoside (IPTG) were from Sigma. The chemiluminscence blotting substrate (ECL+) was obtained from Amersham Corp., and the Immobilon-P polyvinyldene difluoride (PVDF) membrane was from Millipore. Plasmid DNA was purified by using Qiagen columns (Qiagen, Inc.) or a Wizard minipreparation kit (Promega, Inc.). The Gene-Clean Kit used for isolation of DNA fragments was from Bio 101, Inc. All chemicals and reagents were used according to instructions provided by the manufacturer.
Bacterial strains, plasmids, and growth conditions
The E. coli K-12 strains and plasmids used in this study are listed in Table 1. LacZ fusion reporter strains MBN7, MBN8, MBN31, MBN32, MBN34, MBN35, MBN37, and MBN38 were constructed by infection of DV206 and ÅF1 with a λ phage lysate harboring the appropriate construct. Monolysogeny was confirmed by PCR (Powell et al. 1994). The λΦ(katE–lacZ) construct was from Ohnuma et al. (2000), and the λΦ(rpoS–lacZ) constructs were from strains RO200 (OF fusion) (Lange and Hengge-Aronis 1994), CU264 (PF212, transcriptional fusion) (Ueguchi et al. 2001), and CU263 (PF977, translational fusion) (Ueguchi et al. 2001). To generate strains ÅF80, ÅF81, ÅF82, ÅF84, ÅF132, and ÅF133, clpA319∷kan (Katayama et al. 1988), rssA2∷cam (from strain NR419; N. Ruiz), and sprE∷tet (from strain NR253; N. Ruiz) were introduced into DV206 and ÅF1 by P1 transduction. Strains MBN19 and MBN20 were similarly generated by transduction of ΔclpX1∷kan (Katayama et al. 1988) into MBN7 and MBN8. Strain ÅF39 was constructed by transformation of ÅF1 with plasmid pBB535 (Tomoyasu et al. 2001) and ÅF142 and ÅF 143 by transformation of DV206 with plasmid pGFP-ssrA (a kind gift from B. Bukau) (Dougan et al. 2003). The F′lacIq was introduced via mating with strain XL-1 Blue. The cloned clpP was confirmed by sequencing plasmid pÅF2. A clpP under control of the IPTG-inducible Plac was constructed by PCR amplification of the clpP gene, cleavage with SacI and KpnI, and cloning into the SacI–KpnI fragment of pUC18. Strains ÅF134, ÅF135, ÅF138, and ÅF139 were constructed by transformation of DV206 and ÅF132 with plasmid pBB528 followed by transformation with pUC18 and pÅF2.
Table 1.
E. coli strains used in this work
Cultures were grown aerobically or anaerobically at 37°C in Erlenmeyer flasks in a rotary shaker in liquid Luria-Bertani (LB) or minimal M9 defined medium (Miller 1972). For glucose-starvation experiments, the defined M9 medium was used with reduced glucose concentrations (typically 0.05%) such that glucose was the first nutrient to become depleted, upon which the cells entered carbon starvation. When indicated, the M9 media was supplemented with thiamine (10 μM), all 20 amino acids in excess, and glucose (0.1% or 0.4% for anaerobic/aerobic shift experiments). When appropriate, the media were supplemented with kanamycin (50 μg/mL), chloramphenicol (30 μg/mL), rifampicin (150 μg/mL), tetracycline (20 μg/mL), canavanine (12.8 mg/mL), carbenicillin (100 μg/mL), and/or IPTG at concentrations indicated for each experiment. Anaerobic conditions were achieved by constant bubbling of the cultures with a gas mixture consisting of 5% CO2 and 95% N2 as described (Valadi et al. 2001). To overproduce DnaK and DnaJ, 200 μM IPTG was added to exponentially growing cultures of strains ÅF38 and ÅF39 at an OD420 of 0.05 to induce expression from the plasmid pBB535, and to induce clpP expression from the plasmid pÅF2, 50 μM IPTG was added at an OD420 of 0.1. To overproduce the ssrA-tagged GFP, 100 μM IPTG was added to exponentially growing cultures of strain ÅF142 at an OD420 of 0.05.
General methods
P1 transductions, plasmid transformations, and λ-phage lysogeny were performed as described by Miller (1972) and Sambrook and Russell (2001). Protein extracts where prepared according to Sambrook and Russell (2001). σS, DnaK, and SprE levels were determined by gel electrophoresis and immunoblotting according to standard procedures using 11.5% SDS–polyacrylamide gels and mouse monoclonal antibodies directed toward σS, or mouse monoclonal antibodies directed toward DnaK. For detection, the ECL-plus blotting kit was used with horseradish peroxidase-conjugated anti-mouse IgG as secondary antibody. Blots were subsequently exposed in the Fuji Film Image Reader LAS-1000 Pro. For quantitative analyses of the blots, the Image Gauge 3.46, Science Lab 99 software was used. Measurements of β-galactosidase activity from lacZ–gene reporter constructs were performed as described (Miller 1972) with modifications (Albertson and Nyström 1994). All experiments were repeated several times to ensure reproducibility, and the variation was <10%.
Mistranslation assay
Nonsense suppression was determined by measuring a stop codon readthrough in a lacI–lacZ fusion as described in Andersson et al. (1982). The frequency of nonsense suppression was calculated based on the β-galactosidase produced by the wild-type allele (transcribed from the same promoter) under the same conditions. Thus, a value of 0.01 indicates that one out of 100 transcripts generates a full-length protein due to nonsense readthrough. Both alleles were carried on F′ factors in the wild-type strain and the rpsL141 mutant.
Catalase activity
Catalase activity in bacterial extracts was determined by measuring the decrease in the A240nm of hydrogen peroxide as described previously (Gonzalez-Flecha et al. 1993). One unit of catalase is defined as the amount of enzyme that degrades 1 μmol of hydrogen peroxide in 1 min at 25°C (Dukan et al. 2000).
Superoxide dismutase activity
Superoxide dismutase activity was assayed using the xantine oxidase/cytochrome c method (Imlay and Fridovich 1991). One unit of superoxide dismutase is defined as the amount of enzyme that inhibits the rate of cytochrome c reduction by 50% at 25°C.
Protein stability
σS stability measurements were performed as described (Zhou and Gottesman 1998). Briefly, cells were grown exponentially at 37°C. After 1 h of glucose starvation, protein synthesis was blocked by addition of spectinomycin (400 μg/mL) or chloramphenicol (30 μg/mL), and samples were withdrawn at indicated times and resuspended in SDS gel loading buffer, and subjected to SDS-PAGE and quantitative Western blotting as described above. The stability of the ssrA-GFP fusion was analyzed in a similar fashion using antibodies directed against GFP. The ssrA-GFP fusion was not overproduced in this experiment to avoid titration of ClpP. The stability of preOmpA (OmpA with signal sequence) was analyzed, after inhibition of protein synthesis, using two-dimensional gel electrophoresis, and preOmpA was identified on the gels using the gene–protein database (VanBogelen et al. 1997).
Canavanine exposure
A wild-type (MC4100) culture was grown to early exponential phase in LB and divided into two cultures, with one half receiving 12.8 mg/mL canavanine and the other half receiving only LB. Forty-five minutes later, protein synthesis was inhibited by the addition of chloramphenicol, and the half-life of σS was determined by Western blotting as described above.
Anaerobic/aerobic shifts
Cells were grown in M9 media supplemented with thiamine and amino acids in Erlenmeyer flasks anaerobically as described above. The exponentially growing cultures were shifted to aerobic conditions by pouring them into prewarmed Erlenmeyer flasks and aerated by rotary shaking at 240 rpm. Immediately before (sample “zero”) and after the shift, samples were removed at the indicated times and precipitated with 10% trichloroacetic acid. The precipitates were washed with cold 80% acetone, resuspended in SDS loading buffer, and subjected to quantitative Western blotting.
Acknowledgments
Bernd Bukau and Axel Mogk are acknowledged for providing the strain and plasmids essential for this work. The Swedish Research Council, VR, is gratefully acknowledged for long-term support of the cellular senescence project. In addition, this work was funded by the Göran Gustafsson award in Molecular Biology, and by the Swedish Foundation for Strategic Research. T.J.S. was supported by a grant from NIGMS (GM065216).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.409407
References
- Albertson N.H., Nyström T., Nyström T. Effects of starvation for exogenous carbon on functional mRNA stability and rate of peptide chain elongation in Escherichia coli. FEMS Microbiol. Lett. 1994;117:181–187. doi: 10.1111/j.1574-6968.1994.tb06762.x. [DOI] [PubMed] [Google Scholar]
- Andersson D.I., Bohman K., Isaksson L.A., Kurland C.G., Bohman K., Isaksson L.A., Kurland C.G., Isaksson L.A., Kurland C.G., Kurland C.G. Translation rates and misreading characteristics of rpsD mutants in Escherichia coli. Mol. Gen. Genet. 1982;187:467–472. doi: 10.1007/BF00332630. [DOI] [PubMed] [Google Scholar]
- Ballesteros M., Fredriksson Å., Henriksson J., Nyström T., Fredriksson Å., Henriksson J., Nyström T., Henriksson J., Nyström T., Nyström T. Bacterial senescence: Protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J. 2001;20:5280–5289. doi: 10.1093/emboj/20.18.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gallant J., Lindsley D., Kwieciszewki B., Heidel D., Gallant J., Lindsley D., Kwieciszewki B., Heidel D., Lindsley D., Kwieciszewki B., Heidel D., Kwieciszewki B., Heidel D., Heidel D. Enhanced ribosome frameshifting in stationary phase cells. J. Mol. Biol. 1996;263:140–148. doi: 10.1006/jmbi.1996.0565. [DOI] [PubMed] [Google Scholar]
- Battesti A., Bouveret E., Bouveret E. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 2006;62:1048–1063. doi: 10.1111/j.1365-2958.2006.05442.x. [DOI] [PubMed] [Google Scholar]
- Becker G., Klauck E., Hengge-Aronis R., Klauck E., Hengge-Aronis R., Hengge-Aronis R. Regulation of RpoS proteolysis in Escherichia coli: The response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc. Natl. Acad. Sci. 1999;96:6439–6444. doi: 10.1073/pnas.96.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougdour A., Wickner S., Gottesman S., Wickner S., Gottesman S., Gottesman S. Modulating RssB activity: IraP, a novel regulator of σS stability in Escherichia coli. Genes & Dev. 2006;20:884–897. doi: 10.1101/gad.1400306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M.J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage λ and Mu. J. Mol. Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gentry D., Hernandez J., Vinella D., Gentry D., Hernandez J., Vinella D., Hernandez J., Vinella D., Vinella D. The stringent response. In: Neidhardt F.C., editor. Escherichia coli and Salmonella cellular and molecular biology. ASM Press; Washington, DC: 1996. pp. 1458–1496. [Google Scholar]
- Dougan D.A., Weber-Ban E., Bukau B., Weber-Ban E., Bukau B., Bukau B. Targeted delivery of an ssrA-tagged substrate by the adaptor protein SspB to its cognate AAA+ protein ClpX. Mol. Cell. 2003;12:373–380. doi: 10.1016/j.molcel.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Dukan S., Nyström T., Nyström T. Bacterial senescence: Stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes & Dev. 1998;12:3431–3441. doi: 10.1101/gad.12.21.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S., Nyström T., Nyström T. Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem. 1999;274:26027–26032. doi: 10.1074/jbc.274.37.26027. [DOI] [PubMed] [Google Scholar]
- Dukan S., Farewell A., Ballesteros M., Taddei F., Radman M., Nyström T., Farewell A., Ballesteros M., Taddei F., Radman M., Nyström T., Ballesteros M., Taddei F., Radman M., Nyström T., Taddei F., Radman M., Nyström T., Radman M., Nyström T., Nyström T. Protein oxidation in response to increased transcriptional or translational errors. Proc. Natl. Acad. Sci. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A., Calcutt M.J., Becker-Hapak M., Ivanova A., Calcutt M.J., Becker-Hapak M., Ivanova A., Becker-Hapak M., Ivanova A., Ivanova A. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic. Biol. Med. 1996;21:975–993. doi: 10.1016/s0891-5849(96)00154-2. [DOI] [PubMed] [Google Scholar]
- Flynn J.M., Neher S.B., Kim Y.I., Sauer R.T., Baker T.A., Neher S.B., Kim Y.I., Sauer R.T., Baker T.A., Kim Y.I., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Flynn J.M., Levchenko I., Sauer R.T., Baker T.A., Levchenko I., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. Modulating substrate choice: The SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes & Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson Å., Ballesteros M., Dukan S., Nyström T., Ballesteros M., Dukan S., Nyström T., Dukan S., Nyström T., Nyström T. Induction of the heat shock regulon in response to increased mistranslation requires oxidative modification of the malformed proteins. Mol. Microbiol. 2006;59:350–359. doi: 10.1111/j.1365-2958.2005.04947.x. [DOI] [PubMed] [Google Scholar]
- Gentry D.R., Hernandez V.J., Nguyen L.H., Jensen D.B., Cashel M., Hernandez V.J., Nguyen L.H., Jensen D.B., Cashel M., Nguyen L.H., Jensen D.B., Cashel M., Jensen D.B., Cashel M., Cashel M. Synthesis of the stationary-phase σ factor σS is positively regulated by ppGpp. J. Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Flecha B., Cutrin J.C., Boveris A., Cutrin J.C., Boveris A., Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia–reperfusion. J. Clin. Invest. 1993;91:456–464. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren G., Jarolim S., Laun P., Rinnerthaler M., Stolze K., Perrone G.G., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Jarolim S., Laun P., Rinnerthaler M., Stolze K., Perrone G.G., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Laun P., Rinnerthaler M., Stolze K., Perrone G.G., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Rinnerthaler M., Stolze K., Perrone G.G., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Stolze K., Perrone G.G., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Perrone G.G., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Kohlwein S.D., Nohl H., Dawes I.W., Breitenbach M., Nohl H., Dawes I.W., Breitenbach M., Dawes I.W., Breitenbach M., Breitenbach M. The role of respiration, reactive oxygen species and oxidative stress in mother cell-specific ageing of yeast strains defective in the RAS signalling pathway. FEM. Yeast Res. 2004;5:157–167. doi: 10.1016/j.femsyr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Heiskanen P., Taira S., Rhen M., Taira S., Rhen M., Rhen M. Role of rpoS in the regulation of Salmonella plasmid virulence (spv) genes. FEMS Microbiol. Lett. 1994;123:125–130. doi: 10.1111/j.1574-6968.1994.tb07211.x. [DOI] [PubMed] [Google Scholar]
- Hekimi S., Guarente L., Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavata L., Aguilaniu H., Pichova A., Nyström T., Aguilaniu H., Pichova A., Nyström T., Pichova A., Nyström T., Nyström T. The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J. 2003;22:3337–3345. doi: 10.1093/emboj/cdg314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogema B.M., Arents J.C., Bader R., Eijkemans K., Yoshida H., Takahashi H., Aiba H., Postma P.W., Arents J.C., Bader R., Eijkemans K., Yoshida H., Takahashi H., Aiba H., Postma P.W., Bader R., Eijkemans K., Yoshida H., Takahashi H., Aiba H., Postma P.W., Eijkemans K., Yoshida H., Takahashi H., Aiba H., Postma P.W., Yoshida H., Takahashi H., Aiba H., Postma P.W., Takahashi H., Aiba H., Postma P.W., Aiba H., Postma P.W., Postma P.W. Inducer exclusion in Escherichia coli by non-PTS substrates: The role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 1998;30:487–498. doi: 10.1046/j.1365-2958.1998.01053.x. [DOI] [PubMed] [Google Scholar]
- Hsu A.L., Murphy C.T., Kenyon C., Murphy C.T., Kenyon C., Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hwangbo D.S., Gershman B., Tu M.P., Palmer M., Tatar M., Gershman B., Tu M.P., Palmer M., Tatar M., Tu M.P., Palmer M., Tatar M., Palmer M., Tatar M., Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Imlay J.A., Fridovich I., Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- Jishage M., Kvint K., Shingler V., Nyström T., Kvint K., Shingler V., Nyström T., Shingler V., Nyström T., Nyström T. Regulation of σ factor competition by the alarmone ppGpp. Genes & Dev. 2002;16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.E., Cypser J., de Castro E., de Castro S., Henderson S., Murakami S., Rikke B., Tedesco P., Link C., Cypser J., de Castro E., de Castro S., Henderson S., Murakami S., Rikke B., Tedesco P., Link C., de Castro E., de Castro S., Henderson S., Murakami S., Rikke B., Tedesco P., Link C., de Castro S., Henderson S., Murakami S., Rikke B., Tedesco P., Link C., Henderson S., Murakami S., Rikke B., Tedesco P., Link C., Murakami S., Rikke B., Tedesco P., Link C., Rikke B., Tedesco P., Link C., Tedesco P., Link C., Link C. Gerontogenes mediate health and longevity in nematodes through increasing resistance to environmental toxins and stressors. Exp. Gerontol. 2000;35:687–694. doi: 10.1016/s0531-5565(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Katayama Y., Gottesman S., Pumphrey J., Rudikoff S., Clark W., Maurizi M., Gottesman S., Pumphrey J., Rudikoff S., Clark W., Maurizi M., Pumphrey J., Rudikoff S., Clark W., Maurizi M., Rudikoff S., Clark W., Maurizi M., Clark W., Maurizi M., Maurizi M. The two-component, ATP-dependent Clp protease of Escherichia coli. Purification, cloning, and mutational analysis of the ATP-binding component. J. Biol. Chem. 1988;263:15226–15236. [PubMed] [Google Scholar]
- Klauck E., Lingnau M., Hengge-Aronis R., Lingnau M., Hengge-Aronis R., Hengge-Aronis R. Role of the response regulator RssB in σ recognition and initiation of σ proteolysis in Escherichia coli. Mol. Microbiol. 2001;40:1381–1390. doi: 10.1046/j.1365-2958.2001.02482.x. [DOI] [PubMed] [Google Scholar]
- Kloting N., Bluher M., Bluher M. Extended longevity and insulin signaling in adipose tissue. Exp. Gerontol. 2005;40:878–883. doi: 10.1016/j.exger.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Kondo M., Senoo-Matsuda N., Yanase S., Ishii T., Hartman P.S., Ishii N., Senoo-Matsuda N., Yanase S., Ishii T., Hartman P.S., Ishii N., Yanase S., Ishii T., Hartman P.S., Ishii N., Ishii T., Hartman P.S., Ishii N., Hartman P.S., Ishii N., Ishii N. Effect of oxidative stress on translocation of DAF-16 in oxygen-sensitive mutants, mev-1 and gas-1 of Caenorhabditis elegans. Mech. Ageing Dev. 2005;126:637–641. doi: 10.1016/j.mad.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kvint K., Farewell A., Nyström T., Farewell A., Nyström T., Nyström T. RpoS-dependent promoters require guanosine tetraphosphate for induction even in the presence of high levels of σS. J. Biol. Chem. 2000;275:14795–14798. doi: 10.1074/jbc.C000128200. [DOI] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R., Hengge-Aronis R. The cellular concentration of the σS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes & Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- Larsen P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko I., Seidel M., Sauer R.T., Baker T.A., Seidel M., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. A specificity-enhancing factor for the ClpXP degradation machine. Science. 2000;289:2354–2356. doi: 10.1126/science.289.5488.2354. [DOI] [PubMed] [Google Scholar]
- Libina N., Berman J.R., Kenyon C., Berman J.R., Kenyon C., Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Regulation of transcription of the glnALG operon of Escherichia coli by protein phosphorylation. Biochimie. 1989;71:1005–1012. doi: 10.1016/0300-9084(89)90104-1. [DOI] [PubMed] [Google Scholar]
- Magnusson L.U., Farewell A., Nyström T., Farewell A., Nyström T., Nyström T. ppGpp: A global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Mandel M.J., Silhavy T.J., Silhavy T.J. Starvation for different nutrients in Escherichia coli results in differential modulation of RpoS levels and stability. J. Bacteriol. 2005;187:434–442. doi: 10.1128/JB.187.2.434-442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler G., Schuller C., Adam G., Ruis H., Schuller C., Adam G., Ruis H., Adam G., Ruis H., Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993;12:1997–2003. doi: 10.1002/j.1460-2075.1993.tb05849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N.D., Savtchenko R.S., Remington S.J., Roseman S., Savtchenko R.S., Remington S.J., Roseman S., Remington S.J., Roseman S., Roseman S. Effects of mutations and truncations on the kinetic behavior of IIAGlc, a phosphocarrier and regulatory protein of the phosphoenolpyruvate phosphotransferase system of Escherichia coli. J. Biol. Chem. 2006;281:11450–11455. doi: 10.1074/jbc.M507417200. [DOI] [PubMed] [Google Scholar]
- Miersch J., Grancharov K., Pajpanova T., Tabakova S., Stoev S., Krauss G.J., Golovinsky E., Grancharov K., Pajpanova T., Tabakova S., Stoev S., Krauss G.J., Golovinsky E., Pajpanova T., Tabakova S., Stoev S., Krauss G.J., Golovinsky E., Tabakova S., Stoev S., Krauss G.J., Golovinsky E., Stoev S., Krauss G.J., Golovinsky E., Krauss G.J., Golovinsky E., Golovinsky E. Synthesis and biological activity of canavanine hydrazide derivatives. Amino Acids. 2000;18:41–59. doi: 10.1007/s007260050004. [DOI] [PubMed] [Google Scholar]
- Mika F., Hengge R., Hengge R. A two-component phosphotransfer network involving ArcB, ArcA, and RssB coordinates synthesis and proteolysis of σS (RpoS) in E. coli. Genes & Dev. 2005;19:2770–2781. doi: 10.1101/gad.353705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Moreno M., Audia J.P., Bearson S.M., Webb C., Foster J.W., Audia J.P., Bearson S.M., Webb C., Foster J.W., Bearson S.M., Webb C., Foster J.W., Webb C., Foster J.W., Foster J.W. Regulation of σS degradation in Salmonella enterica var typhimurium: In vivo interactions between σS, the response regulator MviA(RssB) and ClpX. J. Mol. Microbiol. Biotechnol. 2000;2:245–254. [PubMed] [Google Scholar]
- Muffler A., Fischer D., Altuvia S., Storz G., Hengge-Aronis R., Fischer D., Altuvia S., Storz G., Hengge-Aronis R., Altuvia S., Storz G., Hengge-Aronis R., Storz G., Hengge-Aronis R., Hengge-Aronis R. The response regulator RssB controls stability of the σS subunit of RNA polymerase in Escherichia coli. EMBO J. 1996;15:1333–1339. [PMC free article] [PubMed] [Google Scholar]
- Nair S., Poyart C., Beretti J.L., Veiga-Fernandes H., Berche P., Trieu-Cuot P., Poyart C., Beretti J.L., Veiga-Fernandes H., Berche P., Trieu-Cuot P., Beretti J.L., Veiga-Fernandes H., Berche P., Trieu-Cuot P., Veiga-Fernandes H., Berche P., Trieu-Cuot P., Berche P., Trieu-Cuot P., Trieu-Cuot P. Role of the Streptococcus agalactiae ClpP serine protease in heat-induced stress defence and growth arrest. Microbiol. 2003;149:407–417. doi: 10.1099/mic.0.25783-0. [DOI] [PubMed] [Google Scholar]
- O’Farrell P.H. The suppression of defective translation by ppGpp and its role in the stringent response. Cell. 1978;14:545–557. doi: 10.1016/0092-8674(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Ohnuma M., Fujita N., Ishihama A., Tanaka K., Takahashi H., Fujita N., Ishihama A., Tanaka K., Takahashi H., Ishihama A., Tanaka K., Takahashi H., Tanaka K., Takahashi H., Takahashi H. A carboxy-terminal 16-amino-acid region of σ38 of Escherichia coli is important for transcription under high-salt conditions and σ activities in vivo. J. Bacteriol. 2000;182:4628–4631. doi: 10.1128/jb.182.16.4628-4631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B.J., Ross W., Gaal T., Gourse R.L., Ross W., Gaal T., Gourse R.L., Gaal T., Gourse R.L., Gourse R.L. rRNA transcription in Escherichia coli. Annu. Rev. Genet. 2004a;38:749–770. doi: 10.1146/annurev.genet.38.072902.091347. [DOI] [PubMed] [Google Scholar]
- Paul B.J., Barker M.M., Ross W., Schneider D.A., Webb C., Foster J.W., Gourse R.L., Barker M.M., Ross W., Schneider D.A., Webb C., Foster J.W., Gourse R.L., Ross W., Schneider D.A., Webb C., Foster J.W., Gourse R.L., Schneider D.A., Webb C., Foster J.W., Gourse R.L., Webb C., Foster J.W., Gourse R.L., Foster J.W., Gourse R.L., Gourse R.L. DksA: A critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004b;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Peterson C.N., Ruiz N., Silhavy T.J., Ruiz N., Silhavy T.J., Silhavy T.J. RpoS proteolysis is regulated by a mechanism that does not require the SprE (RssB) response regulator phosphorylation site. J. Bacteriol. 2004;186:7403–7410. doi: 10.1128/JB.186.21.7403-7410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell B.S., Rivas M.P., Court D.L., Nakamura Y., Turnbough C.L., Jr., Rivas M.P., Court D.L., Nakamura Y., Turnbough C.L., Jr., Court D.L., Nakamura Y., Turnbough C.L., Jr., Nakamura Y., Turnbough C.L., Jr., Turnbough C.L., Jr. Rapid confirmation of single copy λ prophage integration by PCR. Nucleic Acids Res. 1994;22:5765–5766. doi: 10.1093/nar/22.25.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers R.W., III, Kaeberlein M., Caldwell S.D., Kennedy B.K., Fields S., Kaeberlein M., Caldwell S.D., Kennedy B.K., Fields S., Caldwell S.D., Kennedy B.K., Fields S., Kennedy B.K., Fields S., Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes & Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L.A., Silhavy T.J., Silhavy T.J. The response regulator SprE controls the stability of RpoS. Proc. Natl. Acad. Sci. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C.D., Dahlberg A.E., Dahlberg A.E. A single base change at 726 in 16S rRNA radically alters the pattern of proteins synthesized in vivo. EMBO J. 1990;9:289–294. doi: 10.1002/j.1460-2075.1990.tb08107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruteanu M., Hengge-Aronis R., Hengge-Aronis R. The cellular level of the recognition factor RssB is rate-limiting for σS proteolysis: Implications for RssB regulation and signal transduction in σS turnover in Escherichia coli. Mol. Microbiol. 2002;45:1701–1713. doi: 10.1046/j.1365-2958.2002.03123.x. [DOI] [PubMed] [Google Scholar]
- Repoila F., Majdalani N., Gottesman S., Majdalani N., Gottesman S., Gottesman S. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: The RpoS paradigm. Mol. Microbiol. 2003;48:855–861. doi: 10.1046/j.1365-2958.2003.03454.x. [DOI] [PubMed] [Google Scholar]
- Rockabrand D., Livers K., Austin T., Kaiser R., Jensen D., Burgess R., Blum P., Livers K., Austin T., Kaiser R., Jensen D., Burgess R., Blum P., Austin T., Kaiser R., Jensen D., Burgess R., Blum P., Kaiser R., Jensen D., Burgess R., Blum P., Jensen D., Burgess R., Blum P., Burgess R., Blum P., Blum P. Roles of DnaK and RpoS in starvation-induced thermotolerance of Escherichia coli. J. Bacteriol. 1998;180:846–854. doi: 10.1128/jb.180.4.846-854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde J., Heitman J., Cardenas M.E., Heitman J., Cardenas M.E., Cardenas M.E. The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 2001;276:9583–9586. doi: 10.1074/jbc.R000034200. [DOI] [PubMed] [Google Scholar]
- Ruiz N., Peterson C.N., Silhavy T.J., Peterson C.N., Silhavy T.J., Silhavy T.J. RpoS-dependent transcriptional control of sprE: Regulatory feedback loop. J. Bacteriol. 2001;183:5974–5981. doi: 10.1128/JB.183.20.5974-5981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D., Russell D. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. p. A8.40. [Google Scholar]
- Schmelzle T., Beck T., Martin D.E., Hall M.N., Beck T., Martin D.E., Hall M.N., Martin D.E., Hall M.N., Hall M.N. Activation of the RAS/cyclic AMP pathway suppresses a TOR deficiency in yeast. Mol. Cell. Biol. 2004;24:338–351. doi: 10.1128/MCB.24.1.338-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweder T., Lee K.H., Lomovskaya O., Matin A., Lee K.H., Lomovskaya O., Matin A., Lomovskaya O., Matin A., Matin A. Regulation of Escherichia coli starvation σ factor (σS) by ClpXP protease. J. Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P.J. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: Studies with the continuous-culture technique. J. Bacteriol. 1975;123:407–418. doi: 10.1128/jb.123.2.407-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D.D., Gupta A., Gottesman S., Gupta A., Gottesman S., Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- Thomsen L.E., Olsen J.E., Foster J.W., Ingmer H., Olsen J.E., Foster J.W., Ingmer H., Foster J.W., Ingmer H., Ingmer H. ClpP is involved in the stress response and degradation of misfolded proteins in Salmonella enterica serovar typhimurium. Microbiology. 2002;148:2727–2733. doi: 10.1099/00221287-148-9-2727. [DOI] [PubMed] [Google Scholar]
- Tomoyasu T., Mogk A., Langen H., Goloubinoff P., Bukau B., Mogk A., Langen H., Goloubinoff P., Bukau B., Langen H., Goloubinoff P., Bukau B., Goloubinoff P., Bukau B., Bukau B. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol. Microbiol. 2001;40:397–413. doi: 10.1046/j.1365-2958.2001.02383.x. [DOI] [PubMed] [Google Scholar]
- Ueguchi C., Misonou N., Mizuno T., Misonou N., Mizuno T., Mizuno T. Negative control of rpoS expression by phosphoenolpyruvate: Carbohydrate phosphotransferase system in Escherichia coli. J. Bacteriol. 2001;183:520–527. doi: 10.1128/JB.183.2.520-527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H., Valadi A., Adler L., Blomberg A., Gustafsson L., Valadi A., Adler L., Blomberg A., Gustafsson L., Adler L., Blomberg A., Gustafsson L., Blomberg A., Gustafsson L., Gustafsson L. An improved gas distribution system for anaerobic screening of multiple microbial cultures. J. Microbiol. Methods. 2001;47:51–57. doi: 10.1016/s0167-7012(01)00288-3. [DOI] [PubMed] [Google Scholar]
- VanBogelen R.A., Abshire K.Z., Moldover B., Olson E.R., Neidhardt F.C., Abshire K.Z., Moldover B., Olson E.R., Neidhardt F.C., Moldover B., Olson E.R., Neidhardt F.C., Olson E.R., Neidhardt F.C., Neidhardt F.C. Escherichia coli proteome analysis using the gene–protein database. Electrophoresis. 1997;18:1243–1251. doi: 10.1002/elps.1150180805. [DOI] [PubMed] [Google Scholar]
- Vinella D., Albrecht C., Cashel M., D’Ari R., Albrecht C., Cashel M., D’Ari R., Cashel M., D’Ari R., D’Ari R. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 2005;56:958–970. doi: 10.1111/j.1365-2958.2005.04601.x. [DOI] [PubMed] [Google Scholar]
- Wanner B.L. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 1993;51:47–54. doi: 10.1002/jcb.240510110. [DOI] [PubMed] [Google Scholar]
- Webb C., Moreno M., Wilmes-Riesenberg M., Curtiss R., III, Foster J.W., Moreno M., Wilmes-Riesenberg M., Curtiss R., III, Foster J.W., Wilmes-Riesenberg M., Curtiss R., III, Foster J.W., Curtiss R., III, Foster J.W., Foster J.W. Effects of DksA and ClpP protease on σS production and virulence in Salmonella typhimurium. Mol. Microbiol. 1999;34:112–123. doi: 10.1046/j.1365-2958.1999.01581.x. [DOI] [PubMed] [Google Scholar]
- Weber H., Polen T., Heuveling J., Wendisch V.F., Hengge R., Polen T., Heuveling J., Wendisch V.F., Hengge R., Heuveling J., Wendisch V.F., Hengge R., Wendisch V.F., Hengge R., Hengge R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and σ factor selectivity. J. Bacteriol. 2005;187:1591–1603. doi: 10.1128/JB.187.5.1591-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthzel A.M., Stancek M., Isaksson L.A., Stancek M., Isaksson L.A., Isaksson L.A. Growth phase dependent stop codon readthrough and shift of translation reading frame in Escherichia coli. FEBS Lett. 1998;421:237–242. doi: 10.1016/s0014-5793(97)01570-6. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J., Vieira J., Messing J., Messing J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Gottesman S., Gottesman S. Regulation of proteolysis of the stationary-phase σ factor RpoS. J. Bacteriol. 1998;180:1154–1158. doi: 10.1128/jb.180.5.1154-1158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]