Abstract
Background
Gastrointestinal carcinoid tumors are highly metastatic. Activation of the Raf-1 signaling pathway in carcinoid cells results in morphology changes. These Raf-1-induced structural changes may affect cellular adhesion, thereby altering metastatic potential.
Methods
An estrogen-inducible Raf-1 cell line (BON-raf) was used to study the effects of Raf-1 on cellular adhesion. Cell adhesion was measured before and after Raf-1 induction. Western blot analysis was used to confirm Raf-1 activation and measure levels of an essential adhesion regulator, β-catenin.
Results
Estrogen treatment of BON-raf cells resulted in Raf-1 activation and a marked reduction (68%) in cell adhesion. In the absence of Raf-1 induction, carcinoid cells expressed high levels of β-catenin. Raf-1 activation led to decreased expression of β-catenin.
Conclusions
Raf-1 induction in carcinoid cells results in a significant decrease in adhesion. Furthermore, the important adhesion regulator β-catenin is reduced in activated BON-raf cells. These Raf-1-related changes in adhesion may alter the metastatic phenotype of carcinoid cells.
Keywords: Neuroendocrine tumors, Carcinoid tumors, Raf-1 signaling, β-catenin, Adhesion, Metastasis
Introduction
Gastrointestinal (GI) neuroendocrine tumors (NETs) such as carcinoids and pancreatic islet cell tumors frequently metastasize to the liver. After colorectal carcinoma, GI NETs are the most common source of isolated hepatic metastases [1–4]. About 75% of patients with carcinoid tumors have metastases at the time of initial diagnosis [3–5]. While several intracellular signal transduction pathways have been implicated in the growth of GI NETs [6–12], little is known regarding the factors that regulate the development of their metastases.
Metastasis is a complex process by which cancer cells spread from the original tumor to distant sites. Changes in tumor cell adhesion are known to promote the development of metastases. We have previously observed that activation of the Raf-1 signaling pathway in carcinoid cells results in dramatic morphologic changes [13]. Under normal conditions GI carcinoid (BON) cells grow in clusters which coalesce into confluent sheets. However, after Raf-1 induction, the cells develop distinct cellular borders and do not form sheets. This led us to hypothesize that Raf-1 activation in GI carcinoid cells may alter cellular adhesion, thereby affecting the ability of carcinoid cells to invade and metastasize. To test this hypothesis we used an estrogen-inducible Raf-1 carcinoid cell line to analyze the effects of this signaling pathway on cellular adhesion in vitro.
Materials and Methods
Cell Culture
Metastatic human pancreatic carcinoid cells (BON cells), a gift of Drs. Mark Evers and Courtney Townsend, Jr. (University of Texas, Galveston, TX), were maintained in Dulbecco’s modified eagle medium (D-MEM): nutrient mixture F-12 in a 1:1 ratio (Invitrogen, San Diego, CA), supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen), in a humidified atmosphere of 5% CO2 at 37°C.
Activation of Raf-1 in GI Carcinoid Cells
Activation of the Raf-1/MEK/ERK signaling pathway in metastatic human pancreatic carcinoid cells was achieved utilizing an estrogen-inducible Raf-1 cell line (BON-raf [13]). Briefly, BON cells were stably transduced with the retroviral vector pLNC raf-l:ER (gift of Dr. Martin McMahon, University of California at San Francisco), a fusion molecule containing the ligand-binding domain of the estrogen receptor connected to the raf kinase domain of c-raf-1 [14, 15], to create BON-raf cells. BON-raf cells were maintained in phenol red-free D-MEM/F-12 supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 500 μg/mL G418 (Invitrogen).
To induce raf-1 activity in BON-raf cells, 1 μM β-estradiol (E2, Sigma) was added to the media 24 hours after seeding the flasks. An equivalent dilution of ethanol, the carrier for the E2, was used for control treatments.
Adhesion Assay
To study the effects of Raf-1 induction on cellular adhesion in GI carcinoid cells, a standard adhesion assay [16] was used. BON and BON-raf cells were seeded equally in 10 mL petri dishes and allowed to adhere and proliferate for 24 hours. Forty-eight hours after treatment with control or E2, cells were trypsinized with either 0.1% trypsin alone (T) or 0.1% trypsin attenuated by the addition of 1mM calcium chloride (T-Ca), and incubated at 37°C for five minutes. Cells were pipetted five times gently in 10 mL of 1X phosphate-buffered saline (PBS) and counted. Each sample was done in triplicate and the experiment was repeated three times.
For each cell type (BON or BON-raf) and treatment (control or E2) combination, the number of adhered cells was calculated by subtracting the number of floating cells after T-Ca trypsinization from the total number of cells, which was considered equal to the number of floating cells after T trypsinization. Relative adhesion was calculated by dividing the number of adhered cells after E2 treatment by the number of adhered cells after control treatment. Results were analyzed by ANOVA using statistical software (SPSS version 10.0, SPSS, Chicago, IL). A P value of < 0.05 was considered significant.
Western Blot Analysis
Cell lysates were extracted from BON and BON-raf cells harvested after 48 hours treatment with control or E2 using a standard protocol [7]. Protein concentrations were quantified with a bicinchoninic acid assay kit (Pierce, Rockford, IL). Denatured cellular extracts (40 μg) were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH). After transfer, the membranes were blocked for 1 hour in milk solution (5% nonfat dry milk and 0.05% Tween 20 in 1X PBS) and incubated with the appropriate antibodies as previously described [7]. The following primary antibody dilutions were used: regular and phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2 and phospho-ERK1/2, 1:1,000, Cell Signaling Technology, Beverly, MA), achaete-scute complex-like 1 (ASCL1, also known as MASH1, 1:1,000, BD Pharmingen, San Diego, CA), chromogranin A (CgA, 1:1,000, Zymed Laboratories, San Francisco, CA), β-catenin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), and G3PDH (1:10,000, Trevigen, Gaithersburg, MD). Primary antibody incubations were performed overnight at 4°C. After primary antibody incubation, membranes were washed 3 x 5 min or 3 x 10 min, depending on the antibody, in PBS-T wash buffer (1X PBS and 0.05% Tween 20). Membranes were incubated with a 1:2000 dilution of the appropriate anti-mouse or anti-rabbit horseradish peroxidase-conjugated IgG secondary antibody (Cell Signaling Technology) for 1 hour. The membranes were then washed 3 x 5 min or 3 x 10 min in PBS-T wash buffer and developed by ImmunStar (Bio-Rad; Hercules, CA) for ERK1/2, phospho-ERK1/2, chromogranin A, β-catenin, and G3PDH, or Super West Femto chemiluminescence substrate (Pierce) for mASH1, according to the manufacturers’ directions.
β-catenin and G3PDH protein levels were quantified using computer software (ImageQuant version 5.0, Amersham Biosciences, Buckinhamshire, UK). β-catenin expression was calculated by dividing the quantified β-catenin level by the G3PDH level. Relative β-catenin expression was calculated by dividing β-catenin expression in E2-treated cells by that in control cells.
Results
Effects of Raf-1 Induction on Intracellular Signaling in GI Carcinoid Cells
Estradiol treatment of BON-raf cells resulted in high levels of Raf-1 pathway induction as evidenced by increased levels of phospho-ERK1/2 protein. ERK is a downstream kinase in the Raf-1 pathway that becomes phosphorylated when the pathway is activated [9, 17]. Furthermore, E2-treatment of BON-raf cells led to a decrease in the transcription factor ASCL1, which has been shown to be important in determining the NET phenotype [8, 17, 18]. Finally, E2-treated BON-raf cells had lower levels of CgA, a tumor marker that is co-secreted with a variety of peptide hormones and neurotransmitters by NETs and is thus commonly used in the diagnosis and monitoring of patients with these tumors. These consequences of Raf-1 activation— phosphorylation of ERK1/2 and suppression of ASCL1 and CgA—were seen by Western blotting (figure 1) only in E2-treated BON-raf cells, and not in parental BON cells which lack the E2-inducible Raf-1 construct.
Figure 1. Treatment of BON-raf cells with estradiol results in phosphorylation of ERK1/2 and inhibition of ASCL1 and CgA.
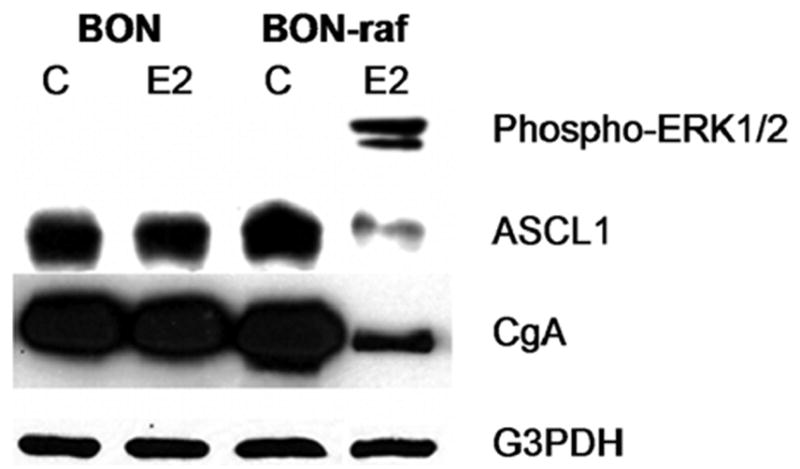
BON and BON-raf cells were treated with control (C) or 1 μM β-estradiol (E2) for 48 hours and total cellular proteins were harvested as described in Materials and Methods. Western blot analysis confirmed the presence of phosphorylated ERK1/2 protein in E2 treated BON-raf cells but not in BON cells or untreated BON-raf cells. Raf-1 activation in BON-raf cells decreased protein levels of ASCL1 and CgA. Equal protein loading was confirmed using antibody against G3PDH. ERK1/2, extracellular signal-regulated kinase 1/2; ASCL1, achaete-scute complex-like 1; CgA, chromogranin A.
Effects of Raf-1 Induction on GI Carcinoid Cellular Appearance
Treatment of BON cells with E2 had no effect on cell shape or size. Like BON-raf cells treated with control, BON cells grew in clusters and confluent sheets, with indistinct cellular margins. However, treatment of BON-raf cells with E2 resulted in dramatic changes in cellular appearance: the cells became flatter and developed distinct margins with gaps between adjacent cells (figure 2). Instead of the round shape of BON cells, the Raf-1-activated BON-raf cells assumed a stellate configuration, with long thin cell projections. The fact that the Raf-1 activated cells did not readily join together to form sheets suggested a change in cell-to-cell adhesion.
Figure 2. Raf-1 activation in BON-raf cells results in changes in cell shape.
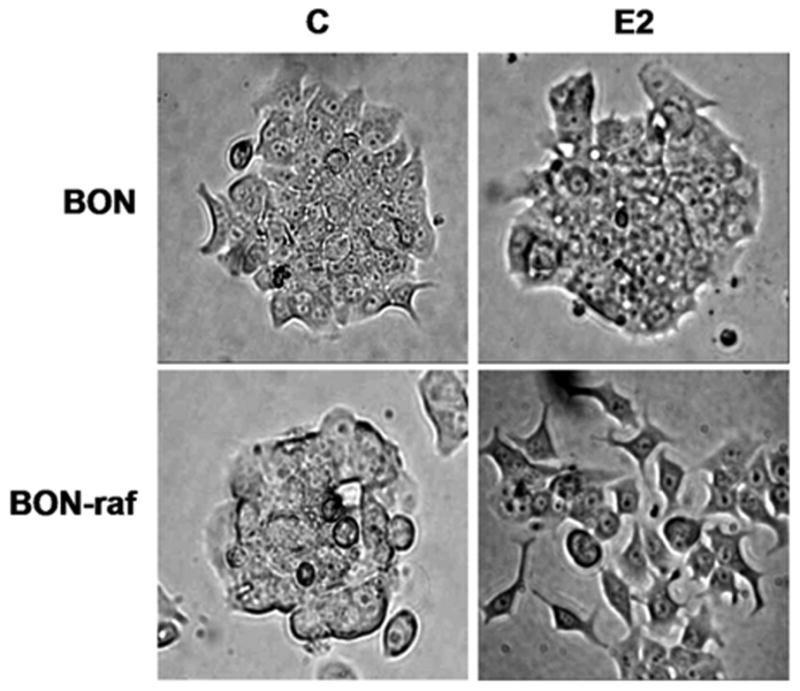
BON and BON-raf cells were treated with control (C) or 1 μM β-estradiol (E2) for 48 hours and examined under light microscopy for changes in cell shape and size. Only E2-treated BON-raf cells developed sharp cellular margins and projectile extensions.
Effects of Raf-1 Induction on Cell Adhesion
The observed changes in cell morphology—i.e, the development of distinct cellular margins and gaps between neighboring cells—led us to hypothesize that Raf-1 induction was altering the ability of the carcinoid cells to adhere together. After Raf-1 induction in BON-raf cells was confirmed by Western analysis as described above, we analyzed the effects of Raf-1 activation on cell adhesion using a standard attenuated-trypsin assay. Activation of the Raf-1 signaling pathway via E2 treatment in BON-raf cells led to a marked reduction (68%, P <0.05) in adhesion compared to cells treated with control. By contrast, no significant difference in adhesion was seen between control and E2 treated BON cells (figure 3). Taken together, the observed changes in cell shape and organization and the decrease in cellular adhesion suggest that Raf-1 activation leads to important structural changes in BON cells.
Figure 3. Raf-1 activation in BON-raf cells results in decreased cellular adhesion.
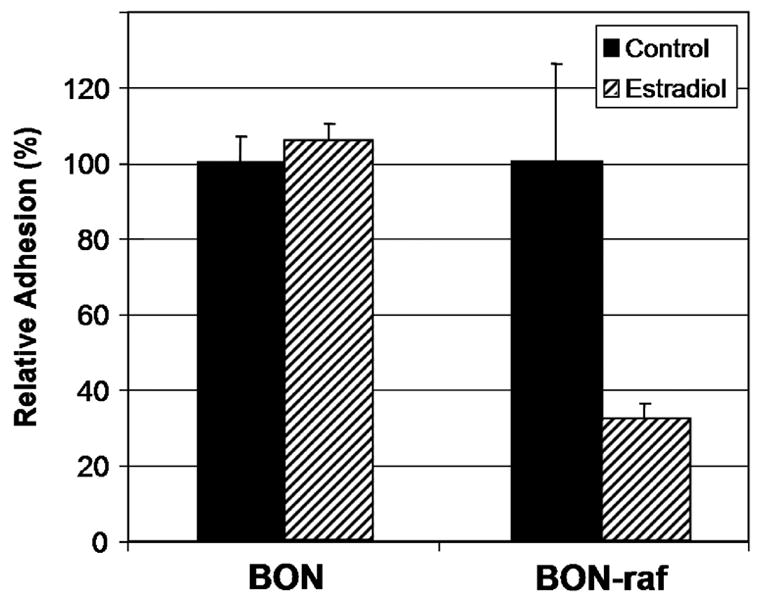
Adhered BON and BON-raf cells were treated with control or 1 μM β-estradiol (E2) for 48 hours and then exposed to 0.1% trypsin attenuated with 1mM calcium chloride for 5 minutes. The relative adhesion rate for each cell line was calculated by dividing the number of adhered cells after control treatment by the number after E2 treatment and multiplying by 100%. A significant (P < 0.05) decrease in adhesion was observed in E2 treated BON-raf cells but not BON cells.
Effects of Raf-1 Induction on Levels of β-catenin
β-catenin is known to play an important role in both cell adhesion and signal transduction. Since Raf-1 activation led to cell shape changes as well as decreased cellular adhesion, we analyzed the effect of Raf-1 induction in GI carcinoid cells on β-catenin expression by Western blotting. As shown in figure 4A, in the absence of Raf-1 pathway activation, BON and BON-raf cells expressed high levels of β-catenin protein. Interestingly, E2-treatment of BON-raf cells, but not BON cells, resulted in decreased levels of β-catenin. The quantified relative β-catenin expression of Raf-1-activated cells compared to control cells was 54% (figure 4B). This 46% drop in β-catenin expression may account for some of the 68% decrease in adhesion seen after E2 treatment of BON-raf cells. We conclude from these data that changes in cellular adhesion after Raf-1 pathway activation could be mediated in part by changes in β-catenin.
Figure 4. Raf-1 activation in BON-raf cells results in decreased protein expression of β-catenin.
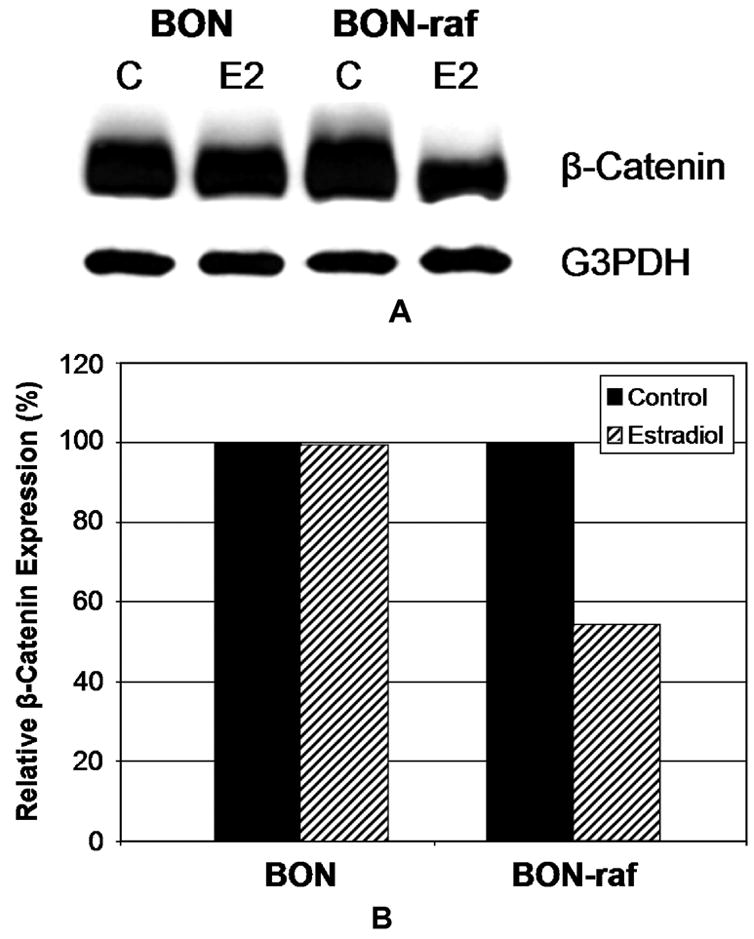
A: Western blot analysis using cell lysates from BON and BON-raf cells treated with control (C) or 1 μM β-estradiol (E2) for 48 hours demonstrated a decrease in β-catenin protein only in E2 treated BON-raf cells. G3PDH was used as a loading control. B: When quantified, the relative β-catenin expression in E2-treated BON-raf cells compared to control cells was 54%, representing a 46% decrease. By contrast, the relative β-catenin expression for E2-treated BON cells was 99.6%.
Discussion
As for most other types of cancer, patients who die from GI NETs usually do so because of their metastases, not the primary tumor. Metastasis is a complex cascade of many steps in which cancer cells leave the original tumor, enter blood or lymphatic vessels, migrate to distant sites in the body, exit the vessel, invade the host organ, establish a blood supply, and grow into a new tumor. Changes in cell adhesion are crucial to several steps in tumor invasion and metastasis. In the present study we report that Raf-1 induction in GI carcinoid cells results in a decrease in cell adhesion and β-catenin protein expression. These adhesion-related changes may affect the metastatic phenotype of these tumor cells.
The catenins, along with their binding partners, cadherins, are important in cancer progression and metastasis [19–22]. β-catenin is mutationally activated in several cancers (e.g., 90% of hepatoblastomas and 10% of colon adenocarcinomas [22, 23]). β-catenin plays a dual role in cell adhesion and transcriptional activation. The molecule interacts with cadherin adhesion receptors and the actin cytoskeleton to form adhesion junctions. Wnt signaling inhibits the degradation of β-catenin, which then can accumulate in the nucleus where it complexes with T cell factor and transactivates target genes known to play a role in cancer progression such as Cyclin D1 and Myc [22–24]. Therefore, downregulation of β-catenin in cancer cells would be expected to have important effects on both cell adhesion and transcriptional activity.
In this study we report for the first time that Raf-1 activation in GI carcinoid cells results in significantly decreased cellular adhesion. Furthermore, Raf-1 activation leads to decreased protein expression of β-catenin, an important cell adhesion and Wnt pathway signaling molecule. These Raf-1 related changes in cellular adhesion and β-catenin expression may alter the metastatic phenotype of carcinoid tumor cells. Further studies are warranted to explore the effects of Raf-1 activation on other adhesion molecules such as E- and N-cadherin, neural cell adhesion molecule (NCAM), and the fibroblast growth factor receptors (FGFRs). In vivo studies using a murine model of hepatic NET metastases may help elucidate the effects of Raf-1 activation on the ability of these tumors to metastasize.
We have previously reported that the pharmacologic Raf-1 activator ZM336372 decreases growth and inhibits hormone production in NETs [17, 25]. Raf-1 activating drugs such as ZM336372 represent a potential novel therapy for patients with NETs. Besides affecting NET growth and hormone production, Raf-1 activators may also change cellular adhesion in tumor cells and thus alter their metastatic potential.
7 - RAF-1 ACTIVATION IN GASTROINTESTINAL CARCINOID CELLS REDUCES TUMOR CELL ADHESION
D.J. Greenblatt, M. Kunnimalaiyaan, H. Chen
DR. CHRISTOPHER R. McHENRY (Cleveland, Ohio): How will you determine if these adhesion related changes actually affect the metastatic phenotype of the GI carcinoid tumor cells? What is the relationship between the Raf-1 signaling pathway and beta-catenin? Is the observed decrease in beta-cantenin expression the result of stimulation of the Raf-1 signaling pathway or is it due to some other estrogen-induced cellular effect? Finally, do all GI carcinoid tumor cells have the Raf-1 signaling pathway, and is there any way to explain the differences in metastatic potential for different carcinoid tumors based on this pathway?
Dr. GREENBLATT: Our results have shown that Raf-1 pathway activation does decrease adhesion and decrease expression of beta-catenin, but we have not definitively answered the question, does it decrease the ability of these cells to metastasize. We do know that adhesion is a crucial step in the metastatic cascade, and we hope that by blocking one step, we may inhibit the entire process. Our lab is currently conducting further studies to answer the question of whether Raf-1 activation decreases metastasis. Preliminary results using an in vitro matrigel migration assay do show that Raf-1 activation decreases the ability of the cells to invade. Furthermore, we intend to perform in vivo experiments using a mouse model of carcinoid liver metastases to answer the question if Raf-1 activation does decrease metastases.
As far as the relationship between Raf-1 and beta-catenin, we think that Raf-1 may modulate beta-catenin by inhibiting a third signaling pathway called GSK three beta.
Do all GI carcinoid tumors have the Raf-1 pathway, and is there any relationship between the activity of this pathway and the different levels of metastatic behavior that we see clinically? It’s important to understand that Raf-1 is a protein that is ubiquitous. It is found in every body in our cells, however, activation of the pathway is highly context dependent. We have started to analyze a collection of human tumor samples taken from patients in the OR, and of all the GI carcinoids that we have looked at to-date, none have shown any evidence of Raf-1 pathway activation. As far as the correlation between Raf-1 pathway activity and metastatic phenotype, we have analyzed about 20 GI neuroendocrine tumors to-date, and none of the metastatic tumors have any Raf-1 activation, and indeed only one tumor sample has any activated Raf at all, and that is a benign insulinoma. While these results are preliminary, we do think that there is some connection between activation of this pathway and metastatic potential, therefore, these results have both diagnostic and therapeutic implications. Measuring Raf-1 activity in tumors may have some prognostic value in predicting which patients will go on to develop hepatic metastases. Our main goal is to try to develop new treatments, targeted therapies, based on Raf-1 activation. We do have one drug, ZM33672, currently in pre-clinical trials, and in our in vitro experiments, as wells in vivo studies, we have been able to inhibit the growth of carcinoid tumors in mice. There is still a lot of work to do before we can think about human clinical trials, but I think this is a promising line of inquiry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA. Isolated liver metastases from neuroendocrine tumors: Does resection prolong survival? J Am Coll Surg. 1998;187:88–92. doi: 10.1016/s1072-7515(98)00099-4. discussion 92–3. [DOI] [PubMed] [Google Scholar]
- 2.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, et al. Resection of liver metastases from a noncolorectal primary: Indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187:487–493. doi: 10.1016/s1072-7515(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 3.Van Gompel JJ, Sippel RS, Warner TF, Chen H. Gastrointestinal carcinoid tumors: Factors that predict outcome. World J Surg. 2004;28:387–392. doi: 10.1007/s00268-003-7019-3. [DOI] [PubMed] [Google Scholar]
- 4.Lal A, Chen H. Treatment of advanced carcinoid tumors. Curr Opin Oncol. 2006;18:9–15. doi: 10.1097/01.cco.0000198018.53606.62. [DOI] [PubMed] [Google Scholar]
- 5.Musunuru S, Carpenter JE, Sippel RS, Kunnimalaiyaan M, Chen H. A mouse model of carcinoid syndrome and heart disease. J Surg Res. 2005;126:102–105. doi: 10.1016/j.jss.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Carson-Walter EB, Baylin SB, Nelkin BD, Ball DW. Differentiation of medullary thyroid cancer by C-raf-1 silences expression of the neural transcription factor human achaete-scute homolog-1. Surgery. 1996;120:168–72. doi: 10.1016/s0039-6060(96)80284-4. discussion 173. [DOI] [PubMed] [Google Scholar]
- 7.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–54. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Kunnimalaiyaan M, Van Gompel JJ. Medullary thyroid cancer: The functions of raf-1 and human achaete-scute homologue-1. Thyroid. 2005;15:511–521. doi: 10.1089/thy.2005.15.511. [DOI] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Chen H. The raf-1 pathway: A molecular target for treatment of select neuroendocrine tumors? Anticancer Drugs. 2006;17:139–142. doi: 10.1097/00001813-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 11.Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy enhancer of split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–42. doi: 10.1016/j.surg.2005.05.027. discussion 1142. [DOI] [PubMed] [Google Scholar]
- 12.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G636–42. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 13.Sippel RS, Chen H. Activation of the ras/raf-1 signal transduction pathway in carcinoid tumor cells results in morphologic transdifferentiation. Surgery. 2002;132:1035–9. doi: 10.1067/msy.2002.128877. discussion 1039. [DOI] [PubMed] [Google Scholar]
- 14.Bosch E, Cherwinski H, Peterson D, McMahon M. Mutations of critical amino acids affect the biological and biochemical properties of oncogenic A-raf and raf-1. Oncogene. 1997;15:1021–1033. doi: 10.1038/sj.onc.1201270. [DOI] [PubMed] [Google Scholar]
- 15.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irby RB, Yeatman TJ. Increased src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 17.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4:910–917. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 18.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Chen H. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134:866–71. doi: 10.1016/s0039-6060(03)00418-5. discussion 871–3. [DOI] [PubMed] [Google Scholar]
- 19.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: Epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 22.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 23.Bienz M, Clevers H. Linking colorectal cancer to wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 24.Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 25.Kappes A, Vaccaro A, Kunnimalaiyaan M, Chen H. ZM336372, a raf-1 activator, inhibits growth of pheochromocytoma cells. J Surg Res. 2006;133:42–45. doi: 10.1016/j.jss.2006.02.002. [DOI] [PubMed] [Google Scholar]


