Abstract
Students’ beliefs and goals can powerfully influence their learning success. Those who believe intelligence is a fixed entity (entity theorists) tend to emphasize ‘performance goals,’ leaving them vulnerable to negative feedback and likely to disengage from challenging learning opportunities. In contrast, students who believe intelligence is malleable (incremental theorists) tend to emphasize ‘learning goals’ and rebound better from occasional failures. Guided by cognitive neuroscience models of top–down, goal-directed behavior, we use event-related potentials (ERPs) to understand how these beliefs influence attention to information associated with successful error correction. Focusing on waveforms associated with conflict detection and error correction in a test of general knowledge, we found evidence indicating that entity theorists oriented differently toward negative performance feedback, as indicated by an enhanced anterior frontal P3 that was also positively correlated with concerns about proving ability relative to others. Yet, following negative feedback, entity theorists demonstrated less sustained memory-related activity (left temporal negativity) to corrective information, suggesting reduced effortful conceptual encoding of this material–a strategic approach that may have contributed to their reduced error correction on a subsequent surprise retest. These results suggest that beliefs can influence learning success through top–down biasing of attention and conceptual processing toward goal-congruent information.
Keywords: Dm, episodic memory, P3a, TOI, achievement motivation
Most students aim to succeed on academic tests. Yet, there is increasing evidence that the likelihood of their success is influenced not only by actual ability, but also by the beliefs and goals that they bring to the achievement situation (Elliot and Dweck, 2005). One framework that has been informative in addressing not only how these beliefs affect overall performance, but also how they affect rebound following failure, concerns individuals’ theories of intelligence (TOI; Dweck and Sorich, 1999). Previous behavioral studies have shown that students who believe that intelligence is a fixed quantity (‘entity theorists’) are particularly vulnerable to decreased performance when they realize they are at risk of failing, whereas students who view intelligence as acquirable (‘incremental theorists’) appear better able to remain effective learners.
These outcomes may be rooted in the different goals that follow from holding either a fixed or an acquirable view of intelligence (Dweck and Leggett, 1988; Hong et al., 1997; Mueller and Dweck, 1998; Sorich-Blackwell, 2001). Entity theorists tend to be more concerned with besting others in order to prove their intelligence (‘performance goals’), leaving them highly vulnerable to negative feedback. As a result, these individuals are more likely to shun learning opportunities where they anticipate a high risk of errors, or to disengage from these situations when errors occur. Indeed, when areas of weakness are exposed, they often will forego remedial opportunities that could be critical for future success (Chiu et al., 1997). In contrast, incremental theorists are more likely to endorse the goal of increasing ability through effort and are more likely to gravitate toward tasks that offer real challenges (‘learning goals’). In addition, in line with their view that there is always potential for intellectual growth, they are more willing to pursue remedial activities when they experience academic difficulty.
Can one better understand the mechanisms underlying these differences by directly examining how motivation influences attention and strategic processing in a difficult academic situation? We propose that self-beliefs about ability and their allied goals can influence both where attention will be biased and what type of processing will be conducted on information entering the focus of attention via the tonic influence of these beliefs on top–down control processes (Dweck et al., 2004). Many current neurocognitive models operationalize top–down control as a process that biases attention toward goal-relevant stimulus and response representations via a network of lateral prefrontal and parietal regions (Corbetta and Shulman, 2002; Miller and Cohen, 2001). According to these models, top–down control is particularly apparent in situations where information is detected that conflicts with current goals, as this will signal a reactive response to reorient attention and direct strategic processes toward maintaining goal-consistent behavior (i.e. reactive control, see Braver et al., in press). The role of monitoring for goal-conflicting information has typically been ascribed to regions of anterior cingulate cortex (ACC; Botvinick et al., 2001; Ridderinkhof et al., 2004), and indeed, interactions between ACC and frontal-parietal regions have been proposed by functional magnetic resonance imaging (fMRI) and event-related potential (ERP) studies to underlie increased behavioral control in situations where the potential for conflict is high (e.g. Egner and Hirsch, 2005; West, 2003; West and Moore, 2005). Thus, given that top–down monitoring and control processes can be linked to particular patterns of neural activity, we argue that such patterns can be used to reveal how self-beliefs impact learning success in challenging academic tasks.
The present study examines whether beliefs about intelligence influence: (i) how students respond to negative performance feedback signaling an error in the accuracy of general knowledge, and (ii) how reactive control (in response to errors) is subsequently engaged toward feedback that could assist students in learning new information that could correct that error. To this aim, students first provided answers to general knowledge questions (e.g. ‘What is the capital of Australia?’), then indicated their confidence in the accuracy of their response. They were then given two successive pieces of feedback, during which ERPs were recorded. The first provided only information about response accuracy (negative or positive performance-relevant feedback), whereas the second provided the correct answer (learning-relevant feedback). Negative feedback signals a conflict with the general goal of doing well, which is something important to both entity and incremental theorists. Yet, because entity theorists tend to emphasize the goal of doing well relative to others (performance goals), we predicted that these theorists would find negative feedback to be more salient, and even threatening, to the goal of proving ability. Subsequently, however, we predicted that incremental theorists, given their bias toward learning and challenge, would be more likely than entity theorists to engage reactive control processes toward encoding the correct answer, thereby increasing the likelihood that they would correct their answers on a subsequent retest. In contrast, entity theorists might subsequently engage attention toward self-critical rumination about their performance and abilities (Molden and Dweck, 2006), sacrificing attention toward the learning-relevant information, and thereby increasing the likelihood that they will repeat these errors.
We predicted that the greater salience of the negative performance-relevant feedback for entity theorists would be evidenced by a greater frontal P3 response to this information. Using a paradigm identical to the one used in the present study, Butterfield and Mangels (2003) found that the amplitude of a frontal P3 waveform was greatest when performance feedback signaled a conflict between expected outcome (as indicated by the subject's confidence that they would be correct or incorrect) and actual outcome (actual accuracy). In other words, it was sensitive to mismatch between an individual's metacognitive beliefs regarding accuracy on a particular trial and the actual outcome of that trial. TOI can also be viewed as a type of metacognitive process, in that it is a belief about one's cognitive abilities (i.e. whether they are fixed or malleable). Thus, this P3 may also index conflict with goals stemming from the individual's pervasive beliefs about intelligence. Notably, the spatiotemporal distribution of the anterior P3 in Butterfield and Mangels (2003) strongly resembles the novelty P3a (e.g. Courchesne et al., 1975; Friedman et al., 2001), a potential that is reliably elicited in response to stimuli that are unexpected within a given task context. This potential has been hypothesized to index the interruption of ongoing processes and reorienting of attention to the unexpected event, subserved by an anterior attentional system that includes both ACC and lateral prefrontal regions (Baudena et al., 1995; Bledowski et al., 2004; Daffner et al., 2000; Knight and Scabini, 1998; Yamasaki et al., 2002; Crottaz-Herbette and Menon, 2006).
Butterfield and Mangels (2003) also described a midline frontal negativity preceding the frontal P3 that was greater overall for negative than positive feedback, consistent with the spatio-temporal distribution of the feedback error-related negativity (FRN; Miltner et al., 1997; Nieuwenhuis et al., 2004). It has been suggested that the FRN indexes mismatch between expected and actual reward (Holroyd and Coles, 2002). Yet, across two separate experiments, Butterfield and Mangels (2003) found the FRN to be less sensitive to subjects’ beliefs than the P3. Rather, results from that study were more consistent with the view that the FRN indexes the initial detection of outcome valence in a binary fashion (good–bad; Hajcak et al., 2005; Yeung et al., 2004), whereas the subsequent P3 registers the effects of conflict between this outcome and prior expectations held in conscious awareness. Studies of response-locked error processing similarly suggest that the positivity (Pe) following the error-related negativity (ERN) is more sensitive to strategy, awareness and other factors related to the allocation of attention to errors (Mathewson et al., 2005; Nieuwenhuis et al., 2001). Thus, for the purposes of the present study, we will focus our analyses on the frontal P3, given its consistent relationship to beliefs and expectations. Nonetheless, given recent studies suggesting that mood and personality variables can in some cases also influence the FRN (Hajcak et al., 2003; Dikman and Allen, 2000; Santesso et al. 2005), we will also analyze the effects of TOI on the midline frontal negativity preceding the P3.
To examine the effects of TOI on the processing of learning-relevant information, we backsorted ERPs to feedback as a function of whether the item was later retrieved on the surprise retest or was forgotten (e.g. ‘differences due to memory’ or Dm effects; Paller and Wagner, 2002). We focused this analysis on the processing of the learning-relevant feedback, reasoning that any TOI differences in the pattern of Dm effects might reveal differences in how entity and incremental students engage reactive control toward the processing of corrective information. We first analyzed an inferior fronto-temporal Dm effect lasting 200–600 ms post-stimulus that had predicted successful encoding in Butterfield and Mangels (2003). Inferior temporal negativities in this latency range have been observed in studies capturing lexical and semantic processing of verbal information (Mangels et al., 2001; Nessler et al., 2006; Nobre et al., 1994; Nobre and McCarthy, 1995; Pickering and Schweinberger, 2003), consistent with generators in ventral stream regions associated with word identification and ‘deep’ conceptual processing (McCandliss et al., 2003; Posner et al., 1999; Rossell et al., 2003). Additionally, we explored Dm effects in other regions, given that entity theorists may also differ qualitatively in their approach to learning, perhaps processing information at a more ‘shallow,’ perceptual level than do incremental theorists (Grant and Dweck, 2003).
In summary, given increasing evidence that the likelihood of academic success is influenced not only by actual ability, but also by the beliefs and goals that individuals bring to an achievement situation, we aim to investigate whether this relationship is mediated by differences in responses to information that conflicts with the goal of performing well (negative feedback). We will focus both on the initial response to error information and the manner in which reactive control processes subsequently engage attention toward corrective information. An understanding of how TOI modulates ERP waveforms previously shown to be associated with the detection and correction of errors on a test of general knowledge (Butterfield and Mangels, 2003) will provide an initial step in building a neurocognitive model of how these beliefs influence learning outcomes.
METHODS
Participants
Participants were drawn from a database of 535 Columbia undergraduates who had consented to future EEG studies in our laboratory. For this particular study, we first selected students based on their average scores across four TOI questions framed from an entity perspective (e.g. ‘You have a certain amount of intelligence and you can't do much to change it.’). For each question, ratings of 1–3 (1 = strongly agree, 3 = somewhat agree) were consistent with an entity view, whereas ratings of 4–6 (4 = somewhat disagree, 6 = strongly disagree) were consistent with an incremental view. Only students whose average scores were unambiguous (entity: ≤ 3, incremental: ≥ 4) were eligible for the study. This left 464 participants from which we recruited a representative sample of 22 entity and 25 incremental students, all of whom also met inclusion criteria for an EEG study with visual–verbal stimuli (18–35 years old, right-handed, fluent English speakers, normal or corrected-to-normal vision/hearing, not currently taking psychoactive medications, no history of neurological or substance abuse disorders). All tested students gave informed consent and were compensated $10/h for their participation.
As shown in Table 1, the tested sample was highly representative of the larger population from which it was drawn. TOI groups within the sample also were well-matched to each other on multiple demographic and affective measures. Although intelligence was not directly assessed, a titration algorithm employed during the experiment ensured that entity and incremental theorists achieved a similar level of performance regardless of background knowledge.
Table 1.
Group characteristics as a function of theory of intelligence (TOI) in both the tested sample and the population from which this sample was drawn. Standard error of the mean (SEM) is shown in parentheses
| Tested sample (n = 47) |
Population (n = 464) |
|||
|---|---|---|---|---|
| Entity | Incremental | Entity | Incremental | |
| n | 22 | 25 | 196 | 268 |
| TOI | 2.5 (0.12) | 4.7 (0.13)** | 2.4 (0.04) | 4.8 (0.04)** |
| Demographic | ||||
| Age (years) | 21.0 (0.64) | 21.6 (0.56) | 21.8 (0.26) | 22.3 (0.25) |
| Education (years) | 14.5 (0.36) | 14.8 (0.26) | 14.6 (0.09) | 14.8 (0.08) |
| Percentage of females | 50.0 | 56.0 | 50.5 | 54.5 |
| Mooda | ||||
| BDI-II | 5.5 (0.86) | 5.2 (0.90) | NA | NA |
| STAI (trait) | 1.8 (0.09) | 1.6 (0.10) | NA | NA |
| STAI (state) | 1.6 (0.08) | 1.5 (0.09) | NA | NA |
| Goalsb | ||||
| Performance | 4.4 (0.42) | 3.8 (0.38) | 4.5 (0.13)* | 4.1 (0.11) |
| Learning | 5.5 (0.31) | 6.2 (0.30)† | 5.8 (0.01) | 6.1 (0.08)* |
| Outcome | 6.4 (0.29) | 6.5 (0.36) | 6.5 (0.09) | 6.7 (0.08)† |
Entity vs incremental t-tests (two-tailed): *P < 0.05; **P < 0.01; †P < 0.1.
aMood measures are not available (NA) for the population because they were taken only on the day of the test. BDI-II: Beck Depression Inventory; STAI: State-Trait Anxiety Inventory.
bGoal subscores reflect the mean rating of how much each of the three statements described how they ‘thought and acted in general’ on a scale of 1 (strongly disagree) to 8 (strongly agree).
Results from an achievement goals questionnaire modified from Grant and Dweck (2003) confirmed that incremental theorists more strongly endorsed learning goals (e.g. ‘It is very important to me to feel that my coursework offers me real challenges’) than entity theorists, and entity theorists more strongly endorsed performance goals (e.g. ‘When I take a course in school, it is very important for me to validate that I am smarter than other students’) than incremental theorists. These differences were significant in the population and in the same direction in the sample. Additionally, in line with the view that both groups value a positive achievement outcome, tested groups did not differ with regard to outcome goals (i.e. ‘It is very important for me to do well in my courses’), although a trend for incremental theorists to endorse these goals to a greater extent was found in the population.
Materials
The stimuli consisted of a pool of 476 general knowledge questions drawn from a variety of academic domains, including literature, art and music history, world and US history, religion, geography, mathematics, and the natural and physical sciences. All correct responses were single words, 3–8 letters in length, unique to a particular question and rated as familiar by four independent raters (all Columbia undergraduates) with ≥75% agreement.
Design and procedure
At first test, students were presented with the general knowledge questions on a computer. For each question, they typed in either their best answer or ‘xxx’ if they felt that they could not take an educated guess (i.e. ‘omit responses’). Except for omit responses, they then rated their confidence that the response was correct on a 7-point scale ranging from 1 (sure wrong) to 7 (sure right). Students had an unlimited amount of time to make both responses.
Next, the feedback sequence began with a 2 s period during which the screen was blank. A fixation crosshair then appeared centrally for 2.5 s followed by the performance-relevant feedback, which consisted of a green asterisk/high tone (positive feedback) or red asterisk/low tone (negative feedback), presented for 1 s. Another crosshair then was presented for 2.5 s, allowing students to prepare for the learning-relevant feedback, which consisted of the correct answer to the question, presented in white for 2 s. This information appeared regardless of whether the students’ initial answer had been correct or incorrect. To ensure that at first test participants in both TOI groups experienced a similar level of subjective difficulty and had similar baseline performance, problems were presented in such a way as to ensure ∼40% accuracy overall. [See Butterfield and Mangels (2003) for a description of the titration algorithm.]
This first portion of the experiment concluded when the student had completed a minimum of 10 trials in all conditions (described below), or had been tested for 3 h, whichever came first. The EEG cap was then removed. After ∼8 min, the student returned to the computer to begin the second phase, which consisted of a surprise retest on all the questions they had answered incorrectly at the first test. Only at the start of the retest were students told that the questions they would be answering were those they had initially gotten wrong. During debriefing, all participants reported being surprised about the retest.
ERP recording and data reduction
Continuous EEG was recorded during the first test only with a sintered Ag/AgCl 64-electrode Quick-Cap and amplified using Neuroscan Synamps 2 with an A/D conversion rate of 500 Hz and bandpass of DC-100 Hz. Impedance was kept below 11 kΩ. EEG was initially referenced to Cz and then converted to an average reference off-line. We compensated for blinks and other eye movement artifacts using 2–6 PCA-derived ocular components. Off-line, the EEG was cut into epochs time-locked to feedback presentation (performance-relevant feedback: −100–1000 ms post-stimulus; learning-relevant feedback: −100–1500 ms post-stimulus). We could not analyze the final 500 ms of the learning-relevant feedback because of increased eye and muscle noise during that part of the epoch.
Following baseline correction to the 100 ms interval preceding the stimulus, epochs containing excessive noise (±100 mV) were rejected and the remaining epochs were averaged to create the event-related potentials (ERPs). A 35 Hz low-pass filter was applied before averaging. ERPs to performance-relevant feedback were averaged as a function of accuracy (correct, incorrect) and confidence (higher, lower; see data analysis for further explanation). ERPs to learning-relevant feedback were averaged for incorrect first test responses only, as a function of whether that item was corrected or not on the retest. To simplify this analysis, we included all trials regardless of initial response confidence, given that there was no interaction between TOI and this variable in the behavioral data.
Data analysis
There was substantial variability in trials to completion (minimum = 219, maximum = 476), most likely due to differences in the rate at which subjects reached the criterion of 10 trials/condition. Because students who took longer to complete the experiment might have had difficulty staying engaged in the task simply due to fatigue, we limited analysis of both behavioral and EEG data to the first 200 trials, reasoning that during this time period, students’ experiences would have been the most comparable.
Differences in the length of the experiment were due largely to how students used the confidence ratings, with some students avoiding low or high extremes. Ultimately, in order to obtain a sufficient number of trials for EEG analysis that still accurately represented the ‘lower’ and ‘higher’ range of confidence responses of each student, we divided trials on the basis of individual's median confidence. On average, the median confidence of incorrect responses was lower than for correct responses (incorrects: M = 3.1, SEM = 0.14; corrects: M = 5.7, SEM = 0.13, P < 0.001), but there were no TOI differences (P > 0.7). High confidence corrects and low confidence errors had a minimum of 25 trials per subject. Although conditions in which expectation and accuracy were ‘mismatched’ contained lower number of trials overall (lower confidence corrects: M = 31.7, min = 13; higher confidence errors: M = 34.0, min = 9), trial counts did not differ between TOI groups (all ts < 1.2, all P-values > 0.2).
Behavioral data were analyzed with a 2 (group) × 3 (confidence: higher, lower, omits) mixed measures analysis of variance (ANOVA). ERP components associated with the performance-relevant feedback were analyzed using 2 (group) × 2 (confidence: higher, lower) mixed-measures ANOVAs on the mean window extending one SD above and below the latency calculated from the Fz/FCz peak in individual subject data [average peak = 380 ms (SD = 20)]. ERPs associated with learning-relevant feedback were evaluated using the mean activity over 250 or 500 ms windows in a series of 2 (group) × 2 (confidence) × 2 (electrode) × 2 (hemisphere) mixed-measures ANOVAs.
RESULTS
Behavioral results
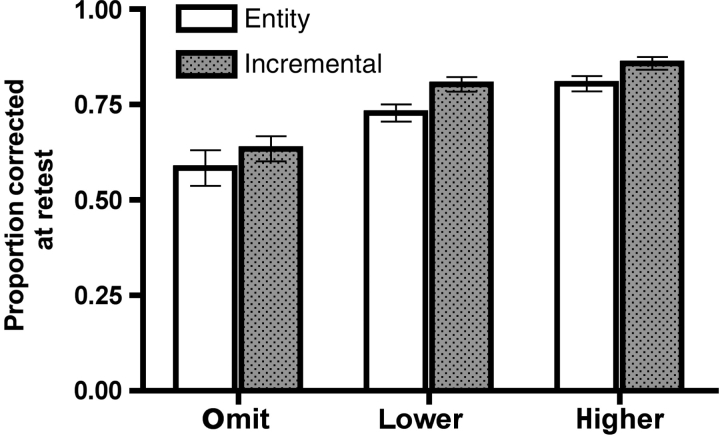
Despite similar first-test performance across groups (entity: M = 40.8%, SEM = 0.01; incremental: M = 41.5%, SEM = 0.01), incremental theorists demonstrated significantly greater improvement on the retest than did the entity theorists. As shown in Figure 1, both groups were able to correct the majority of their errors at retest, yet incremental theorists corrected significantly more errors than did entity theorists overall, F(1, 44) = 4.1, P < 0.05. There also was a significant main effect of response confidence, F(1.4, 60.3) = 50.1, P < 0.0001. Specifically, errors were more likely to be corrected when they were initially made with higher, as compared with lower, confidence (i.e. the ‘hypercorrection’ effect; Butterfield and Metcalfe, 2001). There was no interaction between theory of intelligence and response confidence (P = 0.8), indicating that incremental theorists’ advantage on the retest was similar across all levels of response confidence.
Fig. 1.
Proportion of errors of each confidence type (omits, lower confidence, higher confidence) that were corrected at retest, as a function of theory of intelligence (entity, incremental). Error bars in this and all subsequent figures represent the standard error of the mean (SEM).
Electrophysiological results
Performance-relevant feedback
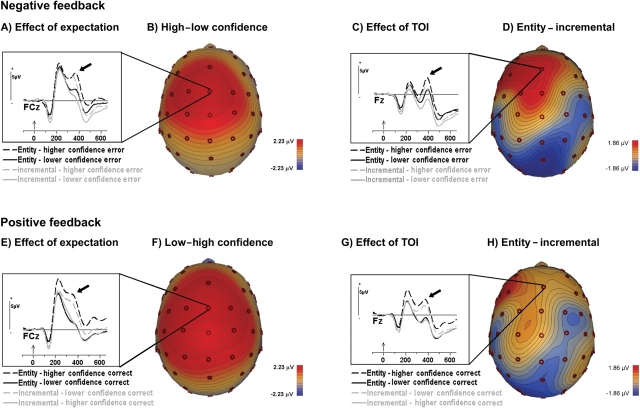
These data represent neural activity time-locked to when the students first learned about the accuracy of their response. Given our hypothesis that TOI effects would be most apparent under conditions of failure, we focused our initial analyses on the frontal P3 following negative feedback at its FCz maximum. As in Butterfield and Mangels (2003), the peak amplitude of this component was greater following higher-confidence responses than lower-confidence responses, F(1, 45) = 57.8, P < 0.0001, replicating its sensitivity to expectation (Figure 2A–B). Yet, at this site, there was only a trend toward a TOI group difference, F(1, 45) = 2.4, P = 0.1. Visual inspection of nearby waveforms suggested that differences between entity and incremental groups instead were maximal just anterior to the FCz site where effects of expectancy were found (Figure 2C–D). Indeed, analysis of the P3 at the anterior frontal midline (Fz) revealed a robust effect of TOI, F(1, 45) = 4.1, P < 0.05, in addition to a significant effect of expectancy, F(1, 45) = 33.6, P < 0.001. TOI did not interact with confidence at either the fronto-central (FCz: P = 0.6) or anterior frontal (Fz: P = 0.9) sites.
Fig. 2.
ERPs to negative and positive performance-relevant feedback. (A) Grand mean averaged waveforms of entity and incremental theorists to negative feedback (feedback following errors) as a function of the participant's confidence that his/her answer was correct (lower, higher), shown at FCz, where effects of expectation were maximal. Waveforms in this and all subsequent figures were low-pass filtered at 15 Hz. The zero point in the timeline marks the feedback onset. Positive is plotted up. (B) Scalp topography of the difference between high and low confidence errors (i.e. the expectancy effect), collapsed over group. Top–down view with nose pointed toward the top of the page. (C) Same grand mean average waveforms as in (A), but shown at Fz, where effects of theory of intelligence (TOI) were more prominent. (D) Scalp topography of the difference between the entity and incremental responses to the negative feedback at 380 ms (peak of the P3), collapsed over confidence (weighted average). (E–F) Same as in (A–B), except for positive feedback. (G–H) Same as in (C–D), except for positive feedback.
To confirm the more anterior frontal topography of the TOI effect to negative feedback, we analyzed its distribution across the following regions: anterior frontal (FP1/2, AF7/8), frontal (F3/4, F5/6), central (C3/4, C5/6), parietal (P3/4, P5/6), occipital (O1/2, CB1/2) and temporal (T7/8, TP7/8). Analysis of the mean amplitude of a latency window bracketing the peak of the anterior P3 (360–400 ms) in a 2 (group) × 2 (confidence) × 6 (region) × 2 (hemisphere) × 2 (electrode) ANOVA revealed only one higher order interaction involving group—a region × hemisphere × group interaction, F(3.18, 143.3) = 2.75, P < 0.05. Further investigation of group differences as a function of region and hemisphere indicated that this interaction arose from the relative specificity of this effect to the left anterior frontal region (P < 0.001). The only other region to demonstrate an effect of group was the left occipital region (P < 0.05), where entity theorists elicited more negative-going activity than did incremental theorists. Although this increased negativity may represent a separate posterior process that is also sensitive to TOI, it may also represent the inverse of the dipole generating the anterior frontal P3.
Modulation of the anterior frontal P3 by TOI was specific for negative feedback. Although a significant effect of expectation was also found for positive feedback (Figure 2E–H), in that the P3 was more positive following lower-confidence than higher-confidence responses at both FCz, F(1, 45) = 95.2, P < 0.0001, and Fz, F(1, 45) = 64.3, P < 0.001, at neither site did we observe a reliable effect of TOI (P-values > 0.3; Figure 2G–H), or interaction of TOI and confidence (P-values > 0.3).
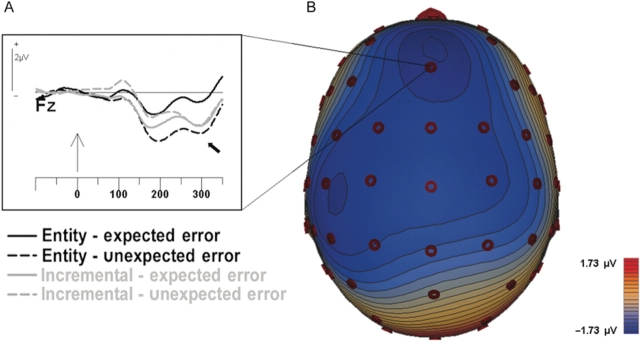
As can be seen by comparing Figure 2C and G, negative feedback was also associated with a larger negativity at ∼300 ms post-stimulus than was positive feedback, suggestive of a feedback-locked error-related negativity (FRN). However, analyses of the effects of TOI and expectancy on the FRN were complicated by its overlap with the subsequent P3, which evidenced significant effects of these factors, but in a positive-going direction. To mitigate the effects of the P3, we attempted to extract the FRN using difference waves in which we subtracted the waveforms for positive feedback that were matched to the negative feedback with regard to probability [i.e. (Low-confidence errors, LCE) − (High-confidence corrects, HCC), (High-confidence errors, HCE) − (Low-confidence corrects, LCC)]. These difference waves could potentially highlight the FRNs associated with negative feedback to expected errors (LCE − HCC) and unexpected errors (HCE−LCC), respectively (for a similar analytic approach, see Butterfield and Mangels, 2003).
As shown in Figure 3, incremental theorists appeared to elicit significant FRNs, defined as negative deflections significantly different from zero in a one-sample t-test (P-values < 0.01), for both expected and unexpected errors. In contrast, entity theorists only elicited a significant FRN following unexpected errors (unexpected: P < 0.001; expected: P = 0.2). These TOI-related differences were supported by a significant interaction of TOI and expectation, F(1, 45) = 4.1, P < 0.05. These results suggest that the FRN of entity theorists was modulated to a greater extent by expectation. Yet, it was their expected errors, rather than unexpected errors, that seem to be responsible for this difference. Even this effect should be interpreted with caution, however, because a TOI by expectancy interaction observed at the P2 to positive feedback, F(1, 45) = 4.3, P < 0.05, may have influenced the FRN difference wave.
Fig. 3.
The feedback-locked negativity (FRN). (A) Difference waveforms associated with negative feedback to unexpected errors (HCE − LCC) and expected errors (LCE − HCC) for entity and incremental theorists. The black arrow points to the part of the waveform corresponding to the peak of the negativity in the raw waveforms (300 ms; see Figure 2C and G). (B) Scalp topography of the FRN difference wave at its peak latency, collapsed across group and expectancy.
Performance-relevant feedback: relationship to goals
TOI differences were relatively specific to midline and left anterior frontal sites. To explore whether group differences arose from conflict with entity theorists’ goal of demonstrating ability relatively to others, we examined the relationship between the peak amplitude of the anterior frontal P3 to negative feedback and achievement goals in a series of Spearman's correlations conducted separately for entity and incremental groups. As shown in Table 2, the endorsement of performance goals was positively related to the amplitude of the anterior frontal P3 to high-confidence errors in both groups; a similar, but less robust relationship was found for low-confidence errors in the incremental group. In other words, the more strongly that subjects felt that statements such as ‘When I take a course in school, it is very important for me to validate that I am smarter than other students’ described them, the larger the magnitude of the anterior frontal P3 response to negative feedback. A similar pattern of significant correlations was also found when analyzing activity measured at left anterior frontal sites.
Table 2.
Spearman's ρ correlations of achievement goals with peak amplitude of the anterior frontal P3 (at Fz) for low-confidence errors (LCE) and high-confidence errors (HCE) for both entity and incremental theorists
| Performance goals | Learning goals | |
|---|---|---|
| Entity n = 22 | ||
| LCE | 0.20 | −0.26 |
| HCE | 0.51* | −0.25 |
| Incremental n = 25 | ||
| LCE | 0.39* | −0.33 |
| HCE | 0.43* | −0.47* |
*P < 0.05.
In contrast, endorsement of learning goals demonstrated a negative correlation with the P3 to high-confidence errors in incremental theorists, suggesting that incremental students who gravitated toward challenging situations found unexpected negative feedback to be less salient or threatening. It is worth noting that although these correlations tended to be strongest for high-confidence errors, which are relatively infrequent, there were no significant correlations between goals and the number of high-confidence errors in either group (P-values > 0.3). Thus, individual differences in the probability of making high-confidence errors were unlikely to be mediating these significant correlations. In addition, the relationship of brain activity to achievement goals was only robust for P3 activity measured at anterior frontal sites. No significant correlations were found between goals and peak amplitude of the P2, FRN (either raw waveforms or difference waves), or the P3 at fronto-central (FCz) or parietal (Pz) sites (all P-values > 0.2).
Performance-relevant feedback: relationship to subsequent retest performance
Analyzing P3 activity as a function of subsequent memory (later corrected vs. later not corrected) revealed a significant Dm effect at the fronto-central site (FCz), F(1, 45) = 11.24, P < 0.01. This result replicates Butterfield and Mangels (2003), who similarly found that activity at this site was greater for items corrected on an immediate retest. Interestingly, activity at Fz failed to significantly predict subsequent memory performance, F(1, 45) = 2.7, P = 0.11, despite the fact that large effects of expectancy had been found at both this site and at FCz. Neither site exhibited a significant interaction between Dm and group.
Learning-relevant feedback: relationship to subsequent retest performance
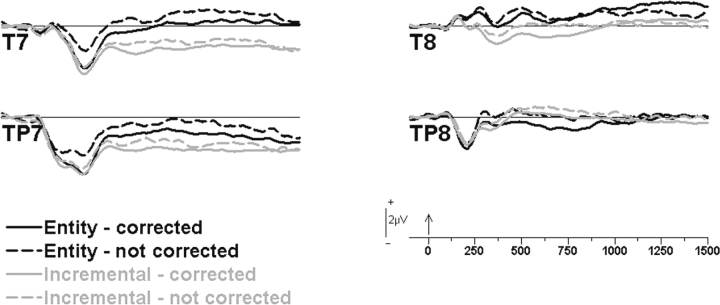
Our initial analysis of subjects’ processing of the learning-relevant feedback focused on the negative-going activity over temporal regions, proximal to where Dm effects had been found in Butterfield and Mangels (2003). As shown in Figure 4, the broad deflection observed from 250 to 500 ms was more negative-going over the left hemisphere, F(1, 45) = 40.5, P < 0.001, and was enhanced for items later corrected, F(1, 45) = 31.2, P < 0.001. Incremental theorists also exhibited greater negativity during this period, F(1, 45) = 4.5, P < 0.05, consistent with the prediction that incremental theorists would engage conceptual processes to a greater extent. Although it appeared that the difference between later corrected and not corrected items was larger for entity than incremental theorists, the TOI × memory interaction was only marginal, F(1, 45) = 2.7, P = 0.1.
Fig. 4.
ERPs to learning-relevant feedback. Grand mean waveforms at temporal sites as a function of theory of intelligence (entity, incremental) and subsequent memory performance (corrected, not corrected).
As this negative-going activity became more sustained (500–1000 ms), it remained more negative over the left hemisphere, F(1, 45) = 13.0, P < 0.001, and was still predictive of later memory performance, F(1, 45) = 11.6, P < 0.001. In addition, incremental theorists continued to exhibit significantly more negativity than entity theorists, F(1, 45) = 4.9, P < 0.05, particularly over the left hemisphere, as indicated by a hemisphere × TOI interaction, F(1, 45) = 4.5, P < 0.05. From 1000 to 1500 ms, although this activity was no longer significantly related to memory performance, F(1, 45) = 1.0, it continued to be left lateralized, F(1, 45) = 18.4, P < 0.001, and enhanced in the incremental group, F(1, 45) = 6.6, P < 0.05.
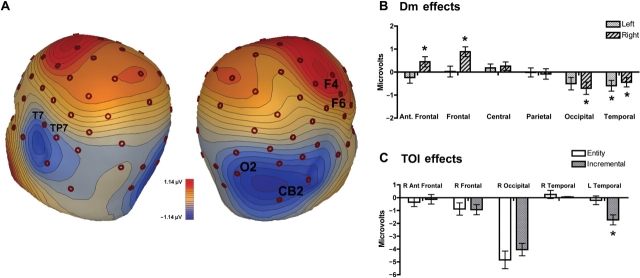
To determine whether Dm and/or TOI effects might be present over other scalp regions during the period when they were most pronounced over temporal sites, we conducted a regional analysis of the mean amplitude from 500 to 1000 ms across anterior frontal (FP1/2, AF7/8), frontal (F3/4, F5/6), central (C3/4, C5/6), parietal (P3/4, P5/6), occipital (O1/2, CB1/2) and temporal (T7/8, TP7/8) sites. No significant effects involving the group were found; however, we did observe a region × hemisphere × memory interaction, F(5, 255) = 3.0, P < 0.05, which subsumed significant two-way interactions involving all possible combinations of these variables. Exploration of the three-way interaction indicated that Dm effects were found in many regions in addition to the temporal regions, including right anterior frontal, right frontal and right occipital sites (Figure 5A, B), consistent with the view that Dm effects have multiple generators (Friedman and Johnson, 2000). Nonetheless, when we examined whether group differences were found at these sites, significant TOI differences were only found over the left temporal region (Figure 5C; P < 0.01; all other P-values > 0.3).
Fig. 5.
(A) Scalp topography illustrating the left and right hemisphere Dm effects (difference of later corrected vs. later not corrected) at 750 ms, collapsed over group. (B) Regional distribution of the Dm effect from 500–1000 ms (collapsed over group). Regions with significant memory-related differences are noted with asterisks. (C) Mean activity for entity and incremental groups in regions were memory-related differences were found in (B). Significant TOI differences (collapsed over subsequent memory) are noted with asterisks.
DISCUSSION
The present study aimed to understand how factors other than ability influence learning success under challenge. Using theories of intelligence to represent these factors, we found that incremental theorists demonstrated significantly greater overall gains in knowledge than did entity theorists, in that they demonstrated greater remediation of errors regardless of confidence with which the error was initially made. As failures were experienced and opportunities to learn from these failures presented themselves, our use of ERPs allowed us to track the neural dynamics of attentional and conceptual processes that we hypothesized to underlie the relationship between TOI and retest outcome.
Our findings suggest that entity and incremental theorists oriented in a somewhat different manner to performance-relevant information. Although entity and incremental theorists exhibited a similar modulation of the more fronto-centrally distributed P3—a potential that primarily indexed mismatch between expected and actual outcome, entity theorists exhibited an enhanced anterior frontal P3 to both expected and unexpected negative performance-relevant feedback. In addition, entity theorists appeared less likely to engage in sustained semantic processing of the learning-relevant feedback when it arrived, as evidenced by differences in the duration of an inferior fronto-temporal negativity that serves as a putative marker of encoding-relevant processes associated with the activation of pre-existing representations in semantic memory (Mangels et al., 2001; Butterfield and Mangels, 2003; Nessler et al., 2006). Given the importance of attention in successful encoding for later recall and recognition tests (Craik et al., 1996), group differences in the degree of sustained ‘deep’ semantic processing of learning-relevant information may explain in part why incremental theorists are often able to better rebound academically following failure.
One question that arises concerns the functional significance of differences between the anterior frontal distribution of the P3 sensitive to TOI effects and the more fronto-central distribution of the P3 sensitive to violations of expectation. Although source localization was beyond the scope of the present study, the more anterior distribution of the P3 differentiating the two groups could be consistent with a dipole in a region of ACC that is oriented rostrally (i.e. rACC) to the more dorsal regions of ACC (dACC) likely to be eliciting the fronto-central P3 (Crottaz-Herbette and Menon, 2006). The rACC has been characterized as the ‘emotional’ subdivision of the ACC based on anatomical connectivity with limbic regions, including the amygdala, anterior insula and orbitofrontal cortex, as well as its outflow to autonomic, visceromotor and endocrine systems (Devinsky et al., 1995). In contrast, the dACC, which exhibits connectivity with lateral prefrontal, parietal and premotor regions (Devinsky et al., 1995), has been characterized as the ‘cognitive’ subdivision. Correspondingly, a meta-analysis concluded that the rACC was particularly involved in orienting attention internally in order to assess ‘the salience of emotional or motivational information and (regulate) emotional responses’, whereas the dACC was better situated to orient attention externally for the purpose of regulating ‘sensory or response selection’ (Bush et al., 2000). Interestingly, in our study it was only the fronto-central P3 that was predictive of subsequent error correction, which requires engaging with external stimuli (i.e. the learning-relevant feedback).
By this view, it is possible that TOI-related differences observed at the anterior frontal P3 index the greater affective salience of negative feedback to the entity theorists, perhaps because they were more likely to view this information as a threat to self-perceptions about ability (Molden and Dweck, 2006). In support of this hypothesis, we found a positive correlation between the amplitude of the anterior frontal P3 and performance goals (concerns about performance relative to others) in both TOI groups. This relationship was particularly strong when accuracy also had been inaccurately overestimated (i.e. high-confidence errors). Yet, the negative correlation of the P3 with learning goals exhibited by incremental theorists suggests that emphasizing a positive approach toward difficulty (i.e. challenge) may mitigate the affective impact of negative feedback.
Relevant to our interpretation of the anterior frontal P3, a recent fMRI study found greater rACC activity overall when errors resulted in a monetary loss, as compared with errors that led only to a failure to gain (Taylor et al., 2006). Moreover, in that study, an analysis of individual subjects’ data indicated that a subgroup of subjects showed greater rACC to all errors, suggesting that some individuals subjectively experience any errors as a loss, regardless of how they are framed experimentally. Nonetheless, while these findings provide some converging support for our interpretation of the TOI differences at the anterior frontal P3, future TOI studies that directly assess threat vs challenge patterns of cardiovascular reactivity (Blascovich, 2000), or that utilize the spatial resolution of fMRI, would be instrumental in pursuing this hypothesis further.
Finally, we note that in contrast to the robust effects of TOI observed at the anterior frontal P3, the effects of beliefs on the FRN that preceded it were more ambiguous. Thus far, however, clear results of personality variables only have been observed for the response-locked ERN. Specifically, the response-locked ERN has been shown to be larger in individuals with higher ratings of negative affect (Hajcak et al., 2004; Luu et al., 2000), or general anxiety (Hajcak et al., 2003), but smaller in individuals scoring low on measures of socialization (Dikman and Allen, 2000; Santesso et al. 2005). There was no evidence that the entity and incremental theorists differed on these affective variables, as they were matched on ratings of depression and anxiety, both of which were in normal range. Although it could be argued that concerns about performance relative to others might modulate even an automatic, affective response to the negative feedback, only the anterior frontal P3 was correlated with performance goals. The clearer modulation of the anterior frontal P3 amplitude by TOI and performance goals is more consistent with the view that internalized beliefs influence the affective appraisal of information relative to the self, after initial valence (good–bad) has been assessed.
Following appraisal of negative feedback, TOI differences in the neural response to learning-relevant feedback provide insight into how students engaged reactive control processes toward information that could potentially ameliorate these errors. From 250 to 500 ms, both entity and incremental theorists evidenced a memory-related left inferior temporal negativity, although this activity was enhanced for incremental theorists overall. Furthermore, whereas for entity theorists this activity generally reverted toward baseline, for incremental theorists, it was sustained for an additional 500 to 1000 ms. Thus, these data clearly indicate that TOI also influences how individuals process learning-relevant information.
There is now substantial evidence accruing that negative-going potentials over inferior temporal sites index activation of semantic representations that subsequently enhance episodic memory for that item. First, the broad negative-going inferior temporal waveform that was a robust predictor of subsequent immediate and delayed error correction in Butterfield and Mangels (2003) was specific for words that the subject had rated as ‘familiar.’ Items for which the subjects did not have a pre-existing semantic representation elicited a similar amplitude as familiar items that were subsequently forgotten, regardless of whether they were later retrieved or not. Second, Nessler et al. (2006) found that the extent to which an inferior fronto-temporal negativity was enhanced during semantic retrieval was positively correlated with successful episodic retrieval of those items on a later recognition test. Third, a recent study found that this waveform could be enhanced simply when attention was successfully biased toward conceptual processing of a verbal stimulus, rather than toward its location (Stern and Mangels, 2006a). Finally, it is worth noting that much of this evidence comes from recent studies using high-density montages and an average reference, most likely because the traditional linked mastoid reference used in earlier Dm studies effectively minimized or eliminated activity at these sites.
Whereas activity at inferior temporal sites from 200 to 400 ms may be sufficient for conceptual fluency (Mangels et al., 2001; Pickering and Schweinberger, 2003; Summerfield and Mangels, 2005), recent studies suggest that it may be the duration over which conceptual representations are activated (perhaps by working memory processes) that best predicts later recollection or retrieval with associated contextual information (Mangels et al., 2001; Summerfield and Mangels, 2005; Ranganath et al., 2005). Thus, although entity theorists might have processed the learning-relevant feedback at a lexical and/or item-specific conceptual level to some extent, they may have been disadvantaged on the retest if they were less likely to sustain attention to the types of associative conceptual processes that would be especially valuable for integrating the question and answer.
Interestingly, sustained processing over other regions, including right frontal and occipital regions, also was predictive of subsequent memory, but did not differ between TOI groups. It is possible that the right frontal sustained positivity indexed control processes involved in maintaining attention to the stimulus (Stern and Mangels, 2006b), but that for entity theorists this attention was directed more toward perceptual features of the stimulus, indexed by sustained negativity over occipital sites (Takashima et al., 2005). For incremental theorists, it was directed toward both perceptual and conceptual processing.
The findings from the present study are consistent with the view that entity and incremental theorists differ in how they appraise performance-relevant information and attend to learning-relevant information. To the extent that entity theorists may have viewed negative feedback as a threat to self-perceptions about ability, rather than as a challenge to improve, they may have engaged less effort in ‘deep,’ semantic processing of the learning-relevant feedback, ultimately compromising their ability to correct as many errors on the subsequent retest. Thus, these findings complement a recent longitudinal study in which a positive relationship between learning goals and final course grade was mediated by self-reported deeper processing of course material; conversely, performance goals were negatively correlated with deeper processing and associated with poorer course outcome (Grant and Dweck, 2003). Nonetheless, whereas self-reports provide introspective insight into task-general strategies, the ERPs used in the present study provided covert measurement of how beliefs influenced attention on a moment-to-moment basis, providing support for a neurocognitive model of the mechanism underlying a relationship between beliefs about ability and achievement success. This model can serve as a basis for future work that seeks to foster learning in vulnerable students.
The direction of causality between students’ beliefs and their neural responses following negative feedback cannot be fully specified, as this was a quasi-experimental design examining effects in two pre-specified groups. Indeed, the manner in which an individual naturally responds to negative feedback may play a role in forming some of the individual's beliefs and goals, even if these beliefs and goals may then go on to be reinforced further via top–down control of subsequent experiences. To demonstrate that activation of a particular belief or goal can actually induce a particular way of processing information, Dweck and colleagues have experimentally manipulated TOI (e.g., via a scientific essay promoting a fixed vs malleable view : Dweck and Leggett, 1988; Hong et al., 1999). These studies typically find that ‘induced theories’ bias preferences in a manner consistent with findings from individual difference studies (Molden and Dweck, 2006). Thus, although one can never rule out the idea that pre-existing tendencies foster the adoption of consistent ideas, these studies support the view that theories themselves can influence patterns of information- and experience-seeking.
In conclusion, top–down control has been a useful construct for understanding the basis of selective attention in both cognitive (Desimone and Duncan, 1995; Kastner and Ungerleider, 2001; Miller and Cohen, 2001) and emotional domains (Mather and Carstensen, 2005; Ochsner and Gross, 2005). Here, we consider how conflict and control processes, guided by individual differences in internalized beliefs and goals, influence the ability to rebound from failure. Thus, these findings add to a growing literature that aims to integrate social, cognitive and neuroscience data by considering how personality variables engage top–down control processes to modulate bottom–up stimulus processing (Amodio et al., 2004; Mathews et al., 2004; Ray et al., 2005).
Acknowledgments
This research was supported by a Cognition and Student Learning (CASL) grant from the Institute for Educational Sciences (IES) to J.A.M., C.S.D. and C.G., as well as NIH grant R21MH066129 to J.A.M. We are grateful to the many students who dedicated their time and support to running subjects and analyzing data for this project, including Aaron Fischer, Mariely Hernandez, Jennifer Mesrie, Adrienne Moll, Tara Patterson, Alison Schulman, Michael Summers and Lynne Taylor, as well as to Matt Greene who provided assistance with programming. Aspects of this data were presented at the 2005 meeting of the Cognitive Neuroscience Society (CNS), New York, NY and the 2005 meeting of the Association for Psychological Science (APS), Los Angeles, CA, USA.
REFERENCES
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Hartley SL, Covert AE. Neural signals for the detection of unintentional race bias. Psychological Science. 2004;15:88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- Baudena P, Halgren E, Hiet G, Clarke JM. Intracerebral potentials to rare targets and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalography and Clinical Neurophysiology. 1995;94:251–64. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Blascovich J. Using physiological indexes of psychological processes in social psychological research. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. New York: Cambridge University Press; 2000. pp. 117–37. [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DE. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22:530–40. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Variation in Working Memory. Oxford University Press; Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butterfield B, Mangels JA. Neural correlates of error detection and correction in a semantic retrieval task. Cognitive Brain Research. 2003;17:793–817. doi: 10.1016/s0926-6410(03)00203-9. [DOI] [PubMed] [Google Scholar]
- Butterfield B, Metcalfe J. Errors committed with high confidence are hypercorrected. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1491–94. doi: 10.1037//0278-7393.27.6.1491. [DOI] [PubMed] [Google Scholar]
- Chiu CY, Hong YY, Dweck CS. Lay dispositionism and implicit theories of personality. Journal of Personality and Social Psychology. 1997;73:19–30. doi: 10.1037//0022-3514.73.1.19. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Review Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance, and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–43. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. Journal of Experimental Psychology: General. 1996;125:159–80. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. Journal of Cognitive Neuroscience. 2006;18:766–80. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, et al. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000;123:927–39. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJ. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Dweck CS, Leggett EL. A social-cognitive approach to motivation and personality. Psychological Review. 1988;95:256–73. [Google Scholar]
- Dweck CS, Mangels JA, Good C. Motivational effects of attention, cognition and performance. In: Dai DY, Sternberg RJ, editors. Motivation, Emotion, and Cognition: Integrated Perspectives on Intellectual Functioning. Mahwah, NJ: Erlbraum; 2004. pp. 41–55. [Google Scholar]
- Dweck CS, Sorich L. Mastery-oriented thinking. In: Synder CR, editor. Coping. New York: Oxford University Press; 1999. pp. 232–51. [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24:539–47. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Elliot A, Dweck CS. Handbook of Competence and Motivation. New York: Guilford; 2005. [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson RJ. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy and Research Techniques. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Grant H, Dweck CS. Clarifying achievement goals and their impact. Journal of Personality and Social Psychology. 2003;85:541–53. doi: 10.1037/0022-3514.85.3.541. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–97. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2005 doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hong Y, Chiu C, Dweck CS. Implicit theories and evaluative encoding of information. Journal of Experimental Social Psychology. 1997;33:296–323. [Google Scholar]
- Hong Y, Chiu C, Dweck CS, Lin DM-S, Wan W. Implicit theories, attributions, and coping: a meaning system approach. Journal of Personality and Social Psychology. 1999;77:588–99. [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–76. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neurophysiology. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Picton TW, Craik FIM. Attention and successful episodic encoding: an event-related potential study. Cognitive Brain Research. 2001;11:77–95. doi: 10.1016/s0926-6410(00)00066-5. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mathews A, Yiend J, Lawrence AD. Individual differences in the modulation of fear-related brain activation by attentional control. Journal of Cognitive Neuroscience. 2004;16:1683–94. doi: 10.1162/0898929042947810. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biological Psychology. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–9. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related potentials following incorrect feedback in a time-estimation task: evidence for a ‘generic’ neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–98. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Molden DC, Dweck CS. Finding ‘meaning’ in psychology: a lay theories approach to self-regulation, social perception, and social development. American Psychologist. 2006;61:192–203. doi: 10.1037/0003-066X.61.3.192. [DOI] [PubMed] [Google Scholar]
- Mueller CM, Dweck CS. Praise for intelligence can undermine children. Journal of Personality and Social Psychology. 1998;75:33–52. doi: 10.1037//0022-3514.75.1.33. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Jr, Bersick M, Friedman D. On why the elderly have normal semantic retrieval but deficient episodic encoding: A study of left inferior frontal ERP activity. Neuroimage. 2006;30:299–312. doi: 10.1016/j.neuroimage.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neuroscience & Biobehavioral Reviews. 2004;28:441–8. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–60. [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–3. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. Journal of Neuroscience. 1995;15:1090–8. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pickering EC, Schweinberger SR. N200, N250r, and N400 event-related brain potentials reveal three loci of repetition priming for familiar names. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:1298–1311. doi: 10.1037/0278-7393.29.6.1298. [DOI] [PubMed] [Google Scholar]
- Posner MI, Abdullaev YG, McCandliss BD, Sereno SC. Neuroanatomy, circuitry and plasticity of word reading. NeuroReport. 1999;10:R12–23. [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. Journal of Cognitive Neuroscience. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive appraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–64. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Sorich-Blackwell L. Doctoral Dissertation. Columbia University; 2001. Theories of Intelligence Predict Motivation and Achievement Across the Adolescent Transition. [Google Scholar]
- Stern E, Mangels JA. Neural correlates of top-down attentional biasing during the spatial Stroop task: an event-related potential (ERP) study. Journal of Cognitive Neuroscience. 2006a doi: 10.1162/jocn.2006.18.6.1004. (in press) [DOI] [PubMed] [Google Scholar]
- Stern ER, Mangels JA. An electrophysiological investigation of preparatory attentional control in a spatial Stroop task. Journal of Cognitive Neuroscience. 2006b;18:1004–17. doi: 10.1162/jocn.2006.18.6.1004. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Mangels JA. Coherent theta-band EEG activity predicts item-context binding during encoding. NeuroImage. 2005;24:692–703. doi: 10.1016/j.neuroimage.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Takashima A, Jensen O, Oostenveld R, Maris E, van de Coevering M, Fernandez G. Successful declarative memory formation is associated with ongoing activity during encoding in a distributed neocortical network related to working memory: a magnetoencephalography study. Neuroscience. 2005;139:291–7. doi: 10.1016/j.neuroscience.2005.05.067. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, et al. Medial frontal cortex activity and loss-related responses to errors. Journal of Neuroscience. 2006;26:4063–70. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia. 2003;41:1122–35. doi: 10.1016/s0028-3932(02)00297-x. [DOI] [PubMed] [Google Scholar]
- West R, Moore K. Adjustments of cognitive control in younger and older adults. Cortex. 2005;41:570–81. doi: 10.1016/s0010-9452(08)70197-7. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences USA. 2002;99:11447–51. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]