Abstract
Esophageal Barrett's adenocarcinoma (BA) develops through a multistage process, which is associated with the transcriptional silencing of tumor-suppressor genes by promoter CpG island hypermethylation. In this study, we explored the promoter hypermethylation and protein expression of proapoptotic deathassociated protein kinase (DAPK) during the multistep Barrett's carcinogenesis cascade. Early BA and paired samples of premalignant lesions of 61 patients were analyzed by methylation-specific polymerase chain reaction and immunohistochemistry. For the association of clinicopathological markers and protein expression, an immunohistochemical tissue microarray analysis of 66 additional BAs of advanced tumor stages was performed. Hypermethylation of DAPK promoter was detected in 20% of normal mucosa, 50% of Barrett's metaplasia, 53% of dysplasia, and 60% of adenocarcinomas, and resulted in a marked decrease in DAPK protein expression (P < .01). The loss of DAPK protein was significantly associated with advanced depth of tumor invasion and advanced tumor stages (P < .001). Moreover, the severity of reflux esophagitis correlated significantly with the hypermethylation rate of the DAPK promoter (P < .003). Thus, we consider DAPK inactivation by promoter hypermethylation as an early event in Barrett's carcinogenesis and suggest that a decreased protein expression of DAPK likely plays a role in the development and progression of BA.
Keywords: Barrett's adenocarcinoma, Barrett's metaplasia, DAPK, reflux esophagitis, inflammation
Introduction
The incidence of Barrett's adenocarcinoma (BA) has increased rapidly in the western world over the past three decades [1,2]. Barrett's carcinogenesis is a multistep process composed of genetic and epigenetic alterations in mismatch repair genes, tumor-suppressor genes, cell cycle regulator genes, protooncogenes, tissue invasion-related genes, or genes essential for cell-cell adhesion [3,4]. Progressive accumulation of gene alterations is postulated for the transition of normal squamous epithelium to metaplastic specialized columnar epithelium [Barrett's metaplasia (BE)] and subsequently through Barrett's dysplasia (DYS) to BA [2]. BE represents the most serious histologic consequence of chronic gastroesophageal reflux. It develops in 5% to 10% of patients with gastroesophageal reflux disease [5] and shows an incidence of malignant transformation between 0.2% and 2% each year [3]. Prolonged chronic inflammation in gastric reflux may contribute to Barrett's carcinogenesis through mechanisms of repetitive tissue damage and regeneration in the presence of reactive phagocyte-derived oxygen and nitrogen species. Locally produced cytokines and acids in the refluxate create a microenvironment that sets the scene for metaplastic and neoplastic transformations of the esophageal epithelium, mainly by directly affecting metaplastic stem cells [2]. It has been postulated that gastric reflux has epigenetic, rather than genotoxic, effects on esophageal transdifferentiation [6]. Indeed, hypermethylation of various genes is a very common event in BA and occurs as early as metaplasia [3,7–13], which supports the precancerous nature of Barrett's specialized intestinal metaplasia.
Death-associated protein kinase (DAPK) is an actinassociated calcium/calmodulin-dependent enzyme with serine/threonine kinase activity [14–16]. DAPK is involved in tumor necrosis factor-α and Fas-induced apoptosis, and has been demonstrated to be an essential mediator in IFN-γ-induced programmed cell death [17]. Furthermore, its proapoptotic function was found to be associated with p19ARF/p53-mediated apoptosis in the rodent model [18]. As disruption of processes involved in programmed cell death is a common feature of human cancers, it is significant that inactivation of DAPK by hypermethylation in the promoter CpG region has been described in a variety of human tumors, including gastrointestinal malignancies, such as carcinomas of the colorectum [19–22], anus [23], esophagus, esophagogastric junction, and stomach [24–28]. DAPK suppresses tumor growth and metastasis by increasing the occurrence of apoptosis in vivo [29]. Loss of DAPK expression is associated with poor overall survival rates of cancer patients (e.g., in primary biliary tract carcinoma and non-small cell lung carcinoma) [30,31].
Because alterations of proapoptotic genes might cause instability in the balance of cell turnover during the chronic inflammatory processes of reflux esophagitis and BE, epigenetic silencing of DAPK might be involved in the early stages of Barrett's carcinogenesis. Although epigenetic changes of the DAPK promoter have been recognized in BA [25,26], data regarding epigenetic abnormalities in its premalignant lesions are limited.
To determine the impact of hypermethylation during multistep Barrett's carcinogenesis, we analyzed the promoter hypermethylation and protein expression of DAPK in nontumorous esophageal squamous mucosa (NT), Barrett's intestinal metaplasia, dysplasia of Barrett's mucosa, and adenocarcinoma.
Materials and Methods
Patients and Tissue Samples
Specimens of non-neoplastic and neoplastic esophageal tissues analyzed in this retrospective study were obtained from two principal sources. First, formalin-fixed paraffin-embedded specimens consisting of 35 BA, 28 BE, 14 lowgrade dysplasia, 7 high-grade dysplasia, and 20 samples of nondysplastic esophageal squamous mucosa (NT) were analyzed by methylation-specific polymerase chain reaction (MSP) and immunohistochemistry. The specimens were obtained from mucosal and surgical resections from 61 patients at the archives of the Institutes of Pathology, Bayreuth and Magdeburg, Germany. This group had an average age of 63.7 years (range, 43–79 years) and consisted of 53 males and 8 females. Twenty-six of 35 BA (74%) represented mucosal carcinoma or carcinoma of early tumor stages (Table 1). Second, formalin-fixed paraffin-embedded specimens of BA and paired noncancerous esophageal mucosa of 66 patients who had undergone surgery at the University of Virginia were submitted to immunohistochemical tissue microarray analysis. This study group comprised 57 male and 9 female patients ranging in age from 51 to 79 years (median, 64.5 years) and included carcinomas of advanced tumor stages (Table 2).
Table 1.
PCR-Specific Promoter Methylation Pattern of DAPK according to Histopathological Diagnosis and Clinical Characteristics.
| Samples | Sex/Age in Years | pTNM | Grading | Reflux | Methylation Pattern | ||||||
| NT | BE | LDys | HDys | BA | |||||||
| B01 | M/63 | 2 | Un | ||||||||
| B02 | M/66 | 1 | Un | ||||||||
| B03 | M/43 | 1 | Un | Un | |||||||
| B04 | M/71 | T1N0Mx | 1 | 1 | Un | Un | |||||
| B05 | M/70 | T1N0Mx | 3 | 3 | Un | Un | |||||
| B06 | F/69 | T1N0Mx | 1 | 2 | Un | ||||||
| B07 | M/73 | T1N0Mx | 2 | 1 | Un | ||||||
| B08 | M/72 | T1N0Mx | 2 | 1 | Un | Un | |||||
| B09 | M/67 | T1N0Mx | 1 | 1 | Un | Un | |||||
| B10 | M/77 | 1 | Un | Un | |||||||
| B11 | M/78 | T1N0Mx | 1 | 2 | Un | Un | |||||
| B12 | M/58 | T1N0Mx | 1 | 2 | Un | Un | |||||
| B13 | M/49 | T1N0Mx | 1 | 2 | Un | Un | |||||
| B14 | M/59 | T1N0Mx | 1 | 1 | Un | Un | |||||
| B15 | M/72 | T1N0Mx | 2 | 1 | Un | Un | Un | ||||
| B16 | M/75 | T1N0Mx | 1 | 1 | Un | Un | |||||
| B17 | F/70 | T1N0Mx | 1 | 2 | Un | ||||||
| B18 | F/70 | T1N0Mx | 1 | 2 | Un | ||||||
| B19 | M/61 | T1N0Mx | 1 | 1 | Un | ||||||
| B20 | M/65 | T1N0Mx | 2 | 0 | Un | ||||||
| B21 | M/69 | T1N0Mx | 1 | 1 | Un | ||||||
| B22 | M/69 | T1N0Mx | 1 | 1 | Un | ||||||
| B23 | M/64 | T1N1M1 | 3 | 0 | Un | ||||||
| B24 | M/62 | T3N1M1 | 3 | 0 | Un | ||||||
| B25 | M/64 | T3N1M0 | 2 | 0 | Un | ||||||
| B26 | M/60 | T3N1M1 | 3 | 0 | Un | ||||||
| B27 | F/72 | 1 | Met | ||||||||
| B28 | M/71 | T4N1M0 | 3 | 3 | Met | ||||||
| B29 | M/46 | 3 | Met | ||||||||
| B30 | F/50 | 3 | Un | Met | |||||||
| B31 | M/74 | T1N0Mx | 1 | 1 | Un | Met | |||||
| B32 | M/50 | 1 | Met | ||||||||
| B33 | F/54 | 3 | Met | ||||||||
| B34 | M/67 | T1N0Mx | 2 | 2 | Met | ||||||
| B35 | M/62 | 1 | Un | Met | Met | ||||||
| B36 | M/64 | T1N0Mx | 1 | 1 | Un | Met | Met | ||||
| B37 | M/71 | T4N1M0 | 3 | 3 | Un | Met | Met | Met | |||
| B38 | M/60 | 1 | Met | ||||||||
| B39 | M/64 | 2 | Met | ||||||||
| B40 | M/49 | T1N0Mx | 1 | 2 | Met | ||||||
| B41 | M/66 | T2N1M0 | 3 | 2 | Met | ||||||
| B42 | M/69 | T2N0M0 | 2 | 2 | Met | ||||||
| B43 | M/73 | T1N0Mx | 1 | 2 | Met | ||||||
| B44 | F/69 | T1N0Mx | 2 | 3 | Met | ||||||
| B45 | M/57 | T1N0Mx | 3 | 3 | Met | ||||||
| B46 | M/69 | T1N0M0 | 2 | 3 | Met | ||||||
| B47 | M/65 | T3N1M0 | 3 | 3 | Met | ||||||
| B48 | M/79 | T1N0Mx | 1 | 2 | Un | Met | |||||
| B49 | M/49 | T1N0Mx | 1 | 2 | Un | Met | |||||
| B50 | M/68 | T1N0M0 | 2 | 2 | Un | Met | |||||
| B51 | M/71 | T1N0Mx | 2 | 3 | Un | Un | Met | ||||
| B52 | M/70 | T1N0Mx | 3 | 3 | Un | Met | Met | ||||
| B53 | M/51 | 2 | Un | Un | Met | ||||||
| B54 | M/51 | T1N0M0 | 1 | 2 | Un | Un | Met | Met | |||
| B55 | M/59 | T1N0M0 | 1 | 3 | Met | Met | |||||
| B56 | F/65 | T3N0M1 | 2 | 3 | Met | Met | |||||
| B57 | M/60 | 3 | Met | Met | |||||||
| B58 | M/48 | T1N0Mx | 1 | 2 | Met | Met | Met | ||||
| B59 | M/75 | T1N0M0 | 1 | 3 | Met | Met | Met | ||||
| B60 | M/50 | T1N0Mx | 1 | 3 | Met | Met | |||||
| B61 | F/53 | T1N0Mx | 1 | 3 | Met | Met | |||||
Methylation analysis of DAPK promoter in esophageal tissues from 61 patients, including nondysplastic esophageal squamous mucosa (NT), BE, low-grade and high-grade dysplasias of Barrett's mucosa (LDys and HDys, respectively), and BA.
pTNM = postsurgical histopathological tumor classification; reflux = severity of gastroesophageal reflux obtained by histologic criteria; Un = unmethylated samples; Met = methylated samples.
Table 2.
Analysis of Immunohistochemical DAPK Protein Expression and Clinicopathological Features of Advanced BAs.
| IRS of DAPK Protein Expression | P | |||
| 0–3 | 4–8 | 9–12 | ||
| Age in years (median ± 8.89) | 64.3 ± 6.53 | 63.3 ± 10.55 | 62.7 ± 6.65 | .947* |
| Sex | ||||
| Male | 14 | 30 | 13 | .296† |
| Female | 1 | 5 | 0 | |
| Tumor differentiation | ||||
| Mild | 0 | 5 | 2 | .127† |
| Moderate | 2 | 14 | 5 | |
| Poor | 13 | 17 | 8 | |
| Depth of invasion | ||||
| pT1, pT2 | 0 | 20 | 13 | .000† |
| pT3, pT4 | 15 | 16 | 2 | |
| Lymph node metastasis | ||||
| Negative | 10 | 30 | 14 | .157† |
| Positive | 5 | 6 | 1 | |
| Lymph invasion | ||||
| Negative | 4 | 17 | 10 | .090† |
| Positive | 11 | 19 | 35 | |
| Metastasis | ||||
| Negative | 11 | 34 | 14 | .071† |
| Positive | 4 | 2 | 1 | |
| Stage | ||||
| I, II | 3 | 26 | 14 | .001† |
| III, IV | 12 | 10 | 1 | |
Correlation of clinicopathological markers and immunohistochemical protein expression of DAPK in a tissue microarray of 66 advanced BAs.
IRS = immunoreactive score, merged into three groups for statistical analyses (0–3, 4–8, and 9–12 points).
One-way analysis ANOVA.
Chi-square test.
Histopathological diagnosis was verified by examining hematoxylin/eosin-stained slides without any knowledge of clinical data (D.K., M.S., and M.V.). Grading of dysplasia, as well as the staging and grading of adenocarcinomas, was performed according to the recent guidelines of the International Union Against Cancer (UICC) Tumor-Node-Metastasis (TNM) classification system [32]. The severity of reflux esophagitis was estimated according to Vieth et al. [33]. The study has been approved by the locally appointed ethics committee and is in accordance with the guidelines of the Declaration of Helsinki for biomedical research. Informed consent was obtained from all patients included in this study.
Tissue Microarray
Representative regions of carcinoma and paired normal esophageal mucosa were selected from hematoxylin/eosin-stained slices for inclusion in a tissue array. Tissue cores with a diameter of 0.6 mm were retrieved from selected regions of donor blocks and punched to a recipient block using a manual tissue array instrument (Beecher Instruments, Silver Spring, MD). Samples were punched in triplicate. The resulting tumor tissue array was used for immunohistochemistry analysis.
DNA Preparation and MSP
After identifying and marking the lesions of interest on hematoxylin/eosin-stained sections (D.K. and M.V.), mirrorimaged areas on 10-µm-thick paraffin-embedded tissue sections were separated macroscopically or, if necessary, by laser capture microdissection (PALM, Bernried, Germany). DNA was prepared using the NucleoSpin Tissue Kit (Macherey and Nagel, Dueren, Germany).
For detection of promoter methylation status, MSP was performed as described recently [22]. Briefly, extracted DNA was subjected to sodium bisulfite modification using the CpGenome DNA modification kit (Intergen, Purchase, NY). Modified DNA was subjected to MSP using specific primers for methylated sequences (sense 5′-GGATAGTCGGATCGAGTTAACGTC-3′ and antisense 5′-CCCTCCCAAACGCCGA-3′) and for unmethylated sequences (sense 5′-GGAGGATAGTTGGATTGAGTTAATGTT-3′ and antisense 5′-CAAATCCCTCCCAAACACCAA-3′), which generates polymerase chain reaction (PCR) products of 114 and 116 bp, respectively. The total 25 µl of PCR mix contained 2 µl of bisulfite-modified DNA, 1x PCR buffer, 3 mM MgCl2, 12.5 pmol of each primer, 160 µM dNTPs, and 0.5 U of Hot-Goldstar Taq polymerase (Eurogentec, Seraing, Belgium). PCR conditions were as follows: 95°C for 10 minutes, 35 cycles of 95°C for 1 minute, annealing with 60°C for 1 minute and 72°C for 1 minute, followed by a final extension step at 72°C for 10 minutes. Methylated standard DNA (Intergen) was used as a positive control for methylation, and placenta DNA was used as a negative control. PCR products were electrophoresed on polyacrylamide gels and visualized by silver staining.
Immunohistochemistry
DAPK protein expression was immunohistochemically analyzed for BE, DYS, BA, and normal esophageal squamous mucosa of all cases investigated by MSP, as well as for the tissue microarray of BA and corresponding NT. For identifying DAPK-expressing cells, five methylated and five unmethylated adenocarcinoma samples were additionally investigated using CD68 antibody. CD68 is a 110-kDa transmembrane glycoprotein associated with lysosomes and is therefore used as a common marker of cells of the monocyte-macrophage lineage, including tissue macrophages. Formalin-fixed paraffin-embedded serial tissue sections (3 µm thick) were dewaxed in xylol and rehydrated by descending concentrations of ethanol. Slices were subjected to antigen retrieval using microwave heating (20 minutes, 450 W, 10 mM EDTA pH 8.0), followed by incubation with specific primary antibodies recognizing DAPK (rabbit polyclonal antibody 44–672, dilution 1:200; BioSource International, Inc., Camarillo, CA) and CD68 (mouse monoclonal antibody M0814, dilution 1:400; DAKO, Hamburg, Germany), respectively, at 37°C for 30 minutes. After phosphate-buffered saline (PBS) washing, incubation with biotinylated secondary antibody was conducted (anti-mouse IgG, anti-rabbit IgG, dilution 1:200; Vector Laboratories, Burlingame, CA) at room temperature for 30 minutes. Detection of the bound antibody was accomplished using the avidin-biotin complex method (Vectastain Elite ABC Kit; Vector Laboratories). A 0.1% solution of 3,3′-diaminobenzidine (5 minutes; Sigma, St. Louis) was used as chromogen. Specificity of immunostaining was checked by omitting single steps in the immunohistochemical protocol and by replacing the primary antibody with nonimmune serum. Positive tissue controls that were stained in parallel with test slides included sections of normal colon mucosa.
Triple Immunofluorescence Analysis
Double labeling of DAPK/CD68 was performed in five representative cases for each methylated and unmethylated adenocarcinoma. Tissue sections were deparaffinized in xylene and dehydrated in ethanol, followed by standard antigen retrieval (microwave, 1 mM EDTA). Nonspecific reactions were blocked with 10% horse serum/PBS. We used specific antibodies as primary antibodies recognizing DAPK and CD68, as described above. Incubation was performed separately for each antibody at 4°C for 16 hours. After washing with PBS and Triton, primary antibodies were detected with FITCconjugated anti-rabbit antibody and Texas red-conjugated anti-mouse antibody, respectively (dilution 1:100, each for 2 hours, at 37°C; Vector Laboratories). Counterstaining and mounting were performed in Vectashield mounting medium with DAPI (Vector Laboratories). Analysis was performed using a fluorescence microscope (Leica DMRE7; Leica Wetzlar, Germany) equipped with a charge-coupled device camera (SPOT RT; Diagnostic Instruments, Burroughs, MI). Separate images were taken in corresponding channels and merged using the SPOT Advanced software (Diagnostic Instruments). Image acquisition of controls and data processing were performed under the same conditions.
Semiquantitative Assessment of Immunohistochemical Results
For DAPK protein expression, staining intensity (SI; 1 = weak, 2 = moderate, 3 = strong) and the percentage of positive cells (PC; 1 = < 10%, 2 = 10–50%, 3 = 51–80%, 4 = > 80%) were semiquantitatively assessed, resulting in an immunoreactive score (IRS = SI x PC) with a maximum of 12 points. The average immunoreactive score was finally estimated for each group. Scoring was performed for the tumor epithelium and stromal macrophages. Immunoreactivity for CD68 was scored as positive or negative, depending on the presence or the absence of cytoplasmatic immunoreactivity. To determine the index of DAPK-expressing macrophages, DAPK-labeled cells per CD68+ stromal cells were counted in 20 high-power fields of tumor adjacent stroma. Samples were examined by two independent pathologists (D.K. and M.V.) who were blinded to other data.
Statistical Analysis
For group comparison, samples with low-grade and highgrade dysplasias were pooled in one group containing all cases with dysplasia (DYS). For statistical analysis, patients were merged into three groups, depending on the immunoreactive score of DAPK estimated by immunohistochemistry (IRS of 0–3, 4–8, and 9–12 points, respectively). For clinicopathological correlation, we merged the TNM and pT categories into early stage (≤ stage II; ≤ pT2) and late stage (≥ stage III; > pT3), and the association of protein expression with TNM stage was investigated using chi-square tests. Correlation between variables was estimated using Fisher's exact test. The linear association between the methylation of DAPK promoter and the average immunoreactive score for DAPK was determined by Pearson's coefficient. For descriptive data analysis and all statistical tests, SPSS software 11.5 for Windows (Chicago, IL) was used. A two-sided value of P ≤ .05 was considered statistically significant.
Results
DAPK Promoter Methylation Presents as an Early Event in Barrett's Carcinogenesis
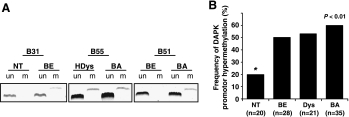
Figure 1A shows representative examples of MSP analysis. Figure 1B summarizes the promoter hypermethylation frequency of DAPK in different groups of esophageal tissue samples. DAPK was hypermethylated in both non-neoplastic and neoplastic samples, with a significant increase in methylation frequency from nondysplastic esophageal squamous cell mucosa to BE. BE without evidence of dysplasia/neoplasia exhibited methylated DAPK in 14 of 28 (50%) cases. In corresponding esophageal squamous cell epithelium, hypermethylation was detected in 4 of 20 cases (20%). Furthermore, MSP of DAPK revealed that 21 of 35 (60%) BA and 11 of 21 (53%) Barrett's mucosa with low-grade and high-grade DYS showed aberrant methylation at the CpG island. The difference between the methylation frequency of normal-appearing squamous mucosa and that of BE, DYS, or BA, respectively, proved to be statistically significant (P < .01). Furthermore, the transition from BE to IN or BA was associated with a further increase in DAPK gene methylation but did not reach statistical significance. An unmethylated PCR product and a methylated PCR product were detected in all methylated samples suggesting monoallelic DAPK promoter hypermethylation. Biallelic methylation was never observed.
Figure 1.
Results of MSP analysis of the DAPK promoter. (A) Representative MSP results of the DAPK promoter of three patients (B31, B55, and B51), including samples of nontumorous squamous epithelium (NT), Barrett's mucosa (BE), high-grade dysplasia (HDys), and BA. Lane un: unmethylated PCR product. Lane m: methylated PCR product. (B) Frequency of hypermethylation of the DAPK promoter in nontumorous, premalignant, and malignant esophageal tissues. Each bar illustrates the portion of samples of a certain lesion classified as “hypermethylated.”.
Hypermethylation of DAPK Is Associated with Severity of Reflux Esophagitis
Statistical analysis of the correlation of methylation status and clinical and demographic characteristics revealed no significant correlations for the frequency of DAPK hypermethylation and patient age or gender and tumor grading [P = .16, P = .29, and P = .13, respectively; one-way analysis of variance (ANOVA) and Fisher's exact test]. Because 26 of 35 carcinomas investigated by MSP were early carcinomas, a correlation of methylation frequency with tumor stage could not be established. By contrast, the severity of reflux esophagitis, scored by histologic criteria, was significantly associated with DAPK promoter hypermethylation (P < .003; Fisher's exact test) (Table 1).
DAPK Promoter Methylation Correlates with Loss of Protein Expression
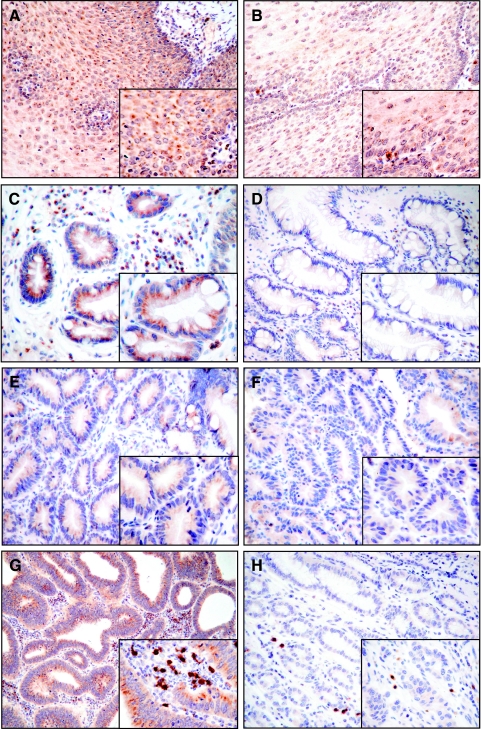
Examples of immunohistochemical protein expression of DAPK in esophageal tissue samples are given in Figures 2 and 3. In cases exhibiting no methylation, cytoplasmatic staining with moderate to strong intensity of immunoreactivity was observed. As expected, we found a significant correlation between DAPK methylation and decrease in immunoreactivity within the non-neoplastic, metaplastic, and dysplastic epithelia (P < .01) (Figure 2). In the group of methylated lesions, the epithelium of each of the samples with BE and low-grade DYS, as well as three cases of BA, appeared to be completely immunonegative. Heterogeneous DAPK expression in dysplastic and neoplastic tissues was not observed.
Figure 2.
Immunohistochemical analysis for the protein expression of DAPK in methylated and unmethylated esophageal tissues representative of non-neoplastic, metaplastic, and neoplastic alterations. Methylation of DAPK promoter resulted in decreased and partial loss of DAPK protein expression (original magnification, x10 and x40). Nontumorous esophageal squamous cell epithelium: unmethylated (A), methylated (B); BE: unmethylated (C), methylated (D); dysplasia of Barrett's mucosa: unmethylated (E), methylated (F); BA: unmethylated (G), methylated (H).
Figure 3.
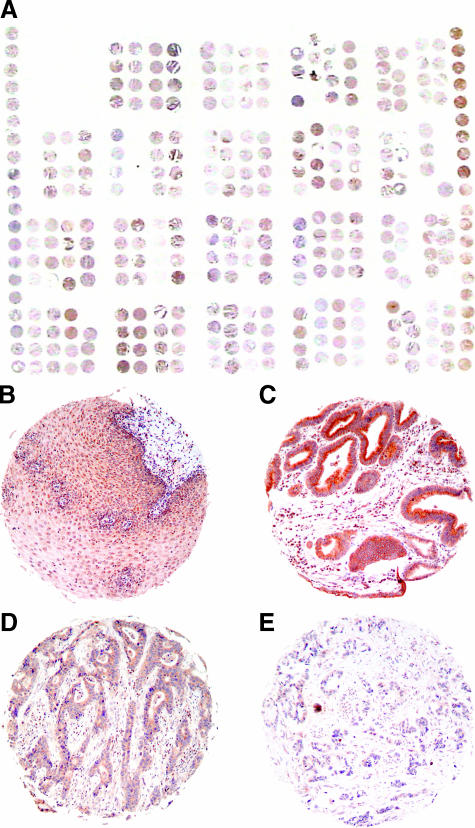
Tissue microarray of BA. Immunohistochemical analysis of DAPK protein expression was conducted on a tissue microarray consisting of 66 BA and corresponding esophageal squamous mucosa specimens (original magnification, x1 and x20). Overview of the tissue microarray slide (A). DAPK-positive sample of non-neoplastic squamous mucosa (B). Examples of tissue microarray core with well-differentiated carcinoma and strong DAPK protein expression (C); moderately differentiated carcinoma and moderate protein expression (D); and poorly differentiated carcinoma with weak protein expression (E).
Loss of DAPK Protein Expression Correlates with Progressively Advanced Stages of Disease
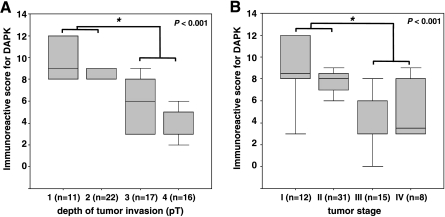
Because the group of BA investigated by MSP consisted mostly of early carcinomas, data correlated with DAPK protein expression in a tissue microarray of 66 additional BAs with heterogeneous clinicopathological characteristics (Table 2, Figure 3). Statistical analysis revealed a significant loss of protein expression with tumor invasion depth (average IRS: pT1/pT2 = 9.9/8.45 vs pT3/pT4 = 5.7/4.1; P < .001) (Figure 4A). Significant loss of protein expression was observed for early-stage tumors compared to nondysplastic squamous epithelium (average IRS = 9.9. vs 11.3; P < .01). Furthermore, there was a significant lower DAPK protein expression in tumor stages III and IV relative to less advanced tumors in stages I and II (average IRS = 9.33 and 7.7 vs 4.4 and 5.12; P < .001) (Figure 4B). There were no significant correlations of immunohistochemically estimated DAPK protein expression with gender, age, tumor differentiation, and lymph node metastasis (Table 2).
Figure 4.
Correlation between immunohistochemical analysis of DAPK protein expression and clinicopathological data. Immunohistochemical protein expression of DAPK was significantly altered depending on tumor invasiveness (pT) (A) and tumor stage (B). A marked decrease in DAPK protein expression correlates with progressively advanced stages of tumor disease.
Tumor-Associated Macrophages Express DAPK Protein and Are Rarely Detectable in Cases of Adenocarcinoma with DAPK Hypermethylation
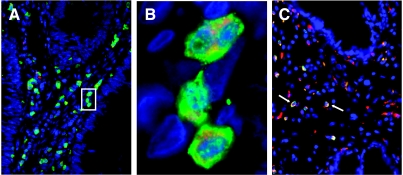
In all unmethylated samples of BE, DYS, and BA, we noted strong immunohistochemical reactivity for a group of inflammatory cells of the surrounding stroma. An immunohistochemical analysis of the DAPK and CD68 protein expressions of serial slices revealed a concordant expression pattern of both proteins. Using triple immunofluorescence, DAPK-expressing stromal cells were identified as macrophages (Figure 5, A and B). Interestingly, DAPK-positive macrophages were accentuated at the tumor invasion front of unmethylated carcinoma, whereas only a minor fraction of the macrophages expressed DAPK in cases with promoter methylation and decreased protein expression (Figure 5C). The differences in the expression pattern of the macrophages between methylated and unmethylated BA proved to be statistically significant (P < .01).
Figure 5.
Triple immunofluorescence of DAPK and CD68. Triple immunofluorescence of macrophages of BA for the visualization of DAPK (FITC)/CD68 (Texas red) (original magnification, x10 and x100). Colocalization of DAPK and CD68 in unmethylated BA proves the expression of DAPK protein in tumor-associated macrophages (A). Enlargement of unmethylated carcinoma shows DAPK clearly confined to the cell membrane, whereas CD68 demonstrates a characteristic cytoplasmatic appearance within macrophages (B). For methylated BA, only a few DAPK-positive macrophages were observed at the tumor invasion front (C).
Discussion
Whereas aberrant methylation of the DAPK promoter has been reported frequently in early preneoplastic lesions of the gastrointestinal tract, such as in the colon, stomach, and anus [19,20,22,23,27,28], studies of DAPK inactivation in esophageal tissue have focused primarily on BA so far [24–26]. Here, we report a study on the promoter methylation status and protein expression of DAPK in matched samples of nondysplastic esophageal squamous epithelium, BE, lowgrade and high-grade DYS, and BA of 127 patients. To our knowledge, this is the most comprehensive methylation study of DAPK ever performed, involving so many distinct histologic stages of disease progression in Barrett's carcinogenesis. Hypermethylation of the promoter region of the DAPK gene occurred in a high percentage of BA (60%) and could be detected in precursor lesions as well (53% for DYS and 50% for BE, respectively). A significant difference in methylation frequency between NT (20%) and BE was established (P < .01). There was a tendency toward the accumulation of DAPK promoter methylation in the Barrett carcinogenesis model. Interestingly, loss of DAPK protein expression was associated with advanced tumor invasiveness and tumor stage.
Eads et al. [10] postulated that DNA hypermethylation is an early epigenetic alteration in Barrett's carcinogenesis. Epigenetic studies of the model of Barrett's carcinogenesis with regard to preneoplastic lesions have been limited to the DNA methylation analysis of a few different genes. Similar to our study, these analyses reported an accumulation of hypermethylation events for genes such as CDKN2A/p16INK4a, GPX, TIMP3, RUNX3, and HPP1 along Barrett's carcinogenesis, whereas the timing and the frequency of hypermethylation varied according to gene [7,8,10,12,13].
The methylation of CpG islands located within the promoter element is generally associated with a decrease in protein expression or a loss of protein expression [34,35]. In fact, in this study, the occurrence of DAPK promoter hypermethylation correlated strongly with a marked decrease in protein expression. Furthermore, for some methylated cases, a complete absence of DAPK protein expression was demonstrated, even though biallelic methylation was never observed by PCR. In these cases, accompanying unmethylated PCR products might be the result of contamination with inflammatory cells and surrounding stroma. This strong association between promoter hypermethylation and loss of the protein expression of DAPK suggests that hypermethylation may be the main inactivation mechanism of this gene in BA and its precursor lesions.
Furthermore, our observation that hypermethylation and loss of DAPK protein expression were already present in BE and DYS and in four cases of NT, and the lack of a significant difference in methylation frequency between BE and DYS or BA suggest that hypermethylation of the DAPK promoter may be an early acquired epigenetic event in the tumorigenic process of BA. DAPK hypermethylation as an early event of gastrointestinal malignancies and its precursor lesions has already been reported for gastric and colorectal carcinomas [20,22,27] and indicates that the impairment of the proapoptotic function of DAPK could be important in malignant transformation.
Two biologic issues have been identified in the carcinogenesis of BA: the balance between cell proliferation and apoptosis in determining the clonal expansion of metaplastic or malignant cells, and the role of altered cell adhesion in remodeling inflamed BE [3]. The development of Barrett's esophagus represents an acquired response to gastroesophageal reflux, thereby providing greater resistance to the effects of chronic mucosal inflammation. DAPK might be involved in the repair process of mucosal damage mediated by gastroesophageal reflux and caused by chronic inflammation. Here, the kinase could function as a caretaker gene by inducing apoptosis, thus eliminating premalignant cells in chronically inflamed and damaged esophageal mucosa. Until now, it has not been shown mechanistically that bile or acid modulates chromatin-modifying enzymes and is capable of inducing hypermethylation of genes. It should be interesting to investigate in a future study whether there is an association between high acid exposure and DAPK inactivation by promoter hypermethylation. We hypothesize that, in Barrett's esophagus, some epithelial cells may acquire DAPK CpG island hypermethylation, which 1) leads to an increased proliferative potential, so that these cells are vulnerable to additional somatic genome alterations; and 2) predisposes them to malignant transformation. The possible protective function of DAPK in the inflammatory process of reflux esophagitis is supported by the results of a quantitative real-time reverse transcription PCR analysis performed by Brabender et al. [25]: This study describes an “on-off” regulation of DAPK during progression to cancer, starting with significant upregulation of gene expression from normal esophagus to BE. In a next step, DAPK is significantly downregulated between BE and BA, which is in line with the results of our study presented here.
Altered expression of DAPK, presumably caused by chronic inflammation in the context of reflux esophagitis, was also observed in tumor-invading macrophages of our BA samples. Interestingly, hypermethylation of the tumor epithelium was strongly associated with a marked loss of DAPK protein expression in macrophages of the tumor invasion front. We have recently reported a similar observation for colorectal carcinomas [22], where DAPK hypermethylation of tumor cells was strongly associated with hypermethylation and a decrease in the FASL expression of macrophages. The methods used in this study cannot satisfactorily answer the question of whether decreased DAPK protein expression in macrophages invading BA is caused by hypermethylation of the macrophages itself or by signaling between tumor cells and macrophages. Further studies are needed to address this issue.
In 4 of 20 cases (20%) of the present study, we demonstrated that CpG island hypermethylation of the DAPK gene occurs as early as the normally appearing esophageal squamous mucosa on histologic investigation. This raises the question as to whether these methylation events represent normal methylation patterns in non-neoplastic tissues or whether they reflect methylation changes that predispose to further progression. However, as in the majority of cases, nondysplastic squamous epithelium was mainly obtained from areas within a few millimeters' distance from metaplastic/neoplastic lesions and thus located in regions affected by reflux. Indeed, three of four cases of NTexhibiting DAPK hypermethylation showed histologic signs of reflux esophagitis. Furthermore, a significant correlation between histologic signs of reflux and increased methylation frequency was confirmed. Therefore, NT tissue with methylation of DAPK rather seems to reflect epigenetic changes caused by gastroesophageal reflux and might be at elevated risk for developing into metaplastic and neoplastic lesions.
A potential criticism of the present analysis is that the majority of carcinomas investigated for methylation status were in a very early tumor stage. Therefore, we performed an immunohistochemical analysis for DAPK protein expression on a tissue microarray of 66 additional cases of advanced BA. Here, we could show a significantly weaker DAPK protein expression in tumor stages III and IV relative to less advanced stage I and II tumors. This implies, based on our observation of a correlation between immunohistochemical expression and MSP analysis, that there might be a general increase in the frequency of CpG island hypermethylation of the DAPK promoter with histopathological and clinical progression of the disease.
This is in line with studies on head, neck, and lung carcinomas that detected a higher frequency of DAPK promoter hypermethylation for more advanced tumor stages, lymph node involvement, and increased tumor size [36,37]. Furthermore, promoter hypermethylation of DAPK appeared to be a significant prognostic factor in primary biliary tract carcinoma [30]. This increased loss of protein expression with advanced tumor invasiveness and tumor stage supports the potential protective function of DAPK against tumor progression and metastasis in Barrett's carcinogenesis.
In conclusion, our data demonstrated that CpG island hypermethylation of DAPK promoter occurs in early-step lesions and revealed a decrease in protein expression along the multistep Barrett's carcinogenesis. Based on the positive association between a gradual increase in DAPK hypermethylation, a decrease in protein expression, and progression of the neoplastic process, we suggest that inhibition of de novo methylation at any stage could be important for the prevention of cancer development and disease progression.
Acknowledgements
The authors thank Michael Vieth (Department of Pathology, Bayreuth, Germany) for assistance with verifying histopathological diagnoses and marking lesions of interest on hematoxylin/eosin-stained sections. The authors are grateful to Simone Staeck, Hiltraud Scharfenort, Nicole Wolf, Nadine Wiest, Claudia Miethke, and Carola Ku"gler for their skillful technical assistance.
Abbreviations
- BA
Barrett's adenocarcinoma
- BE
Barrett's metaplasia
- DAPK
deathassociated protein kinase
- DYS
Barrett's dysplasia
- MSP
methylation-specific polymerase chain reaction
- NT
nontumorous esophageal squamous mucosa
Footnotes
This work was supported, in part, by financial aid to the “Spitzenbonusprojekt” of the Medical Faculty, Otto-von-Guericke University, Magdeburg, Germany (institutional grant to the Department of Pathology, Magdeburg; M.V. and R.S.S.) and a grant award from the National Cancer Institute CA106176 (W. El-Rifai).
References
- 1.Devesa SS, Blot WJ, Fraumeni JF., Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 2.Jankowski JA, Wright NA, Meltzer SJ, Triadafilopoulos G, Geboes K, Casson AG, Kerr D, Young LS. Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol. 1999;154:965–973. doi: 10.1016/S0002-9440(10)65346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett's metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 4.Tannapfel A. Molecular findings in Barrett's epithelium. Dig Dis. 2004;22:126–133. doi: 10.1159/000080311. (review) [DOI] [PubMed] [Google Scholar]
- 5.Vieth M, Stolte M. Barrett's esophagus and neoplasia: data from the Bayreuth Barrett's archive. Gastroenterology. 2002;122:590–591. doi: 10.1053/gast.2002.31600. [DOI] [PubMed] [Google Scholar]
- 6.Spechler SJ. The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology. 1999;117:218–228. doi: 10.1016/s0016-5085(99)70571-8. (review) [DOI] [PubMed] [Google Scholar]
- 7.Bian YS, Osterheld MC, Fontolliet C, Bosman FT, Benhattar J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett's esophagus. Gastroenterology. 2002;122:1113–1121. doi: 10.1053/gast.2002.32370. [DOI] [PubMed] [Google Scholar]
- 8.Vieth M, Schneider-Stock R, Rohrich K, May A, Ell C, Markwarth A, Roessner A, Stolte M, Tannapfel A. INK4a-ARF alterations in Barrett's epithelium, intraepithelial neoplasia and Barrett's adenocarcinoma. Virchows Arch. 2004;445:135–141. doi: 10.1007/s00428-004-1042-0. [DOI] [PubMed] [Google Scholar]
- 9.Eads CA, Lord RV, Kurumboor SK, Wickramasinghe K, Skinner ML, Long TI, Peters JH, DeMeester TR, Danenberg KD, Danenberg PV, et al. Fields of aberrant CpG island hypermethylation in Barrett's esophagus and associated adenocarcinoma. Cancer Res. 2000;60:5021–5026. [PubMed] [Google Scholar]
- 10.Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L, Peters JH, DeMeester SR, DeMeester TR, Skinner KA, et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–3418. [PubMed] [Google Scholar]
- 11.Klump B, Hsieh CJ, Holzmann K, Gregor M, Porschen R. Hypermethylation of the CDKN2/p16 promoter during neoplastic progression in Barrett's esophagus. Gastroenterology. 1998;115:1381–1386. doi: 10.1016/s0016-5085(98)70016-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, Vieth M, Moskaluk CA, El-Rifai W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett's tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulmann K, Sterian A, Berki A, Yin J, Sato F, Xu Y, Olaru A, Wang S, Mori Y, Deacu E, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett's-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138–4148. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 14.Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider-Stock R, Roessner A, Ullrich O. DAP-kinaseprotector or enemy in apoptotic cell death. Int J Biochem Cell Biol. 2005;37:1763–1767. doi: 10.1016/j.biocel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Cohen O, Inbal B, Kissil JL, Raveh T, Berissi H, Spivak-Kroizaman T, Feinstein E, Kimchi A. DAP-kinase participates in TNF-alpha- and Fas-induced apoptosis and its function requires the death domain. J Cell Biol. 1999;146:141–148. doi: 10.1083/jcb.146.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raveh T, Droguett G, Horwitz MS, DePinho RA, Kimchi A. DAP kinase activates a p19ARF/p53-mediated apoptotic checkpoint to suppress oncogenic transformation. Nat Cell Biol. 2001;3:1–7. doi: 10.1038/35050500. [DOI] [PubMed] [Google Scholar]
- 19.steller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 20.Mittag F, Kuester D, Vieth M, Peters B, Stolte B, Roessner A, Schneider-Stock R. DAPK promoter methylation is an early event in colorectal carcinogenesis. Cancer Lett. 2006;240:69–75. doi: 10.1016/j.canlet.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Anacleto C, Leopoldino AM, Rossi B, Soares FA, Lopes A, Rocha JC, Caballero O, Camargo AA, Simpson AJ, Pena SD. Colorectal cancer “methylator phenotype”: fact or artifact? Neoplasia. 2005; 7:331–335. doi: 10.1593/neo.04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider-Stock R, Kuester D, Ullrich O, Mittag F, Habold C, Boltze C, Peters B, Krueger S, Hintze C, Meyer F, et al. Close localization of DAP-kinase positive tumor-associated macrophages and apoptotic colorectal cancer cells. J Pathol. 2006;209:95–105. doi: 10.1002/path.1951. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Martins CR, Fansler ZB, Roemer KL, Kincaid EA, Gustafson KS, Heitjan DF, Clark DP. DNA methylation in anal intraepithelial lesions and anal squamous cell carcinoma. Clin Cancer Res. 2005;11:6544–6549. doi: 10.1158/1078-0432.CCR-05-0374. [DOI] [PubMed] [Google Scholar]
- 24.Brock MV, Gou M, Akiyama Y, Muller A, Wu TT, Montgomery E, Deasel M, Germonpre P, Rubinson L, Heitmiller RF, et al. Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res. 2003;9:2912–2919. [PubMed] [Google Scholar]
- 25.Brabender J, Marjoram P, Lord RV, Metzger R, Salonga D, Vallbohmer D, Schafer H, Danenberg KD, Danenberg PV, Selaru FM, et al. The molecular signature of normal squamous esophageal epithelium identifies the presence of a field effect and can discriminate between patients with Barrett's esophagus and patients with Barrett'sassociated adenocarcinoma. Cancer Epidemiol Biomark Prev. 2005;14:2113–2117. doi: 10.1158/1055-9965.EPI-05-0014. [DOI] [PubMed] [Google Scholar]
- 26.Schildhaus HU, Krockel I, Lippert H, Malfertheiner P, Roessner A, Schneider-Stock R. Promoter hypermethylation of p16INK4a, E-cadherin, O6-MGMT, DAPK and FHIT in adenocarcinomas of the esophagus, esophagogastric junction and proximal stomach. Int J Oncol. 2005;26:1493–1500. [PubMed] [Google Scholar]
- 27.Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847–2851. [PubMed] [Google Scholar]
- 28.Kim HC, Kim JC, Roh SA, Yu CS, Yook JH, Oh ST, Kim BS, Park KC, Chang R. Aberrant CpG island methylation in early-onset sporadic gastric carcinoma. J Cancer Res Clin Oncol. 2005;131:733–740. doi: 10.1007/s00432-005-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inbal B, Cohen O, Polak-Charcon S, Kopolovic J, Vadai E, Eisenbach L, Kimchi A. DAP kinase links the control of apoptosis to metastasis. Nature. 1997;390:180– 184. doi: 10.1038/36599. [DOI] [PubMed] [Google Scholar]
- 30.Tozawa T, Tamura G, Honda T, Nawata S, Kimura W, Makino N, Kawata S, Sugai T, Suto T, Motoyama T. Promoter hypermethylation of DAP-kinase is associated with poor survival in primary biliary tract carcinoma patients. Cancer Sci. 2004;95:736–740. doi: 10.1111/j.1349-7006.2004.tb03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X, Khuri FR, Lee JJ, Kemp BL, Liu D, Hong WK, Mao L. Hypermethylation of the death-associated protein (DAP) kinase promoter and aggressiveness in stage I non -small-cell lung cancer. J Natl Cancer Inst. 2000;92:1511–1516. doi: 10.1093/jnci/92.18.1511. [DOI] [PubMed] [Google Scholar]
- 32.Wittekind C, Meyer HJ, Bootz F. Berlin: Springer; 2004. TNM Classification of Malignant Tumors. [Google Scholar]
- 33.Vieth M, Peitz U, Labenz J, Kulig M, Naucler E, Jaspersen D, Meyer-Sabellek W, Willich S, Lind T, Malfertheiner P, et al. What parameters are relevant for the histological diagnosis of gastroesophageal reflux disease without Barrett's mucosa? Dig Dis. 2002;22:196–201. doi: 10.1159/000080319. [DOI] [PubMed] [Google Scholar]
- 34.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 35.Delgado S, Gomez M, Bird A, Antequera F. Initiation of DNA replication at CpG islands in mammalian chromosomes. EMBO J. 1998;17:2426–2435. doi: 10.1093/emboj/17.8.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosas SL, Koch W, da Costa Carvalho MG, Wu L, Califano J, Westra W, Jen J, Sidransky D. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and deathassociated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61:939–942. [PubMed] [Google Scholar]
- 37.Kim DH, Nelson HH, Wiencke JK, Christiani DC, Wain JC, Mark EJ, Kelsey KT. Promoter methylation of DAP-kinase: association with advanced stage in non-small cell lung cancer. Oncogene. 2001;20:1765–1770. doi: 10.1038/sj.onc.1204302. [DOI] [PubMed] [Google Scholar]