Abstract
Objective
We have measured the concentration of immunoreactive neutrophil elastase (ir-NE) in the tumor extracts of 313 primary human breast cancers. Sufficient time has elapsed, and we are now ready to analyze its prognostic value in human breast cancer.
Methods
ir-NE concentration in tumor extracts was determined with an enzyme-linked immunosorbent assay that enables a rapid measurement of both free-form ir-NE and the A1-protease inhibitor-complexed form of ir-NE. We analyzed the prognostic value of this enzyme in human breast cancer in univariate and multivariate analyses.
Results
Patients with breast cancer tissue containing a high concentration of ir-NE had poor survival compared to those with a low concentration of ir-NE at the cutoff point of 9.0 µg/100 mg protein (P = .0012), which had been previously determined in another group of 49 patients. Multivariate stepwise analysis selected lymph node status (P = .0004; relative risk = 1.46) and ir-NE concentration (P = .0013; relative risk = 1.43) as independent prognostic factors for recurrence.
Conclusions
Tumor ir-NE concentration is an independent prognostic factor in patients with breast cancer who undergo curative surgery. This enzyme may play an active role in tumor progression that leads to metastasis in human breast cancer.
Keywords: Neutrophil elastase, breast cancer, prognosis, multivariate analysis
Introduction
During invasion and metastasis formation, tumor cells confront a variety of natural tissue barriers in vivo, such as basement membranes and surrounding tissue stromal matrices composed of elastins, collagens, and proteoglycans. It is thus necessary for tumor cells to elaborate a battery of extracellular matrix (ECM)-degradative enzymes to achieve metastatic invasion. Many different types of ECMdegradative enzymes have been implicated in the invasive growth and metastasis of cancer cells [1–3].
The production of tumor cell proteases, including collagenase [4,5], plasminogen activator [6,7], and cathepsin B [8], has been implicated in tumor cell invasion into adjacent tissues and metastasis. Another proteolytic enzyme thought to be involved in this process is elastase, which is the only protease that is able to degrade insoluble elastin—a structural component of elastic tissues such as blood vessel, skin, lung, and breast tissues.
There are three well-characterized mammalian elastases. The best characterized is porcine pancreatic elastase I, first described by Balo and Banga [9], which is a serine protease secreted in zymogen form by pancreatic acinar cells. The second class of mammalian elastase is neutrophil elastase (NE), the neutral protease found in granules of human polymorphonuclear leukocytes [10,11]. The third mammalian elastase is a metalloprotease, which is secreted by inflammatory macrophages [12]. Of these elastases, NE exhibits the most proteolytic activity under physiological conditions.
The presence of elastinolytic activities in human breast cancer tissue has been demonstrated by Hornebeck et al. [13]; however, in their study, it was not determined whether the activity could be attributed specifically to breast cancer cells. Thereafter, several investigators have described elastinolytic enzyme production by human and rodent mammary tumor cells [14–16], although these enzymes have not been isolated or characterized.
In this connection, we previously have reported that NE is produced by human breast cancer cell lines, using a highly specific and sensitive enzyme immunoassay [17]. In addition, we conducted a prognostic study of 313 patients with breast cancer who underwent curative mastectomy and have reported a preliminary result suggesting that the concentration of immunoreactive neutrophil elastase (ir-NE) in tumor extracts may affect prognosis in human breast cancer [18]. Sufficient time has elapsed, and we are now ready to analyze prognosis in patients with breast cancer.
Materials and Methods
Patients
Three hundred thirteen patients with breast cancer in the present analysis constitute 100% of our previous study population [18]. These patients underwent curative mastectomy with lymph node dissection at the Department of Surgery II, Kumamoto University Hospital, between March 1982 and April 1989. The median follow-up period for patients was 18.5 years (range, 14.0–21.1 years). The clinicopathological characteristics of the 313 patients are summarized in Table 1.
Table 1.
Relation between ir-NE Content in Tissue Extracts and Clinicopathological Factors of Human Breast Cancer (n = 313).
| Characteristic Content (µg/100 mg Protein) | n (%) | ir-NE (Mean ± SD) |
| Menstrual status | ||
| Premenopause/perimenopause | 179(57) | 5.24 ± 4.11 |
| Postmenopause | 134 (43) | 4.32 ± 3.50 |
| Tumor size (cm) | ||
| < 2.0 | 57 (18) | 3.38 ± 1.13 |
| 2.0–5.0 | 176 (56) | 4.55 ± 3.25 |
| > 5.0 | 80 (26) | 7.20 ± 5.12* |
| Lymph node status | ||
| Node-negative | 178 (57) | 2.54 ± 1.90 |
| Node-positive | 135 (43) | 5.47 ± 4.24′ |
| Histologic type‡ | ||
| Papillotubular | 72 (23) | 3.26 ± 3.01 |
| Solid-tubular | 117 (37) | 4.66 ± 3.86 |
| Scirrhous | 111 (35) | 5.11 ± 3.92 |
| Other | 13 (4) | 4.28 ± 3.99 |
| Histologic grade§ | ||
| I | 90 (29) | 4.42 ± 3.76 |
| II | 122 (39) | 4.63 ± 3.73 |
| III | 101 (32) | 5.89 ± 5.01 |
| Vessel involvement | ||
| Absent | 194 (62) | 4.22 ± 4.05 |
| Present | 119 (38) | 4.96 ± 3.97 |
| Estrogen receptor¶ | ||
| Positive | 159 (51) | 5.52 ± 4.03 |
| Negative | 124 (40) | 4.14 ± 3.63 |
| Unknown | 30 (10) | 4.68 ± 4.00 |
| Progesterone receptor¶ | ||
| Positive | 94 (30) | 5.38 ± 4.24 |
| Negative | 178 (57) | 4.29 ± 3.62 |
| Unknown | 41 (13) | 4.21 ± 3.97 |
| Type of surgery# | ||
| Halsted | 75 (24) | 4.51 ± 3.93 |
| Modified | 238 (76) | 5.72 ± 4.10 |
| Adjuvant therapy | ||
| Endocrine therapy | 75 (24) | 3.45 ± 3.19 |
| Chemotherapy | 45 (14) | 4.51 ± 4.13 |
| Both | 92 (29) | 3.96 ± 3.22 |
| None | 101 (32) | 4.07 ± 3.18 |
| Immunoreactive NE | ||
| < 9.0 | 261 (83) | |
| ≥9.0 | 52 (17) |
P < .002, compared with <2.0 and 2.0 to 5.0 cm.
P < .001, compared with node-negative.
Breast tumor was analyzed according to the classification of the Japanese Breast Cancer Society [19]. When histologic typing was performed according to World Health Organization classification [20], all tumors were classified as invasive ductal carcinoma.
Breast tumor was graded according to the criteria described by Bloom and Richardson [21].
Estrogen receptor and progesterone receptor were determined by a dextrancoated charcoal method [22]. Tumors were considered hormone receptor-positive if they contained at least 10 fmol of specific binding sites per milligram of protein.
During the mid-1980s, Halsted mastectomy or modified radical mastectomy preserving the pectoral muscles was widespread in Japan, although breast conservation surgery for breast cancer had become common in Western countries.
Assay for ir-NE
Breast cancer specimens were homogenized and extracted with 50 mM Tris-HCl buffer (pH 7.4) containing 0.25% Triton X-100, as described previously [23]. The resulting supernatant was assayed for ir-NE concentration as described below.
The concentration of ir-NE in tumor extracts was determined with a newly established enzyme immunoassay kit (Mochida Pharmaceutical Co., Tokyo, Japan). This is a sensitive assay that enables a rapid measurement of both NE-complexed a1-protease inhibitor (α1-PI) and free-form NE [24]. When 0.1 ml of tissue extract was used, the detection limit of ir-NE was 0.063 µg/100 mg protein. The intraassay and interassay coefficients of variation were 3.2% to 5.6% and 5.1% to 8.7%, respectively.
To measure the level of free-form and α1-PI-complexed form in tissue extracts, we determined the concentration of ir-NE in all samples in the presence and in the absence of an excess amount (100 µg/ml) of α1-antitrypsin (Sigma, St. Louis, MO) using the conventional Merck kit (E. Merck, Darmstadt, Germany) according to the method of Neumann et al. [25]. Because the Merck kit detects only NE complexed with α1-PI, the difference between these concentrations was regarded as free-form ir-NE, and the concentration in the absence of α1-antitrypsin was regarded as the α1-PI-complexed form of ir-NE.
Survival Analysis
Routine postoperative follow-up consisted of clinical evaluations every month for the first 2 years and every 3 to 6 months thereafter. Disease recurrence was documented by physical examination, roentgenographic and laboratory tests, and other relevant diagnostic procedures. The major statistical endpoint of this study was disease recurrence (distant recurrences only) and was calculated from the day of operation to the day of discovery of recurrence or the last known date alive. Event time distribution was estimated with the method of Kaplan and Meier [26]. Differences between death distributions were tested for statistical significance with the log-rank test [27]. For simultaneous control of the effects of many variables on differences in death rates, a multivariate proportional hazards regression model [28] was used. P < .05 was considered significant.
Results
Relation of ir-NE Content to Clinicopathological Factors
Table 1 shows the correlation between ir-NE content and the characteristics of the patients in this series. When ir-NE content was compared in terms of menstrual status, histologic type, histologic grade, vessel involvement, estrogen receptor, and progesterone receptor, no significant association was found between ir-NE content and any of these features. However, ir-NE content was significantly higher in tumors with a size of > 5.0 cm than in those with < 5.0 cm (P < .002). Similarly, ir-NE content was significantly higher in patients who were node-positive than in those who were node-negative.
Univariate Analysis
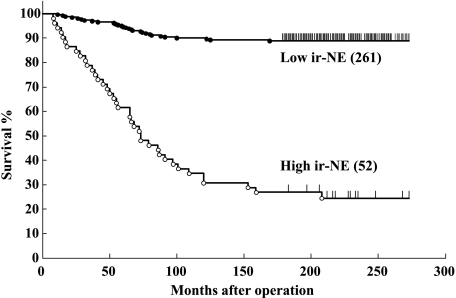
As expected, lymph node status, tumor size, histologic grade, vessel involvement, and adjuvant therapy were found to have a significant effect on disease-free survival when evaluated in a univariate analysis. When patient prognosis was analyzed in terms of the results of ir-NE, patients with breast cancer tissues containing a high concentration of ir-NE had a disease-free survival time significantly shorter than that in patients with a low content of ir-NE (P = .0012; Figure 1 and Table 2). In this analysis, the cutoff point of 9.0 µg/100 mg protein was used because our preliminary study of another 49 patients [17] revealed that this cutoff point could give a statistically significant separation for risk of relapse, according to the method of Tandon et al. [29]. This cutoff point identified 16.6% (52 of 313) of the patients as having high ir-NE levels in the present series.
Figure 1.
Relapse-free survival curves in 313 patients with breast cancer in terms of ir-NE concentration in tumor extracts. The major statistical endpoint of this study was disease recurrence (distant recurrences only). The cutoff point between high and low enzyme levels is 9.0 µg/100 mg protein. Numbers in parentheses show the total number of patients per group.
Table 2.
Univariate and Cox Regression Analyses as Prognostic Factors for Relapse in Patients with Breast Cancer.
| Variable | Univariate Analysis |
Multivariate Analysis |
Relative Risk | |||
| P | Z | SE | P | |||
| Independently associated with relapse | ||||||
| Lymph node status (node-negative vs node-positive) | .0012 | -1.68 | 0.54 | .0001 | 1.62 | |
| Associated with relapse only when evaluated alone | ||||||
| Tumor size (< 2.0 vs 2.0–5.0 vs > 5.0 cm) | .0431 | 0.46 | 0.32 | .324 | 1.04 | |
| Histologic grade (I vs II vs III) | .0088 | -0.74 | 0.39 | .163 | 0.62 | |
| Vessel involvement (absent vs present) | .0315 | -0.62 | 0.24 | .112 | 1.51 | |
| Adjuvant therapy (endocrine vs chemotherapy vs both vs none) | .0048 | 0.53 | 0.33 | .103 | 0.54 | |
| ir-NE (< 9.0 vs − 9.0) | .0012 | 0.45 | 0.31 | .062 | 1.50 | |
| Not associated with relapse | ||||||
| Menstrual status (premenopause/perimenopause vs postmenopause) | .4226 | -0.70 | 0.40 | .504 | 1.57 | |
| Histologic type (papillotubular vs solid-tubular vs scirrhous vs other) | .1003 | 0.04 | 0.45 | .754 | 0.17 | |
| Estrogen receptor (positive vs negative vs unknown) | .7314 | -1.47 | 0.33 | .214 | 1.08 | |
| Progesterone receptor (positive vs negative vs unknown) | .1571 | a0.79 | 0.38 | .133 | 1.52 | |
| Type of surgery (Halsted vs modified) | .9174 | 0.61 | 0.44 | .417 | 0.81 | |
Multivariate Analysis
To verify the independent nature of the prognostic value of ir-NE concentration, we used multivariate analysis. Cox regression analysis of overall survival, allowing for menstrual status, tumor size, lymph node status, histologic type, histologic grade, vessel involvement, estrogen receptor, progesterone receptor, type of surgery, adjuvant therapy, and ir-NE, showed that lymph node status is the single independent prognostic factor of disease-free survival (P = .0012; relative risk = 1.62; Table 2). To eliminate the effect of the inclusion of not so important variables into the model, we also performed stepwise regression analysis with a 5% significance level. Through a stepwise method, the model selected lymph node status (P = .0004; relative risk = 1.46) and ir-NE concentration (P = .0013; relative risk = 1.43) (Table 3). Menstrual status, tumor size, histologic type, histologic grade, vessel involvement, estrogen receptor, progesterone receptor, type of surgery, and adjuvant therapy were not independent prognostic factors.
Table 3.
Final Stepwise Regression Analysis.
| Variable | Z | SE | P | Relative Risk |
| Lymph node status | -1.55 | 0.49 | .0004 | 1.46 |
| ir-NE | 0.39 | 0.11 | .0013 | 1.43 |
Discussion
The purpose of identifying prognostic factors in breast cancer is to provide a sound basis for the rational management of the disease. Reliable predictors of cancer recurrence and death in patients with breast cancer may help to determine the selection of adjuvant chemotherapy or endocrine therapy. Classic prognostic factors, such as age, tumor size, lymph node involvement, histologic grade, and hormone receptor status, assist in predicting the patient's outcome or response to treatment, but they are not entirely dependable. Several enzymes or biologic factors determined in the cytoplasm and organelles of tumor cells have been found to have prognostic value in human breast cancer [29,30].
In the present study, we have demonstrated that the ir-NE concentration in tumor extracts is an independent prognostic factor that clearly identifies patients at high risk and at low risk for the disease, indicating that this enzyme level can be added to the list of second-generation prognostic factors in human breast cancer [31–33]. NE is the only neutral protease that is able to degrade insoluble elastin [11,34]. NE can also hydrolyze other ECM proteins, including type IV collagens [35], fibronectins [36], and proteoglycans [37], and has been reported to potentiate the conversion of plasminogen to plasmin by urokinase-type plasminogen activator [38]—an enzyme that has been postulated to play a role in cancer spread [39]. Thus, tumor NE may play a pathologic role in facilitating cancer cell invasion and metastasis, either directly by the dissolution of the tumor matrix or indirectly through such a protease cascade. The results presented here—demonstrating that free-form (active form) NE, but not the β1-PI-complexed form (inactive form), contributes to the prognostic value in human breast cancer—may support the above assumption.
The interactions between tumor and normal cells are complex events that occur continuously throughout the entire invasion process. A wide variability in the relative proportions of tumor and host cells has been observed at the zone of tumor invasion. Breast tumors also are heterogenous, with varying tumor cellularities and amounts of stroma. It is therefore possible that some of the NE proteins detected in this study that assayed tumor cytosols were extracted from infiltrating inflammatory cells and that this inflammatory cell involvement correlated with poor prognosis. Normal cells, such as neutrophils, fibroblasts, macrophages, and lymphocytes, all of which appear in the tumor invasion zone, may cooperate for the destruction of the host ECM. In fact, inflammatory cell infiltration has been reported to be associated with poor prognosis in human breast cancer [40].
In conclusion, tumor NE, whatever the cellular origin, may play an active role in the tumor progression that leads to metastasis in human breast cancer. The long-term follow-up results presented here, demonstrating that free-form NE is a strong and independent prognostic factor in human breast cancer, may support the above assumption.
Acknowledgements
We are grateful to Makiko Terashita for her support with the preparation of this manuscript.
Footnotes
This work was supported, in part, by grants from the Yuumi Memorial Foundation and the Nagoya Medical Foundation in Japan.
References
- 1.Schmitt M, Janicke F, Graeff H. Proteases, matrix degradation and tumor cell spread. Fibrinolysis. 1992;6(Suppl 4):1–170. [Google Scholar]
- 2.Nakajima M and Chop AM. Tumor invasion and extracellular matrix degradative enzymes: regulation of activity by organ factors. Semin Cancer Biol. 1991;2:115–127. [PubMed] [Google Scholar]
- 3.Carter RL. Some aspects of the metastatic process. J Clin Pathol. 1982;35:1041–1049. doi: 10.1136/jcp.35.10.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wooley DE. Collagenolytic mechanisms in tumor cell invasion. Cancer Metastasis Rev. 1984;3:361–372. doi: 10.1007/BF00051460. [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, Thorgeirsson UP, Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;2:277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita J, Ogawa M, Yamashita S, Nakashima Y, Saishoji T, Nomura K, Inada K, Kawano I. Differential biological significance of tissue-type and urokinase-type plasminogen activator in human breast cancer. Br J Cancer. 1993;68:524–529. doi: 10.1038/bjc.1993.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossowski L and Reich E. Antibodies to plasminogen activator inhibit tumor metastasis. Cell. 1983;35:611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- 8.Recklies AD, Tiltman KJ, Stoker AM, Poole AR. Secretion of proteinases from malignant and non-malignant human breast cancer. Cancer Res. 1980;40:550–556. [PubMed] [Google Scholar]
- 9.Balo J, Banga I. Elastase and elastase inhibitor. Nature. 1949;164:491–493. doi: 10.1038/164491a0. [DOI] [PubMed] [Google Scholar]
- 10.Janoff A, Schere J. Elastolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968;128:1137–1156. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baugh RJ, Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976;15:836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- 12.Banda MJ, Werb Z. Mouse macrophage elastase. Biochem J. 1981;193:589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornebeck W, Derouette JC, Brechemier D, Adnet JJ, Robert L. Elastogenesis and elastinolytic activity in human breast cancer. Biomedicine. 1977;26:48–52. [PubMed] [Google Scholar]
- 14.Kao RT, Wong M, Stern R. Elastin degradation by proteases from cultured human breast cancer cells. Biochem Biophys Res Commun. 1982;105:383–389. doi: 10.1016/s0006-291x(82)80056-9. [DOI] [PubMed] [Google Scholar]
- 15.Zeydel M, Nakagawa S, Biempica L, Takahashi S. Collagenase and elastase production by mouse mammary adenocarcinoma primary cultures and cloned cells. Cancer Res. 1986;46:6438–6445. [PubMed] [Google Scholar]
- 16.Grant AJ, Lerro KA, Wu CW. Cell associated elastase activities of rat mammary tumour cells. Biochem Int. 1990;22:1077–1084. [PubMed] [Google Scholar]
- 17.Yamashita J, Ogawa M, Ikei S, Omachi H, Yamashita S, Saishoji T, Nomura K, Sato H. Production of immunoreactive polymorphonuclear leucocyte elastase in human breast cancer cells: possible role of polymorphonuclear leucocyte elastase in the progression of human breast cancer. Br J Cancer. 1994;69:72–76. doi: 10.1038/bjc.1994.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita J, Ogawa M, Shirakusa T. Free-form neutrophil elastase is an independent marker predicting recurrence in primary breast cancer. J Leukoc Biol. 1995;57:375–378. doi: 10.1002/jlb.57.3.375. [DOI] [PubMed] [Google Scholar]
- 19.Japanese Breast Cancer Society, author. General Rule for Clinical and Pathological Record of Mammary Cancer. 10th ed. Tokyo: Kanehara; 1989. Histologic classification of breast tumors; pp. 21–57. [Google Scholar]
- 20.World Health Organization, author. International Histologic Classification of Tumours. No. 2. Geneva: World Health Organization; 1981. Histologic typing of breast tumours; pp. 35–62. [Google Scholar]
- 21.Bloom HJG, Richardson WW. Histologic grading and prognosis in breast cancer. A study of 1049 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire WL, De La Garza M, Chamness GC. Evaluation of estrogen receptor assays in human breast cancer tissue. Cancer Res. 1977;37:637–639. [PubMed] [Google Scholar]
- 23.Yamashita J, Horiuchi S, Kimura M, Nishimura R, Akagi M. Plasminogen activator as a functional marker for estrogen dependence in human breast cancer cells. Jpn J Cancer Res (Gann) 1986;77:177–181. [PubMed] [Google Scholar]
- 24.Ikei S, Ogawa M, Samejima H, Arakawa H, Sugita H, Yamashita J, Sato H, Tatematsu A, Sato I. Evaluation of a newly developed enzyme-immunoassay kit for neutrophil elastase. Jpn J Clin Exp Med. 1992; 69:2944–2950. [Google Scholar]
- 25.Neumann S, Hennrich H, Gunzer G, Lang H. Enzyme-linked immunoassay for human granulocyte elastase in complex with α1-proteinase inhibitor. In: Horl W, Heidland A, Gunzer G, Lang H, editors. Proteases: Potential Role in Health and Diseases. New York: Plenum; 1984. pp. 379–390. [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 29.Tandon AK, Clark GM, Chamness GC, Chirgwin JM, McGuire WL. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990;322:297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, Saishoji T, Shin S. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54:1630–1633. [PubMed] [Google Scholar]
- 31.Morimoto-Tomita M, Uchimura K, Bistrup A, Lum DH, Egeblad M, Boudreau N, Werb Z, Rosen SD. Sulf-2, a proangiogenic heparan sulfate endosulfatase, is upregulated in breast cancer. Neoplasia. 2005;7:1001–1010. doi: 10.1593/neo.05496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuefer R, Day KC, Kleer CG, Sabel MS, Hofer MD, Varambally S, Zorn CS, Chinnaiyan AM, Rubin MA, Day ML. ADAM15 disintegrin is associated with aggressive prostate and breast cancer disease. Neoplasia. 2006;8:319–329. doi: 10.1593/neo.05682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghebeh H, Mohammed S, AI-Omair A, Qattan A, Lehe C, AI-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, et al. The B7-H1(PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita J, Ogawa M, Abe M, Hayashi N, Kurusu Y, Kawahara K, Shirakusa T. Tumor neutrophil elastase is closely associated with the direct extension of non-small cell lung cancer into the aorta. Chest. 1997;111:885–890. doi: 10.1378/chest.111.4.885. [DOI] [PubMed] [Google Scholar]
- 35.Mainardi C, Dixit S, Kang A. Degradation of type IV (basement membrane) collagen by a proteinase isolated from human polymorphonuclear leukocyte granules. J Biol Chem. 1980;255:5435–5441. [PubMed] [Google Scholar]
- 36.McDonald JA, Kelley DG. Degradation of fibronectin by human leukocyte elastase: release of biologically active fragments. J Biol Chem. 1980;255:8848–8858. [PubMed] [Google Scholar]
- 37.Heck LW, Caterson B, Christner JE, Baeker JR. The interaction of human neutrophil elastase and cathepsin G with rat chondrosarcoma proteoglycan aggregate. Fed Proc. 1982;41:5065–5066. [Google Scholar]
- 38.Machovich R, Owen WG. An elastase-dependent pathway of plasminogen activation. Biochemistry. 1989;28:4517–4522. doi: 10.1021/bi00436a059. [DOI] [PubMed] [Google Scholar]
- 39.Ossowski L. Invasion of connective tissue by human carcinoma cell lines: requirement for urokinase, urokinase receptor, and interstitial collagenase. Cancer Res. 1992;52:6754–6760. [PubMed] [Google Scholar]
- 40.Rilke F, Colnaghi MT, Cascinelli N, Andreola S, Baldini MT, Bufalino R. Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer. 1991;49:44–49. doi: 10.1002/ijc.2910490109. [DOI] [PubMed] [Google Scholar]