Abstract
Glutamine-rich sequences exist in a wide range of proteins across multiple species. A subset of glutamine-rich sequences has been shown to form amyloid fibers implicated in human diseases. The physiological functions of these sequence motifs are not well understood, partly because of the lack of structural information. Here we have determined a high-resolution structure of a glutamine-rich domain from human histone deacetylase 4 (HDAC4) by x-ray crystallography. The glutamine-rich domain of HDAC4 (19 glutamines of 68 residues) folds into a straight α-helix that assembles as a tetramer. In contrast to most coiled coil proteins, the HDAC4 tetramer lacks regularly arranged apolar residues and an extended hydrophobic core. Instead, the protein interfaces consist of multiple hydrophobic patches interspersed with polar interaction networks, wherein clusters of glutamines engage in extensive intra- and interhelical interactions. In solution, the HDAC4 tetramer undergoes rapid equilibrium with monomer and intermediate species. Structure-guided mutations that expand or disrupt hydrophobic patches drive the equilibrium toward the tetramer or monomer, respectively. We propose that a general role of glutamine-rich motifs be to mediate protein–protein interactions characteristic of a large component of polar interaction networks that may facilitate reversible assembly and disassembly of protein complexes.
Keywords: amyloid, transcription
Histone deacetylases (HDACs) regulate diverse cellular processes through enzymatic deacetylation of both histone and nonhistone proteins (1, 2). Two major classes of this family, class I and class II, share a highly conserved catalytic domain that is targeted by a number of antitumor drugs (3). Class II HDACs, which include HDAC4, HDAC5, and HDAC9, contain an additional N-terminal extension that confers responsiveness to calcium signals and mediates interactions with transcription factors and cofactors (4–7). A prominent feature of this N-terminal region is a highly conserved glutamine-rich domain that can repress transcription independently of the C-terminal catalytic domain (8–10).
Glutamine-rich sequences have been found in a variety of eukaryotic proteins, including transcription activators and repressors (11, 12). Bioinformatics analysis suggests that glutamine-rich sequences appear to undergo positive selection, suggesting that these low-complexity sequences are conserved for functional reasons (13). Although the physiological functions of most glutamine rich sequences are not well understood, it has been widely observed that high content of glutamines and/or asparagines may lead to increased propensity of amyloid formation implicated in human neurodegenerative diseases and prion-like, non-Mendelian inheritance in yeast (14–16).
The intriguing physiological roles of glutamine-rich sequences have attracted much attention to these unusual protein motifs. Max Perutz (15) and others have studied this problem extensively by using x-ray fiber diffraction and modeling. It has been hypothesized that both the physiological and pathological roles of glutamine-rich sequences are related to the unique properties of glutamines and asparagines to engage in polar protein–protein interactions, termed polar zippers (17). Recent structural studies of amyloid peptides indeed revealed extensive interaction networks formed by the amide side chains of glutamines and asparagines (18). However, how glutamine-rich sequences mediate protein–protein interactions in most physiological protein complexes is not well understood.
Apart from the structural studies of amyloid peptides mentioned above, there is no high-resolution structural information about any glutamine rich domain, which is largely due to the fact that most glutamine-rich domains are either insoluble or unstructured in solution. As part of our effort to characterize the mechanism of transcriptional repression by class II HDACs (19, 20), we have determined the crystal structure of a highly conserved N-terminal domain of human HDAC4 that contains 19 glutamines of 68 residues. On the basis of the structure, we have also performed site-specific mutagenesis and analyzed the biochemical properties of both the wild-type protein and the mutants. These studies provide new insights into potential functions of glutamine-rich sequences.
Results and Discussion
Overall Structure of a Glutamine-Rich Four-Helix Bundle.
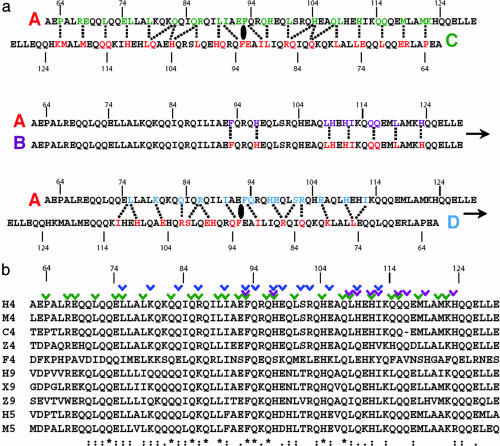
The crystal structure of an N-terminal fragment of human HDAC4 (residues 62–153) containing the glutamine-rich domain was determined by the single-wavelength anomalous diffraction method [supporting information (SI) Table 1]. The asymmetric unit contains four copies of HDAC4, each of which folds into a single α-helix from residues 62–129, whereas residues 130–153 are disordered (Fig. 1). This observation is consistent with circular dichroism analysis that indicates mainly an α-helical secondary structure of HDAC4 (residues 62–153) in solution (SI Fig. 6). The four helices stack together to form a four-helix bundle that is 108 Å long and 20 Å wide. However, the assembly mechanism is distinct from that of many four-helix bundle proteins characterized thus far (21–23). The HDAC4 four-helix bundle has three perpendicular twofold axes intercepting at the center of the complex. Each helix contacts the other three helices differently (Fig. 2a). Helix A interacts extensively with helix C in an antiparallel orientation, burying ≈2,528 Å2 of solvent accessible surface area at the interface. Helix A and helix B contact each other at their C-terminal region and bury ≈1,122 Å2 at the interface, whereas helix A and helix D interact mostly in their middle region and bury ≈1,371 Å2 of surface area. Because of the D2 point group symmetry, the interactions of each helix with its neighboring three helices are the same for all four helices. The total buried surface area for the four-helix bundle is ≈9,500 Å2. On the basis of the size of contacting surface, the hierarchy of the HDAC4 four-helix assembly can be considered as two antiparallel helices, A and C (A/C), and B and D (B/D), stacked onto each other at a crossing angle of 23° (Fig. 1a). The residues mediating the interactions between each helix and its surrounding three helices are highly conserved (Fig. 2b), suggesting that the glutamine-rich domain of other members of the class II HDAC family may fold into a similar structure as observed here.
Fig. 1.
Overall structure of the HDAC4 tetramer. (a) Four copies of the HDAC4 glutamine-rich domain in the asymmetric unit assemble into a four-helix bundle. Shown are monomers A (red), B (magenta), C (green), and D (blue). The three twofold axes are indicated. (b) Side view from a.
Fig. 2.
Conserved assembly interactions in the HDAC4 tetramer. (a) Schematic presentation of interhelical interactions between monomer A and its neighboring helices B, C, and D. (Top) Interactions between A and C. Residues of A interacting with C are in green, whereas residues in C contacting A are in red. The dotted lines indicate approximately the location of contacts. (Middle) Interactions between A and B. Residues of A interacting with B are in magenta, whereas residues in B contacting A are in red. (Bottom) Interactions between A and D. Residues of A interacting with D are in blue, whereas residues in D contacting A are in red. (b) Sequence alignment of the glutamine-rich motif of class II HDACs from a variety of species (H4, human HDAC4; M4, mouse HDAC4; C4, chicken HDAC4; Z4, zebrafish HDAC4; F4, Drosophila HDAC4; H9, human HDAC9; X9, Xenopus HDAC9; Z9, zebrafish HDAC9; H5, human HDAC5; and M5, mouse HDAC5). Numbering is based on human HDAC4. Colored checks above the sequence denote various modes of interhelical interactions: green, A/C; magenta, A/B; blue, A/D.
Detailed Protein–Protein Interactions.
The detailed interactions in the HDAC4 four-helix bundle show many unique features as compared with typical coiled coil proteins (24, 25). Secondary structure prediction suggests that the glutamine-rich region of HDAC4, HDAC5, and HDAC9 forms predominantly α-helices, but there is no discernible pattern of regularly arranged apolar residues in the primary sequences (Fig. 2b). Nevertheless, the “knob-into-hole” type of interactions still account for a major component of the HDAC4 four-helix bundle interface (26) (Fig. 2a). As shown in Fig. 3a, Leu-71 and Leu-78 of helix A and His-111 and Met-118 of helix C act as knobs (position d in the heptad repeat convention) to insert into holes formed by residues in the opposing helix. For example, His-111 of helix C inserts into the hole of Leu-71, Glu-74, Leu-75, and Leu-78 from helix A, whereas Leu-71 of helix A inserts into the hole of His-111, Gln-114, Gln-115, and Met-118 of helix C. To maintain such an interlocked interaction, the two helices would have to coil around each other to keep the knobs and holes in register. However, the HDAC4 helix is approximately straight throughout its entire length. Detailed analysis of the crystal structure reveals that the knob-into-hole mode of interaction breaks down at Leu-78 of helix A and the corresponding layer (Gln-107 and Leu-108) of helix C (Figs. 2a and 3b). Here, His-104 of helix C at an expected d position would normally fill in the hole formed by Leu-78, Lys-81, Gln-82, and Gln-85 of helix A, but this does not occur because of the straight α-helical conformation. Instead, a polar interaction network involving several glutamine residues forms at this junction (Figs. 2a and 3b). Gln-107 of helix C and Gln-82 of helix A form a hydrogen bond with each other, whereas His-104 of helix C forms a hydrogen bond with Arg-86. A similar polar layer made of a Gln–Asn–Gln–Asn tetrad was also observed interrupting the typical coiled coil interactions in the Max homodimer (27, 28). On the other side of the polar junction, a number of apolar residues from helix A and C form a hydrophobic interface (Fig. 3c). Because of the twofold symmetry between Phe-93 of both helix A and helix C, the interactions between the C-terminal half of A and the N-terminal of C is the same as described above. Similarly, the interaction between helix B and helix D is the same as that described above for helix A and helix C (Fig. 2a).
Fig. 3.
Protein–protein interactions in the HDAC4 tetramer. (a) The knob-into-hole type of interactions between helix A and C. (b) His-104 of helix C fails to fill in the hole of Leu-78, Lys-81, Gln-82, and Gln-85 of helix A because of the straight helical conformation. A polar interaction network is formed here (see Detailed Protein–Protein Interactions). (c) Interactions between helix A and helix C near the twofold axis. The extrahelical hydrophobic patch formed by Leu-89, Ile-90, and Phe-93 of helix A and C pack against the corresponding region of helix B and D (behind helix A/C, residues not shown, also see SI Fig. 7). (d) A hydration pocket (blue circle) in the HDAC4 tetramer is surrounded by a polar interaction network.
The HDAC4 four-helix bundle has a relatively small hydrophobic core at the center of the complex, consisting of three nonpolar residues (Leu-89, Ile-90, and Phe-93) from each helix (Fig. 3c and SI Fig. 7a). Interestingly, Phe-93 and His-97 of each helix form a double helical network of aromatic residues that may contribute to the stability of the complex (SI Fig. 7b). The C-terminal region of helix A and helix B interact with each other in a parallel fashion (SI Fig. 8), but the interactions appear to be much looser than those seen in typical coiled coil proteins. Symmetry-related interactions are found in the same region of helix C and helix D. Helix A also interacts with helix D in the HDAC4 four-helix bundle. Most notably, Lys-79, Gln-83, and Arg-86 of helix A interact with Glu-105, Arg-102, and Glu-98 of helix D, respectively (Fig. 3d). Because of the D2 point group symmetry, the same interactions also occur at the A/D interface on the other side of the center of symmetry and at the interface between helix B and helix C. Together, these residues form a polar interaction network around the central hydrophobic core.
Hydrated Cavities at Protein–Protein Interfaces.
Two large cavities are enclosed at the interface of the HDAC4 four-helix bundle (Fig. 3d, one of the two symmetry-related ones is shown). Here, residues with long side chains, such as Lys-79, Gln-83, and Arg-86 from one helix, and Glu-105, Arg-102, and Glu-98 from another, swing out to interact with each other through hydrogen bonds and electrostatic interactions (Fig. 3d). His-104 also swings away to participate in the interhelical interaction as described above (Fig. 3c), whereas the side chain of Ser-101 (data not shown) is too short to fill the cavity. As a result, two hydration pockets are formed at the interface of the HDAC4 complex where the electron densities for solvent molecules are observed (SI Fig. 9). These hydration pockets separate the central hydrophobic core from the C-terminal apolar interaction interface between helix A/B (SI Fig. 8) and C/D (data not shown). Thus, despite the large, buried, solvent-accessible surface area, the HDAC4 four-helix bundle lacks an extended hydrophobic interface. Multiple hydrophobic patches are separated by polar interaction networks and buried hydration pockets. The interaction interfaces are less densely packed as compared with most stable coiled coil proteins. These structural features are similar to those observed at the interface of weak and transient protein–protein complexes (29, 30).
Reversible Assembly of the Glutamine-Rich Four-Helix Bundle.
We were initially drawn to the glutamine-rich domain of HDAC4 by a model wherein class II HDACs repress transcription through a higher-order protein–DNA complex. This hypothesis is based on the observation that full-length class II HDACs form homo- and heterocomplexes in cellular extracts (31). Biochemical studies of glutamine-rich domains from other transcription factors such as Groucho and GAGA also suggest the tendency for these domains to form tetramer or higher-order assembly (11, 12). Using chemical cross-linking, we observed higher molecular weight species corresponding to the dimer, trimer, and tetramer of HDAC4 (residues 62–153) (SI Fig. 10). Similar results were also found with HDAC5 and HDAC9 (data not shown). These observations suggest that the isolated glutamine-rich domain of class II HDACs can form higher-order oligomers in solution. However, our structural analysis suggests that the glutamine-rich domain may not form a stable complex in solution (see above). To further address this question, we analyzed the oligomerization of HDAC4 (residues 62–153) in solution by using multiangle light scattering (MALS). As shown in Fig. 4 (green trace), HDAC4 (residues 62–153) indeed behaves as a polydispersive species that may contain the tetramer, monomer, and intermediate species. The exchange between different species appears too fast to be resolved by the size-exclusion column linked to MALS, suggesting a rapid equilibrium between the tetramer and subtetramer species. Although this behavior complicates the determination of the binding constant of the HDAC4 tetramer, we were able to use structure-guided mutations to show that the interactions observed in the crystal structure are likely responsible for the assembly of the HDAC4 tetramer in solution. A single mutation of Phe93Asp, which is predicted to disrupt the central hydrophobic core, prevents the oligomerization of HDAC4 (residues 62–153) as analyzed by MALS (Fig. 4, magenta trace). The Phe93Asp mutation does not seem to affect the folding of HDAC4 (residues 62–153) because the mutant still retains the α-helix structure, as seen in circular dichroism spectroscopy (data not shown). As described above, His-97 and Phe-93 of each helix form an aromatic cluster at the center of the complex (SI Fig. 7). Another mutation, His97Phe, is designed to extend the central hydrophobic core and further stabilize the complex. Indeed, the His97Phe mutant exists as a stable tetramer as analyzed by MALS (Fig. 4, blue trace). We have determined the crystal structure of the His97Phe mutant (SI Table 2). The larger aromatic ring of phenylalanine is readily accommodated by the loose packing interface. There is little structural change in the four-helix bundle between the wild type and the His97Phe mutant. Previous studies have shown that the N-terminal domain of HDAC4 containing the glutamine-rich sequence can repress MEF2-dependent transcription independently of the C-terminal catalytic domain (8–10). Whereas the His97Phe mutant similarly repressed MEF2-dependent transcription, the Phe93Asp mutant showed decreased ability to repress MEF2-dependent transcription (L.G. and L.C., unpublished results). These studies suggest functional relevance of the interactions observed in the crystal structure. However, whether HDAC4 or other members of the class II HDAC family repress transcription through a higher-order tetramer complex remains to be further investigated.
Fig. 4.
Analysis of the oligomerization state of HDAC4 (residues 62–153) by MALS. Shown are size-exclusion chromatography runs superimposed with the MALS data. The left-hand scale is the MALS data of molecular weight; the right-hand scale is the refractive index signal. The green trace indicates wild type showing a mixture between monomer and tetramer. The blue trace indicates the His97Phe mutant showing a stable tetramer (50.3 kDa, error rate 2.2%). The magenta trace indicates the Phe93Asp mutant showing a single species of monomer (12.5 kDa, error rate 3%). The error rate is given by the MALS software. The calculated molecular weight of the monomer based on the sequence is 13.6 kDa.
Diverse Roles of Glutamine Residues.
We observed several features of glutamine residues in the current structure that may have general implications for understanding the function of other glutamine-rich domains (13). The HDAC4 four-helix bundle contains a total of 76 glutamines of 272 aa residues, a remarkably high percentage (28%) for any given residue in a protein. The distribution of the glutamine residues in the primary sequence appears to be random (Fig. 2). But in the structure, most of these glutamine residues are located on the surface of the complex (Fig. 5 a and b), except for those forming polar interaction networks that divide the HDAC4 interface into patches (Fig. 5a). Nevertheless, these glutamine residues appear to contribute to the stability of the HDAC4 tetramer through a number of mechanisms. First, glutamine residues mediate a large number of intrahelical interactions that may stabilize the α-helix conformation of HDAC4. For example, at the N-terminal region of helix A (Fig. 5c), Gln-70 accepts a hydrogen bond from Arg-67 while donating one to Glu-74. Gln-69 and Gln-73 and Glu-68 and Gln-72 also form hydrogen-bonding pairs. There are a total of 28 intrahelical hydrogen bonds mediated by glutamine residues in the four-helix bundle. Second, the glutamine residues also participate in interhelical hydrogen bonding interactions. For example, Gln-82 of helix A forms a hydrogen bond with Gln-107 of helix C (Fig. 3b), Gln-83 of helix A forms a hydrogen bond with Arg-102 of helix D (Figs. 3d), and Glu-92 forms a hydrogen bond with Gln-96 of helix C (Fig. 3c). There are a total of 12 interhelical hydrogen bonds mediated by glutamine residues in the HDAC4 tetramer. Finally, the long side chain of glutamine residues participates in extensive van der Waals contact at the HDAC4 tetramer interface. For example, at the interface between helix A and helix C, Leu-71 of helix A inserts between Gln-114 and Gln-115 of helix C (Fig. 3a). At the interface between helix A and helix B, Gln-115 and Leu-119 of helix A make van der Waals contact to Ile-112 and Gln-116 of helix B, respectively (SI Fig. 8). These structural observations suggest that the diverse roles of glutamine in protein–protein interaction may be attributed to its two unique properties. First, glutamine can form hydrogen bonds through the amide group with a variety of amino acids, including both the positively and negatively charged residues. Second, glutamine can make van der Waals contact through the long aliphatic side chain. However, a tradeoff for the diversity appears to be the stability. The lack of extensive hydrophobic and/or electrostatic interactions in the HDAC4 tetramer may explain partly its modest stability (32). Thus, a likely general role of glutamine-rich domains in eukaryotic proteins may be to mediate diverse and reversible protein–protein interactions involved in the assembly and disassembly of protein complexes in cells. In the case of class II HDACs, the full-length protein may be regulated by signals that either stimulate or repress the formation of the tetramer as part of a mechanism of transcriptional regulation.
Fig. 5.
Structural and chemical features of the HDAC4 glutamine-rich domain. (a) Distribution of glutamine residues on the HDAC4 tetramer structure. Although most of the glutamine residues face outside, four clusters form dividing layers at the protein–protein interface. This view is similar to Fig. 1b. (b) Top view of the glutamine distribution. (c) An example of the diverse roles of glutamine residues in the HDAC4 tetramer. (d) Top view of the distribution of histidine residues at the HDAC4 tetramer interface. This view is similar to b.
Implications for pH Sensitivity and Amyloid Formation.
A notable feature of the HDAC4 four-helix bundle is the large number of histidine residues at the protein–protein interface (Fig. 5d). A single His–His packing interaction was previously observed in the Max homodimer (27). But in the present structure, a total of 20 histidine residues are buried in the HDAC4 four-helix bundle, suggesting that the assembly of the HDAC4 tetramer may be sensitive to pH changes (33). Indeed, we have observed that the HDAC4 tetramer dissociates into monomer and lower order oligomers when the pH is shifted below the pKa (pH 6.5) of histidine (data not shown). This observation raises an intriguing possibility that HDAC4 mediated transcriptional repression may be coupled with pH changes inside cells. Moreover, it has been observed that acidic pH and metal ions such as zinc can promote the formation of certain amyloid fibers (34). Thus, an interesting question to be addressed in the future is whether low pH-induced destabilization of the HDAC4 tetramer may lead to amyloid formation by the glutamine-rich domain.
In summary, we have determined a high-resolution structure of the glutamine-rich domain from human HDAC4. The structure shows that the glutamine-rich α-helix adopts a straight conformation and packs in a unique manner as a tetramer. Glutamine residues play diverse roles in stabilizing the α-helix as well as mediating interhelical interactions. The glutamine-rich, four-helix bundle has limited hydrophobic cores separated by polar interaction networks and hydration pockets. Consistent with these structural features, our biochemical analyses reveal that the HDAC4 tetramer undergoes rapid equilibrium with monomer and intermediate species in solution. On the basis of these observations, we suggest that a general role of glutamine-rich motifs may be to mediate reversible protein–protein interactions in transient protein complexes. Because of their unstable nature, glutamine-rich motifs may also be triggered to form amyloid fibers under certain conditions.
Methods
Sample Preparation and Crystallization.
The glutamine-rich domain of human HDAC4 (residues 62–153) was cloned in pET-28a vector and overexpressed in Escherichia coli BL21 (DE3)pLysS (Stratagene, La Jolla, CA). The His-tagged protein was purified by nickel–nitrilotriacetic acid–agarose affinity resin, anion exchange column, and gel filtration. For crystallization, ≈1 μl of protein at 20 mg/ml in storage buffer [10 mM Hepes (pH 7.6)/150 mM NaCl/2 mM DTT] was mixed with ≈1 μl of reservoir solution [55 mM Hepes (pH 7.5)/0.825M Li2SO4] as a hanging drop. Large diamond-shaped crystals grew at room temperature to full size in ≈1 week (≈2 × 0.5 × 0.5 mm3). Crystals belong to the space group C2 with cell dimensions a = 187.398 Å, b = 60.451 Å, c = 60.450 Å, and β = 108.74°. Selenomethionine labeled HDAC4 (residues 63–153) was prepared as described in ref. 24. The selenomethionine protein was purified and crystallized similarly to the native protein.
Data Collection, Structure Determination, and Analysis.
The HDAC4 (residues 62–153) crystals (native and selenomethionine) were stabilized in the harvest/cryoprotectant buffer [50 mM Hepes (pH 7.5)/1.5 M Li2SO4/10% PEG 400] and flash frozen with liquid nitrogen for cryocrystallography. The native data and the single-wavelength anomalous diffraction data were collected at the Advanced Light Source BL8.2.2 beam line at the Lawrence Berkeley National Laboratory. Data were reduced by using HKL2000 (35). The initial positions of selenomethiones were found by SOLVE (36). Phase calculation, density modification, and model refinement were done with CNS (37). The statistics of crystallographic analysis are presented in SI Table 1. Figures of structure illustration were prepared by using Ribbons (38). Model building was carried out in O (39). The sequence alignment was performed by ClustalW (40). The data were severely anisotropic. On most diffraction images, large streaks of X-shaped diffusive scattering superimpose onto the diffraction pattern in the low- and medium-resolution range (SI Fig. 11). These inherent limitations of crystals are reflected by the high B factors and the relatively poor R factors of the final model (SI Table 1). Refinement strategies were explained in the legend of SI Fig. 11. The His97Phe mutant was crystallized similarly to the wild-type protein (space group C2; cell dimensions, a = 189.885 Å, b = 61.023 Å, c = 60.777 Å, and β = 108.610°). The mutant structure was determined by the molecular replacement method (SI Table 2).
Mutagenesis and MALS Analysis.
Site-specific mutations (Phe93Asp and His97Phe) in HDAC4 (residues 62–153) were made with QuikChange (Stratagene). The mutants were expressed and purified similarly to the wild-type protein. The mutants and the wild-type HDAC4 were also analyzed by MALS. For each protein sample, 40 μl of 5–10 mg/ml sample was used for each run. Multiangle laser light scattering data were collected on a DANM DSP laser photometer emitting light at 690 nm and detecting at 18 fixed angle positions (Wyatt Technologies, Santa Barbara, CA). Molecular mass calculations were performed on the ASTRA 4.90.08 software supplied with the instrument. Samples were run over a preequilibrated Shodex KW-803 size exclusion chromatography column at 25°C before collection of light-scattering data. The running buffer condition was 50 mM Hepes (pH 7.0) and 200 mM Na2SO4.
Supplementary Material
Acknowledgments
We thank Ming Lei, Feng Guo, and Steve Edwards for help in data collection and Xiaojiang Chen, Myron Goodman, and James Stroud for critical reading of the manuscript. L.G. and D.L.B. are supported by National Institutes of Health training grants. This research was supported by National Institutes of Health R01 and Shared Instrument grants (to L.C.).
Abbreviations
- HDAC
histone deacetylase
- MALS
multiangle light scattering.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 2H8N (wild-type) and 2O94 (His97Phe mutant)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0608041104/DC1.
References
- 1.Wolffe AP. Science. 1996;272:371–372. doi: 10.1126/science.272.5260.371. [DOI] [PubMed] [Google Scholar]
- 2.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 3.Marks PA, Richon VM, Rifkind RA. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 4.McKinsey TA, Zhang CL, Olson EN. Curr Opin Genet Dev. 2001;11:497–504. doi: 10.1016/s0959-437x(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 5.Verdin E, Dequiedt F, Kasler HG. Trends Genet. 2003;19:286–293. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 6.Youn HD, Grozinger CM, Liu JO. J Biol Chem. 2000;275:22563–22567. doi: 10.1074/jbc.C000304200. [DOI] [PubMed] [Google Scholar]
- 7.Berger I, Bieniossek C, Schaffitzel C, Hassler M, Santelli E, Richmond TJ. J Biol Chem. 2003;278:17625–17635. doi: 10.1074/jbc.M301646200. [DOI] [PubMed] [Google Scholar]
- 8.Sparrow DB, Miska EA, Langley E, Reynaud-Deonauth S, Kotecha S, Towers N, Spohr G, Kouzarides T, Mohun TJ. EMBO J. 1999;18:5085–5098. doi: 10.1093/emboj/18.18.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JK, Sun L, Yang XJ, Zhu G, Wu Z. J Biol Chem. 2003;278:23515–23521. doi: 10.1074/jbc.M301922200. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Dowling M, Yang XJ, Kao GD. J Biol Chem. 2004;279:34537–34546. doi: 10.1074/jbc.M402475200. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins RC, Lis JT. J Mol Biol. 1999;285:515–525. doi: 10.1006/jmbi.1998.2356. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Hasson P, Paroush Z, Courey AJ. Mol Cell Biol. 2004;24:4341–4350. doi: 10.1128/MCB.24.10.4341-4350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michelitsch MD, Weissman JS. Proc Natl Acad Sci USA. 2000;97:11910–11915. doi: 10.1073/pnas.97.22.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo EH, Lansbury PT, Jr, Kelly JW. Proc Natl Acad Sci USA. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perutz MF. Trends Biochem Sci. 1999;24:58–63. doi: 10.1016/s0968-0004(98)01350-4. [DOI] [PubMed] [Google Scholar]
- 16.Serio TR, Lindquist SL. Annu Rev Cell Dev Biol. 1999;15:661–703. doi: 10.1146/annurev.cellbio.15.1.661. [DOI] [PubMed] [Google Scholar]
- 17.Perutz MF, Johnson T, Suzuki M, Finch JT. Proc Natl Acad Sci USA. 1994;91:5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han A, Pan F, Stroud JC, Youn HD, Liu JO, Chen L. Nature. 2003;422:730–734. doi: 10.1038/nature01555. [DOI] [PubMed] [Google Scholar]
- 20.Han A, He J, Wu Y, Liu JO, Chen L. J Mol Biol. 2005;345:91–102. doi: 10.1016/j.jmb.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Kim KK, Yokota H, Kim SH. Nature. 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 22.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 23.Hill RB, Raleigh DP, Lombardi A, DeGrado WF. Acc Chem Res. 2000;33:745–754. doi: 10.1021/ar970004h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy GA, Spedale EJ, Powell ST, Pillus L, Schultz SC, Chen L. J Mol Biol. 2003;334:769–780. doi: 10.1016/j.jmb.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 25.Burkhard P, Stetefeld J, Strelkov SV. Trends Cell Biol. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- 26.Crick FH. Acta Crystallogr A. 1953;6:689–697. [Google Scholar]
- 27.Ferre-D'Amare AR, Prendergast GC, Ziff EB, Burley SK. Nature. 1993;363:38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- 28.Nair SK, Burley SK. Cell. 2003;112:193–205. doi: 10.1016/s0092-8674(02)01284-9. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Nature. 1998;392:42–48. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Zhang CL, McKinsey TA, Lu JR, Olson EN. J Biol Chem. 2001;276:35–39. doi: 10.1074/jbc.M007364200. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Brown JH, Reshetnikova L, Blazsek A, Farkas L, Nyitray L, Cohen C. Nature. 2003;424:341–345. doi: 10.1038/nature01801. [DOI] [PubMed] [Google Scholar]
- 33.Cabezon E, Butler PJ, Runswick MJ, Walker JE. J Biol Chem. 2000;275:25460–25464. doi: 10.1074/jbc.M003859200. [DOI] [PubMed] [Google Scholar]
- 34.Klug GM, Losic D, Subasinghe SS, Aguilar MI, Martin LL, Small DH. Eur J Biochem. 2003;270:4282–4293. doi: 10.1046/j.1432-1033.2003.03815.x. [DOI] [PubMed] [Google Scholar]
- 35.Otwinowski Z, Minor W. In: Methods in Enzymology. Carter CWJ, Sweet RM, editors. Vol 276. New York: Academic; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 36.Terwilliger TC, Berendzen J. Acta Crystallogr D. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 38.Carson M. J Appl Crystallogr. 1991;24:958–961. [Google Scholar]
- 39.Jones TA, Zou JY, Cowan SW, Kjeldgaard Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 40.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Nucleic Acid Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.