Abstract
mAbs that are sensitive to protein conformation can be helpful in studies of protein structure and function; in particular, mAb fragments are useful reagents in membrane protein crystallization. We immunized mice with the rat 5HT2c serotonin receptor and derived clonal hybridoma cells, which we tested for specific antigen reactivity by using the complementarity of purified protein from bacteria and receptor-embedded mammalian cell membranes. Nine mAbs met our criteria for specificity, affinity, and sensitivity to conformational features. Epitopes were mapped in various additional tests. Five of the nine mAbs have cytoplasmic epitopes, and two of these are sensitive to the ligand state of the receptor. These properties should be useful both for structural analysis and in probes of function.
Keywords: conformational sensitivity, epitope mapping, membrane protein crystallization
Monoclonal antibodies (mAbs) can often provide discriminating probes of protein structure and function. Many have great utility as analytical markers for protein identification or affinity tags for protein purification by virtue of their exquisite specificity. Others are useful in functional studies, such as to characterize a molecular surface with respect to protein interactions or to discern conformational changes in response to activity effectors. Some are even effective as therapeutics, as in the case of herceptin (1, 2). Moreover, mAbs can be effective tools for structural biology in that proteins stabilized in complexes with protein ligands are often made more tractable for structure determination. Whereas mAbs raised against peptides suffice as analytical reagents, those used for functional or structural studies generally require binding to conformation-specific epitopes and must be elicited with intact antigens.
There are several examples of the use of mAbs in functional studies. One of considerable relevance for basic biochemistry and for immunology relates to the HIV-1 envelope glycoprotein gp120. A panel of mAbs able to recognize native gp120 was used to identify two molecular surfaces with immunogenic potential, one competive with the CD4 receptor and another induced by CD4 (3–5) and ultimately shown to be competitive with chemokine coreceptors. Another example concerns the biochemistry of NhaA, a bacterial Na+/H+ antiporter that is strongly regulated by pH (6). A specific mAb was shown able to bind only the alkaline pH state of NhaA (7), and eptiope mapping disclosed a previously unpredicted conformational change upon activation (8).
Applications of mAbs in structural biology include crystallographic analyses of HIV gp120 structures (9–11), which has intrinsic flexibility, and of respiratory proteins (12, 13) and potassium channels (14, 15), which reside in membranes. Membrane proteins are notoriously difficult to crystallize, which may be due both to their instability in detergent-embedded aqueous phase and also to the unsuitability of detergent-coated lipophilic surfaces for the formation of useful crystal lattice contacts. Monovalent antibody fragments have been shown to aid crystallization (16), both by helping to fix the conformation and by increasing hydrophilic surface for crystal contacts.
mAbs can be obtained in a variety of ways. Approaches fall into two categories, the first by screening against libraries of antibodies, typically presented to the antigen by phage-display methods. The advantage of this technique resides in the ease with which mAbs can be isolated and genetically modified according to their intended utilization, for example, cocrystallization attempts with membrane proteins (17). The disadvantage is in the limit imposed by the number of molecules expressed in any given library. The second, more traditional approach involves immunizing live animals with antigen to elicit a response, isolating all antibody-producing cells, and screening for specificity in a subsequent step. This procedure is usually lengthier and critically dependent on whether an immune response is obtained of sufficient titer, but the antibody variability that can be produced in vivo typically exceeds that displayed by libraries.
The most suitable mAbs for crystallization of membrane proteins are those that bind the target molecule with high affinity at a conformation-sensitive epitope, typically one that comprises more then a polypeptide segment (16). This finding has given rise to the generally accepted guideline that these mAbs should be able to immunoprecipitate their target protein (high affinity) but fail to recognize this protein on immunoblots derived from denaturing gels (three-dimensional sensitivity). Dissociation of the antibody–membrane protein complex during crystallization is an issue for low-affinity interacting partners, whereas the potential for introducing detrimental flexibility between the associated partners is present when the epitope recognized by the mAb is on a single stretch of polypeptide chain (linear epitope). Monovalent fragments appropriate for crystallization can be produced either by papain digestion (Fabs) or by recombinant expression (Fvs or Fabs) in a wide variety of cellular hosts (reviewed in ref. 18).
G protein-coupled receptors (GPCRs) are a class of membrane proteins that might especially benefit from availability of conformation-sensitive antibodies. These receptors have been notably recalcitrant in crystallization efforts, perhaps in part due to flexibility when extracted from the lipid bilayer and in part due to the limited amount of extramembranous surface. GPCRs are thought to undergo conformational change upon activation by ligand binding or other stimulation, and intrinsic flexibility may be essential for function. mAbs could be useful both for stabilization and for enhancing crystallization probability. Although there are numerous mAbs against GPCRs, many of these, such as the 1D4 mAb elicited by the nine C-terminal residues of rhodopsin (19), are against linear epitopes. There are few examples of conformational mAbs for GPCRs. Rhodopsin is an exception (20, 21), and recent work has established appropriate mAbs for the neurotensin receptor (22). Our study here emphasizes GPCRs that respond to serotonin, 5-hydroxytryptamine (5-HT).
Mammals have at least 15 structurally and pharmacologically distinct receptors for serotonin, and all but two of these are GPCR receptors (23). These receptors are classified into seven evolutionary subfamilies, members of which share common signaling linkages. Subfamily 2 is composed of three members (2a, 2b, and 2c), that couple to Gαq, leading to activation of the phospholipase C-β pathway. 5HT2c from rat origin, a protein of 460 aa residues (24) is the focus of the work presented here. This molecule has a predicted 7TM topology, with three sites of N-linked glycosylation (one at the N terminus, and two contiguous on the IV–V loop), and longer than average V–VI loops and C-terminal tail.
We have expressed rat 5HT2c receptors both in bacteria and in mammalian cells. Here we describe the complementary use of the two expression systems to raise and efficiently screen for anti-5HT2c antibodies in mice. Nine candidate mAbs meeting our criteria for specificity and sensitivity to three-dimensionality were further characterized in several additional tests with respect to epitopes on the receptor, responsiveness to state of activation, and cross-reactivity with the human 5HT2c receptor.
Results
Antibody Production.
mAbs specific to 5HT2c were generated by immunizing mice with antigen expressed and purified in Escherichia coli as a fusion to periplasmic maltose-binding protein (MBP) (MBP-5HT2c), an established way to generate functional GPCRs in bacteria (25). MBP-5HT2c was purified by means of a streptavidin-binding peptide (26) fused to the C terminus of the receptor. A tobacco-etch virus protease (27) recognition site was introduced to allow cleavage of the fusion. A conventional immunization protocol was followed on four animals. To select the animal best responding to the antigen, reactivity against 5HT2c was assessed by Western blot probed with sera from the different animals over a range of dilutions [supporting information (SI) Fig. 6]. Animal no. 2 was euthanized 4 days after a final immunization, and spleen cells were fused to myeloma NS-1 cell line by using standard procedures (28).
Antibody Screening.
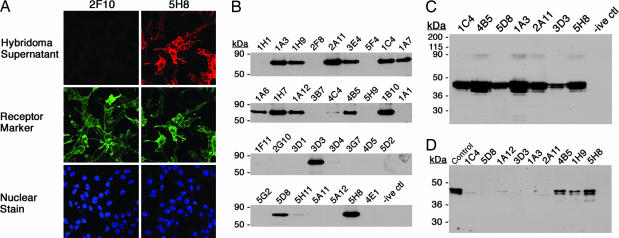
The first objective was to select hybridomas showing specific reactivity toward 5HT2c. To minimize the number of false positives, while at the same time maximizing the selection of antibodies reactive to a receptor in an as close to native conformation as possible, the initial screen was performed on 5HT2c from a different source compared with immunization by immunocytochemistry on mouse fibroblasts (NIH 3T3 cells) stably expressing 5HT2c with a C-terminal KT3 epitope tag (29). These 5HT2c-expressing cells were mixed in a 50:50 ratio with parental cells and fixed only mildly with paraformaldehyde not to compromise the native structure of the receptor, while at the same time assuring sufficient membrane permeabilization for the identification of antibodies reactive to intracellular epitopes. Cells were costained with rabbit anti-KT3 antibody and supernatants from each single hybridoma culture. Only staining patterns that colocalized with that observed with anti-KT3 were deemed as specific, and the hybridoma was maintained for further analyses. A typical example of this screening procedure is shown in Fig. 1A, in which supernatants from one reactive and one nonreactive hybridoma are displayed. Of the original 480 hybridomas, 33 were maintained for further screening.
Fig. 1.
Screening of hybridoma fusions. (A) Screening by immunocytochemistry on NIH 3T3 cells expressing 5HT2c-KT3. Staining patterns from a reactive (5H8) (Top Right) and a nonreactive (2F10) (Top Left) supernatant are shown by labeling the cells, after treatment with the hybridoma supernatants, with CY3-conjugated anti-mouse IgG antibody. (Middle) Simultaneously, expression of 5HT2c was also monitored with rabbit anti-KT3 antibody on the same cells (anti-KT3 Ab) detected with FITC-conjugated anti-rabbit IgG antibody. (Bottom) In addition, in this experiment, cells were stained with nuclear stain TOTO-3. (B) Selection by IP on MBP-5HT2c expressed in E. coli. Supernatants from hybridomas for which specific staining was detected were tested for high affinity to the antigen by IP. The antigen was presented as a detergent solubilized fusion to MBP. The elutions from the IP were run on an SDS/PAGE gel and then transferred to a membrane and probed with rabbit anti-5HT2c Ab (30). The three character codes, shown on top of each lane, refer to the name assigned to that particular hybridoma. Negative control (-ive ctl) was an IP set up with growth medium for the hybridoma cells alone. (C) IP on 5HT2c expressed in mammalian cells. HEK 293 GntI− cells expressing 5HT2c were induced for the expression of this protein. The antigen was presented as a detergent-solubilized membrane fraction. The experiment was performed as discussed in B, and the blot was probed with rabbit anti-5HT2c Ab. (D) mAbs in Western blot analysis. Shown is a screen for conformationally sensitive Abs. Membranes from 293 GntI− cells expressing 5HT2c were solubilized in SDS buffer and loaded (10 μg of protein per lane) for PAGE. The membrane corresponding to each lane was cut and blotted separately with each of the mAbs. The hybridoma supernatants were diluted at 1:2,000, whereas the rabbit anti-2c control Ab was diluted at 1:10,000.
The second objective was to select high-affinity binders to 5HT2c. The 33 hybridomas were tested for their ability to immunoprecipitate receptor from detergent-solubilized E. coli membranes expressing MBP-5HT2c. As shown in Fig. 1B, 14 of the 33 hybridomas had secreted antibodies that could immunoprecipitate the solubilized MBP-5HT2c protein; 12 of these 14 hybridomas were sufficiently stable to allow cloning to single cell purity; and 9 of the 12 corresponding antibodies also had sufficiently high affinity to immunoprecipitate 5HT2c derived from mammalian cells. A subset comprising seven of the immunoprecipitated fractions is displayed in Fig. 1C.
Last, the mAbs were tested for their ability to recognize folded as opposed to denatured receptor. The objective of this investigation was to select the most suitable molecules for cocrystallization experiments. The nine mAbs were tested by Western blot analysis against a receptor presumed to be denatured to an unfolded state by SDS and separated by PAGE. As shown in Fig. 1D, when probed against receptor from mammalian cell origin, six of the nine receptor-specific mAbs failed to show a convincing response, although none of these was completely negative. Complete unfolding cannot be assured in these unboiled SDS experiments with detergent-solubilized receptor, however, so we did not consider this screen to be sufficiently indicative to reduce the numbers further. Thus, all nine mAbs were carried forward for additional characterization.
Epitope Mapping.
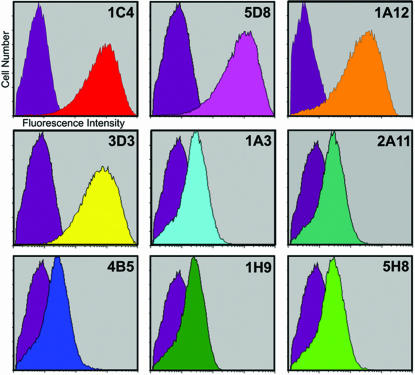
In terms of epitope mapping, the most obvious distinction for an mAb that recognizes a membrane protein is whether it binds to intra- or extracellular regions of its target. To address this question, live mammalian cells, stably transfected with an inducible expression cassette (31, 32) were induced and uninduced for expression of 5HT2c. These were incubated with each individual antibody and subsequently with a fluorescent anti-mouse IgG secondary antibody. As shown in Fig. 2, four reagents to the extra-cellular side of 5HT2c were identified by fluorescence shifts between the induced and uninduced cells by FACS (1C4, 1A12, 3D3, and 5D8). The remaining five mAbs did not produce a substantial shift in fluorescence, and were classified as reactive to the intracellular side of 5HT2c (1A3, 2A11, 1H9, 4B5, and 5H8).
Fig. 2.
Cell sorting to distinguish between extracellular and intracellular reactivity of mAbs to the 5HT2c receptor. HEK 293 GntI− cells induced and uninduced for expression of 5HT2c were incubated separately with each mAb and with secondary FITC-conjugated anti-mouse Ab. One hundred thousand cells for every group were passed through a FACS, recording the fluorescence of each cell. Shown are the two resulting fluorescence plots for nine given antibodies in logarithmic scale, one for uninduced (all in purple) and one for induced (in different colors) cells. Curves from stained cells exhibiting a strong shift in fluorescence upon receptor expression are shown in the first four plots in red, pink, orange, and yellow.
On its intracellular face, 5HT2c has three loops and a tail. The first two loops, between transmembrane helices (TMs) I–II and III–IV are short, whereas the V–VI loop and the C-terminal tail are long, measuring ≈70 and 73 aa residues after the palmitoylation site, respectively. It thus seemed plausible that the mAbs reactive to the intracellular portion of 5HT2c were likely to recognize epitopes in these two latter regions. To verify this hypothesis, constructs expressing 5HT2c in which either the V–VI loop (Δ 244–299) or the tail (387 stop) had been deleted were transiently transfected together with appropriate controls and plated on slides, and the mAbs were tested by immunocytochemistry. A subset of these experiments is shown in Fig. 3A. The data point to the C-terminal region as being a principal target of all intracellular-reacting mAbs and to a lack of V–VI loop epitopes for any of the mAbs.
Fig. 3.
Epitope mapping. (A) Immunocytochemistry on HEK 293 cells transiently transfected with empty vector, wild-type 5HT2c, and with constructs in which either the V–VI intracellular loop or the C-terminal tail were deleted. These were stained with either extracellular reactive 1C4 (Upper) or intracellular reactive 2A11 (Lower) mAbs and with CY3-coupled anti-mouse IgG secondary antibody. Cells were also treated with nuclear stain TOTO-3, shown in blue. Images were captured by confocal microscopy. (B) ELISA comparing reactivity toward 5HT2c of rat and human origin. Membranes (10 μg per well) were plated, and each point was assayed in triplicate. Background, calculated from a control antibody not reactive to 5HT2c, was subtracted from the data. Reactivity toward human and rat protein is shown in blue and red, respectively. 1C4, 3D3, 1A12, and 5D8 recognize the extracellular side of 5HT2c, and 2A11 recognizes the intracellular side. (C) Deglycosylation in the presence of mAbs. Membranes (2.5 μg) from cells induced and uninduced (last two lanes) for expression of 5HT2c were loaded in each lane after preincubation with mAbs and treatment with peptide-N-glycosidase F (PNGase F). The blot was probed with rabbit anti-5HT2c antibody. A sample that was not deglycosylated, a sample that was treated with an intracellular-binding mAb (5H8), and membranes derived from uninduced cells are also shown on the blot.
The C terminus of 5HT2c extends for 73 residues after the putative palmitoylated cysteine (Cys-387). To further narrow the regions along the C terminus responsible for binding of these mAbs, constructs in which stop codons had been inserted along this region at positions 387, 402, 417, 431, and 445 were expressed as fusions to MBP and assayed for antibody binding. Membranes were absorbed to ELISA plates and tested for reactivity against each of the five intracellular-reactive mAbs as well one extracellular mAb (1C4) as a control for expression of these truncated receptors (data not shown). Binding of internal mAbs could only be detected when the C terminus was truncated at position 417 or further toward the C terminus (431 and 445). These data suggest that a predominant recognition region for the mAbs reactive to the intracellular side of 5HT2c must reside in the segment comprising residues between 387 and 417.
We also investigated the ability of these mAbs raised against the rat 5HT2c receptor to recognize 5HT2c of human origin as well. The two proteins share ≈90% sequence identity, and the distinctions may affect reactivity and thereby help to specify epitopes. Immunocytochemistry on cells transiently transfected with 5HT2c from either species, showed indistinguishable staining patterns with all of the intracellular-reactive mAbs. In contrast, when the extracellular mAbs were tested, staining on human protein appeared to be less intense, and the extent of this decrease seemed to vary according to the mAb applied. To confirm and quantify this impression, membranes derived from cells transiently expressing the human and rat forms of 5HT2c were absorbed to a plate and reactivity toward the mAbs assayed by ELISA. Indeed, all extracellular-reactive mAbs showed decreased reactivity toward the human protein (Fig. 3B), with 1C4 and 1A12 exhibiting the most dramatic reductions. Surprisingly, ELISA signal for the intracellular-reactive group appeared to be substantially enhanced for human protein (2A11 is shown in Fig. 3B). Given the indistinguishable staining patterns between the two species, suggesting comparable protein expression levels per cell, this phenomenon could possibly be due to higher transfection efficiency for the construct bearing the human gene. If taken into account, this could result in an even lower affinity for human versus rat protein for the extracellular-reactive mAbs.
Finally, we examined the effect of glycosylation on recognition. The rat 5HT2c receptor has three sites of attachment for N-linked glycosylation: one at the N terminus and two on the IV–V loop, and the mature protein expressed in mammalian cells is fully modified (F.M. and W.A.H., unpublished observation). Essentially, by the definition given our screening procedures, the presence of sugars did not appear to impede or mask antibody–antigen recognition for the selected antibodies (Figs. 1C, 2, and 3A). We contemplated that the presence of bound antibody might nevertheless affect accessibility to specific carbohydrate sites, but, in fact, the converse seemed true. The addition of saturating concentration of antibody did not prevent subsequent enzymatic cleavage of carbohydrates from the receptor at any site of attachment. Treatment of membranes derived from mammalian cells expressing 5HT2c with endoglycosidase F systematically resulted in complete removal of the carbohydrates from all three sites of attachment (data not shown). No differences in the efficiency of deglycosylation could be observed when these membranes were preincubated with an excess of each mAb reactive to the extracellular side of 5HT2c (Fig. 3C).
Functional Testing.
Sensitivity of the mAbs to the activation state of 5HT2c was probed by challenging these to immunoprecipitate the agonist (5-HT) and inverse agonist (ketanserin) bound forms of the receptor. Membranes from mammalian cells expressing 5HT2c were treated with saturating concentrations of either ligand, solubilized, and immunoprecipitation (IP) experiments performed and analyzed as described in Fig. 1C. The resulting Western blots showed that all mAbs recognized the agonist-bound state, as could be predicted given the presence of 5-HT at immunization, circulating at high concentration in the injected animals. In contrast, two mAbs (2A11 and 1H9) failed to recognize the inverse agonist-bound, inactivated form (Fig. 4). 2A11 and 1H9 are both reactive to the C-terminal tail region of 5HT2c. Interestingly, 1H9 was shown to be reactive by Western blot analysis in Fig. 1D and, hence, tentatively classified as insensitive to conformation. These data may shed some doubts on the trustworthiness of probing for conformational sensitivity by Western blotting alone.
Fig. 4.
Ligand-state sensitivity. Shown is IP in the presence of 5-HT and ketanserin. Solubilized, mammalian cell-derived membranes expressing 5HT2c were treated with either agonist or antagonist and immunoprecipitated with 1A3, 2A11, and 1H9. The blot of the resulting fractions was probed with rabbit anti-5HT2c antibody.
Discussion
We find in this study that a mouse immunized with a detergent-solubilized serotonin receptor from the rat can evoke a potent immune response directed against the receptor in membranes on mammalian cells. By immunizing with purified receptor expressed in bacteria and by testing with receptors in mammalian cell membranes, we efficiently identified truly selective antibodies. After screening 480 hybridoma cell lines by using assays with both receptor-expressing cells and purified receptor molecules, we identified and characterized nine monoclonal antibodies against 5HT2c (SI Table 1). The epitopes of these antibodies were delimited in various tests, showing that the set includes diverse binding sites, both extracellular and cytoplasmic. Most, if not all of these antibodies recognize conformational rather than linear epitopes, including ones that can discriminate the state of activity of the receptor. This response against the rat receptor by the mouse, whose own 5HT2c receptor is 98% identical in sequence to that of the rat, is consistent with predominant if not exclusive neuronal expression of the 5HT2c receptor (23), whereby the mouse immune system is naïve to this antigen.
Although the sites of 5HT2c binding for the nine characterized mAbs cannot be defined precisely, certain epitopal aspects are clear. First, by FACS analysis, four of the antibodies definitively bind to the extracellular surface (Fig. 2). That the other five do indeed bind to the cytoplasmic surface is evident from the lack of reactivity to extreme deletions from the C terminus. By contrast, all of the cytoplasmic mAbs bind to a Δ(V–VI) loop deletant and also to the human 5HT2c receptor. Moreover, although there was no binding to rat 5HT2c truncated after residue 387 or 402, binding was restored for the truncations at 417 or thereafter. The human and rat sequences differ at many sites in the V–VI loop and in stretches 384–393 and 425–433 (rat), but they are identical in loops I–II and III–IV and in the 402–417 span identified by deletion analysis as containing epitopes for the five cytoplasmic antibodies. The epitopes for all these cytoplasmic antibodies are surprisingly similar at this level of resolution, but they do clearly differ because there are distinctions in Western blotting efficiency (Fig. 1D) and in response to ketanserin-complexed 5HT2c (Fig. 4). There are fine distinctions in the epitope definition for the extracellular mAbs as well. The selected mAbs bind equally well to receptor produced in bacteria (nonglycosylated) and in mammalian cells (fully glycosylated) (Figs. 1B and 2), and none of these mAbs interfere with deglycosylation (Fig. 3C). The sites of glycosylation are in the N-terminal tail and in loop IV–V, and these segments near these sites are not likely epitopes. There are distinctions in binding to rat versus human 5HT2c receptors, which are identical in extracellular loop II–III but differ in loop VI–VII as well as in a portion of the N-terminal tail. Taken together, these observations point to a likely involvement of the II–III loop in some and of the VI–VII loop in others. A schematic illustration of epitope observations is shown in Fig. 5.
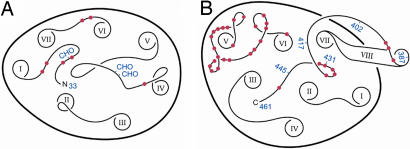
Fig. 5.
Schematic illustration of rat 5HT2c extramembranous surfaces. Drawings are based on the structures of rhodopsin (33, 34), with helix positions and traces for conserved loops based on those structures. The positions of unconserved loops are fanciful. (A) Extracellular surface. Sites of carbohydrate attachment are labeled “CHO” in blue. (B) Cytoplasmic surface. Positions of C-terminal truncations are indicated in blue by numbers. Positions of difference between human and rat 5HT2c are shown in red in both A and B. Helix ends are designated by Roman numerals. The two views are taken perpendicular to the membrane surface as rotated by 180° about the vertical.
GPCR activation, whether by agonist binding as by 5-HT to a serotonin receptor or by photoconversion of 11-cis to all-trans retinal in the case of rhodopsin, leads to changes that must be communicated to the cytoplasmic surface where interactions with the heterotrimeric G protein occur. Biophysical studies, notably EPR labeling experiments, suggest that large conformational changes occur during photoactivation of rhodopsin (35). In contrast, the recent structure of a photoactivated intermediate of rhodopsin shows little change from the inactive state and highly flexible cytoplasmic domains, leading to the suggestion that this plasticity may facilitate an induced fit binding of the G protein transducin (36). Pharmacological agents known as inverse agonists, such as ketanserin in the case of 5HT2c, are thought to lock the receptor in an inactive, nonsignaling state like that of rhodopsin with 11-cis retinal in the dark; true agonists generate an active state that catalyzes the exchange of GTP for GDP in the Gα subunit of an associated heterotrimeric G protein. Antibodies 2A11 and 1H9 cleanly discriminate these two states, binding to receptor in the presence of serotonin but not in the presence of ketanserin. Both of these antibodies engage cytoplasmic surfaces of 5HT2c, including the C-terminal tail, and they clearly see distinct conformations for the two states. How much these might differ remains to be determined, and precise identification of ketanserin with the inactive state may also need work, but our results seem superficially at odds with the suggestion that activated and inactive GPCRs may differ only minimally. Interestingly, none of our four extracellular antibodies discriminate between the serotonin and ketanserin states of 5HT2c.
An important application of monoclonal antibodies, and the main motivation for this study, is their utility in stabilizing flexible proteins and enhancing their probability for crystallization. Conformation-specific antibodies, like natural binding partners, serve to reduce intrinsic flexibility through their interactions. In the case of membrane proteins such as GPCRs, we expect some loss of integrity when removed from their natural lipid bilayer environment into a detergent micelle. Moreover, there is a large entropic penalty for the formation of crystal contacts involving flexible regions. Thus, such regions tend to be excluded from lattice contact and crystallization probability is sharply reduced when a substantial portion of the molecular surface is flexible (37). Because detergent-solubilized surfaces of membrane proteins are inherently flexible, and this is an especially large fraction for many GPCRs, this effect becomes an important factor for GPCR crystallization. The expansion of fixed, water-soluble surfaces through complexation with Fab or Fv antibody fragments increases the probability of membrane-protein crystallization, both theoretically (37) and in practice (16), and there are several successful applications (12, 14, 15, 38). The mAbs identified here have appropriate characteristics (SI Table 1) for such application with the 5HT2c receptor.
Materials and Methods
Receptor Expression in Mammalian Cells.
Stable cell lines were generated by transfection with cDNA for the rat 5HT2c receptor and selection on the basis of introduced markers. NIH 3T3 cell lines stably expressing 5HT2c were generated by antibiotic selection followed by an in vivo amplification step (39). HEK293 GntI− cell lines for inducible expression of 5HT2c were generated and maintained following published protocols (31). Cells were grown in tissue culture at 37°C.
Receptor Expression in E. coli.
An expression construct was introduced into E. coli for the production of 5HT2c as a C-terminal fusion to MBP with a recognition site for tobacco-etch virus protease engineered between these components, as well as a nona-histidine tag at the N terminus of the mature MBP and a streptavidin-binding peptide tag at the receptor C terminus. This fusion protein was under weak Lac promoter control, and cells were grown at 18°C after induction with isopropyl β-d-thiogalactoside.
Isolation of Membranes.
Bacteria resuspended from E. coli cell pellets were broken open in a French press, intact cells and debris were removed by centrifugation, and membranes were then pelleted by ultracentrifugation and resuspended by dounce homogenization. Mammalian cells harvested in PBS were pelleted by centrifugation and then were resuspended in dilute salt for osmotic lysis. After dounce homogenization, nuclei were separated by centrifugation and membranes were pelleted and resuspended as for the bacterial membranes. All steps were performed on ice or at 4°C.
Purification of MBP-5HT2c.
E. coli membranes were solubilized in a dodecylmaltoside/cholesterol hemisuccinate mixture and cleared of insoluble matter by ultracentrifugation. The 5HT2c fusion protein was then captured on streptavidin beads, eluted with desthiobiotin after separation, cleaved with tobacco-etch virus protease, and cleared of protease, MBP, and uncleaved MBP-5HT2c by passage through Ni+2-nitrilotriacetic acid beads. 5HT2c was concentrated to ≈1 mg/ml and used for immunizations.
Production of Monoclonal Antibodies.
Female BALB/c mice were immunized and boosted following established procedures. Animals were tail bled after the third boost, and sera were tested to select the best candidate for fusion. One animal was euthanized 4 days after boosting. Its spleen was removed and spleen cells were fused to myeloma NS-1 cells. Hybridoma cell selection was performed by hypoxanthine, aminopterin, and thymidine selection. Clonal purity was achieved in limiting dilution conditions, and the resulting hybridoma cells were maintained on primary macrophages. All research involving animal subjects was approved by the Institutional Animal Care and Use Committee.
Immunocytochemistry.
NIH 3T3 cells were plated onto glass slides as 50:50 mixtures of parental cells with cells stably transfected with 5HT3c containing a C-terminal KT3 tag, fixed with paraformaldehyde, blocked with goat serum in PBS, and incubated with hybridoma supernatants. Rabbit anti-KT3 antibody (Bethyl Laboratories, Montgomery, TX) was also included in the preparation, and the secondary antibodies used for detection were Cy3-conjugated anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) and FITC-conjugated anti-rabbit IgG (Jackson ImmunoResearch). Labeled cells were screened on a standard fluorescence microscope, and the results documented and confirmed by confocal microscopy. TOTO-3 iodide (Invitrogen, Carlsbad, CA) was used as a nuclear stain.
FACS Analysis.
A Beckman Coulter (Fullerton, CA) Altra flow cytometer was used to detect photoemission from a secondary antibody labeled with R-phycoerythrin upon excitation by a krypton–argon laser. The viable cell population was determined by using the forward- and side-scatter characteristics of the cells, and data from 30,000 viable cells were collected for each assay.
IPs.
Membranes were solubilized in dodecylmaltoside/cholesterol hemisuccinate mixture or digitonin and cleared of the insoluble material by ultracentrifugation, and appropriate volumes of solubilized receptor and of a hybridoma culture supernatant were combined with a mixture of protein A-coupled and protein G-coupled Sepharose beads. Assays were typically incubated overnight at 4°C under gentle agitation and then were washed with buffer and eluted at pH 2.7. The recovered sample was then pH neutralized for SDS/PAGE analysis.
ELISA.
For ELISA based on membranes, the membranes were diluted in PBS and transferred to wells of a high-binding 96-well plate (Corning, Midland, MI) and incubated overnight at 4°C overnight. Immobilized membranes were blocked with nonfat dry milk and BSA, and then tested with antibodies in hybridoma supernatants. An alkaline–phosphatase-conjugated anti-mouse IgG (Sigma, St. Louis, MO) was used as the secondary antibody, and binding was detected by colorimetry from reaction with p-nitrophenyl phosphate in a diethanolamine-based buffer (Pierce, Rockford, IL).
Carbohydrate Cleavage Experiments.
Membranes from HEK293 GntI− cells expressing 5HT2c were suspended either in medium alone or in a hybridoma supernatant and incubated for 2 h at room temperature. Deglyscosylations were then performed with N-glycosidase F (New England Biolabs, Ipswich, MA) for 1 h on ice. Results were analyzed by SDS/PAGE in blots probed with rabbit anti 5HT2c antibody.
Detailed aspects of the Materials and Methods are presented as SI Text.
Supplementary Material
Acknowledgments
We thank Reinhard Grisshammer for helpful comments on the manuscript and Qing Fan for discussions and help with Fig. 5. This work was supported in part by National Institutes of Health Grant GM68671.
Abbreviations
- 5-HT
5-hydroxytryptamine
- GPCR
G protein-coupled receptor
- IP
immunoprecipitation
- MBP
maltose-binding protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700301104/DC1.
References
- 1.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Ditzel HJ, Parren PW, Binley JM, Sodroski J, Moore JP, Barbas CF, III, Burton DR. J Mol Biol. 1997;267:684–695. doi: 10.1006/jmbi.1997.0912. [DOI] [PubMed] [Google Scholar]
- 4.Moore JP, Sodroski J. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 6.Rothman A, Gerchman Y, Padan E, Schuldiner S. Biochemistry. 1997;36:14572–14576. doi: 10.1021/bi971800y. [DOI] [PubMed] [Google Scholar]
- 7.Padan E, Venturi M, Michel H, Hunte C. FEBS Lett. 1998;441:53–58. doi: 10.1016/s0014-5793(98)01524-5. [DOI] [PubMed] [Google Scholar]
- 8.Venturi M, Rimon A, Gerchman Y, Hunte C, Padan E, Michel H. J Biol Chem. 2000;275:4734–4742. doi: 10.1074/jbc.275.7.4734. [DOI] [PubMed] [Google Scholar]
- 9.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA, et al. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong PD, Wyatt R, Majeed S, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure (London) 2000;8:1329–1339. doi: 10.1016/s0969-2126(00)00547-5. [DOI] [PubMed] [Google Scholar]
- 11.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure (London) 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 13.Ostermeier C, Iwata S, Ludwig B, Michel H. Nat Struct Biol. 1995;2:842–846. doi: 10.1038/nsb1095-842. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 16.Hunte C, Michel H. Curr Opin Struct Biol. 2002;12:503. doi: 10.1016/s0959-440x(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 17.Rothlisberger D, Pos KM, Pluckthun A. FEBS Lett. 2004;564:340–348. doi: 10.1016/S0014-5793(04)00359-X. [DOI] [PubMed] [Google Scholar]
- 18.Andersen DC, Reilly DE. Curr Opin Biotechnol. 2004;15:456–462. doi: 10.1016/j.copbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie D, Arendt A, Hargrave P, McDowell JH, Molday RS. Biochemistry. 1984;23:6544–6549. doi: 10.1021/bi00321a041. [DOI] [PubMed] [Google Scholar]
- 20.Adamus G, Zam ZS, Arendt A, Palczewski K, McDowell JH, Hargrave PA. Vision Res. 1991;31:17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
- 21.Molday RS, MacKenzie D. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- 22.Niebauer RT, White JF, Fei Z, Grisshammer R. J Recept Signal Transduct Res. 2006;26:395–415. doi: 10.1080/10799890600928228. [DOI] [PubMed] [Google Scholar]
- 23.Barnes NM, Sharp T. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 24.Julius D, MacDermott AB, Axel R, Jessell TM. Science. 1988;241:558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- 25.Grisshammer R, Duckworth R, Henderson R. Biochem J. 1993;295:571–576. doi: 10.1042/bj2950571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keefe AD, Wilson DS, Seelig B, Szostak JW. Protein Expr Purif. 2001;23:440–446. doi: 10.1006/prep.2001.1515. [DOI] [PubMed] [Google Scholar]
- 27.Dougherty WG, Carrington JC, Cary SM, Parks TD. EMBO J. 1988;7:1281–1287. doi: 10.1002/j.1460-2075.1988.tb02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler G, Milstein C. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 29.MacArthur H, Walter G. J Virol. 1984;52:483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backstrom JR, Sanders-Bush E. J Neurosci Methods. 1997;77:109–117. doi: 10.1016/s0165-0270(97)00102-7. [DOI] [PubMed] [Google Scholar]
- 31.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Proc Natl Acad Sci USA. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves PJ, Kim JM, Khorana HG. Proc Natl Acad Sci USA. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. J Mol Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 34.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 35.Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Adv Protein Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- 36.Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong PD, Wyatt R, Desjardins E, Robinson J, Culp JS, Hellmig BD, Sweet RW, Sodroski J, Hendrickson WA. J Biol Chem. 1999;274:4115–4123. doi: 10.1074/jbc.274.7.4115. [DOI] [PubMed] [Google Scholar]
- 38.Ostermeier C, Essen LO, Michel H. Proteins. 1995;21:74–77. doi: 10.1002/prot.340210110. [DOI] [PubMed] [Google Scholar]
- 39.Julius D, Livelli TJ, Jessell TM, Axel R. Science. 1989;244:1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.