Abstract
There is increasing evidence that species extinctions jeopardize the functioning of ecosystems. Overfishing and other human influences are reducing the diversity and abundance of fish worldwide, but the ecosystem-level consequences of these changes have not been assessed quantitatively. Recycling of nutrients is one important ecosystem process that is directly influenced by fish. Fish species vary widely in the rates at which they excrete nitrogen and phosphorus; thus, altering fish communities could affect nutrient recycling. Here, we use extensive field data on nutrient recycling rates and population sizes of fish species in a Neotropical river and Lake Tanganyika, Africa, to evaluate the effects of simulated extinctions on nutrient recycling. In both of these species-rich ecosystems, recycling was dominated by relatively few species, but contributions of individual species differed between nitrogen and phosphorus. Alternative extinction scenarios produced widely divergent patterns. Loss of the species targeted by fishermen led to faster declines in nutrient recycling than extinctions in order of rarity, body size, or trophic position. However, when surviving species were allowed to increase after extinctions, these compensatory responses had strong moderating effects even after losing many species. Our results underscore the complexity of predicting the consequences of extinctions from species-rich animal communities. Nevertheless, the importance of exploited species in nutrient recycling suggests that overfishing could have particularly detrimental effects on ecosystem functioning.
Keywords: biodiversity, cichlid, nutrient cycling, stoichiometry, species identity
Understanding the consequences of species extinctions for ecosystem functioning is a critical challenge. There is substantial evidence that declining species richness alters ecosystem processes in experimental systems with simple spatial and trophic structure (1), but this relationship remains poorly understood in species-rich, natural ecosystems (2). The large size, high mobility, and complex trophic relationships of vertebrate species make assessing the potential consequences of their extinctions particularly challenging.
Fish are the most species-rich group of vertebrates, and their diversity is threatened worldwide by overfishing, species introductions, and other factors (3–6). Although the collective influence of fish on food web structure (7, 8), nutrient cycling (9, 10), and primary productivity (11) is well documented, the ecosystem-level effects of eroding fish species richness are unclear. Tropical freshwater fish are of special concern because they represent >10% of all vertebrate species (12, 13) and support ≈72% of global fish harvests from inland waters (14). These fisheries provide vital animal protein for hundreds of millions of people in developing countries and benefit terrestrial conservation efforts by alleviating demand for bush meat (15).
Nutrient recycling offers an ideal quantitative basis for directly linking fish species and ecosystem functioning. Fish store a large proportion of ecosystem nutrients in their tissues, transport nutrients farther and faster than other aquatic animals, and excrete dietary nutrients in dissolved forms that are readily available to primary producers (9). Individual species vary widely in their recycling of nitrogen (N) relative to phosphorus (P) (16–18); thus, fish community composition could affect aggregate rates of nutrient recycling as well as the ratio of available N and P, which determines the identity of the nutrient that limits primary productivity (19). At an ecosystem scale, nutrient recycling by fish is often important in tropical waters (17, 18, 20, 21), where rapid turnover of nutrients is required to sustain high primary productivity (22, 23).
We assessed nutrient recycling by the complete fish fauna of a Neotropical river (Rio Las Marias, RLM; 69 species) and by diurnal, nearshore fish in Lake Tanganyika (LT), Africa (36 species). Species-specific contributions were estimated from field measurements of excretion of dissolved N and P by individual fish, and extensive censuses of population densities. Here, we use these data in probabilistic, numerical simulations to test how extinctions would affect the rates and N:P ratio of nutrient recycling by fish at each site.
We apply this simulation approach to four issues that are critical for predicting the ecosystem-level effects of extinctions from natural communities. First, responses by surviving species often moderate the consequences of extinctions (24–27); therefore, we compare random series of extinctions in which populations of surviving species are either held constant or increased to replace extinct species. The replacement scenario uses metabolic scaling rules to estimate the numerical response required to fill the energetic role of extinct species from the same trophic guild. Second, real-world patterns of animal extinctions are generally nonrandom (28, 29) and may produce weaker or stronger effects on ecosystem processes than randomly ordered loss of species (24–27, 30). We evaluate declines in nutrient recycling predicted from the following known correlates of extinction risk in fish: small population size, high trophic position in the food web (3, 4), large body size (5), and high fishing pressure. The implications of these nonrandom scenarios are compared with those of random extinction series, as well as best- and worst-case scenarios where the probability of extinction is negatively or positively related to a species' contribution to aggregate nutrient recycling. Third, species often vary widely in their contributions to ecosystem processes (30–32), making the relationship between biodiversity and ecosystem functioning dependent on the identity of extinct species (25, 27, 30). We analyze how contributions to nutrient recycling are distributed across fish species and quantitatively separate the influence of biomass dominance and species identity on contributions by individual species. Finally, because the consequences of declining biodiversity can differ among ecosystem processes (1), we evaluate whether N and P recycling by fish are affected similarly by extinctions.
Results
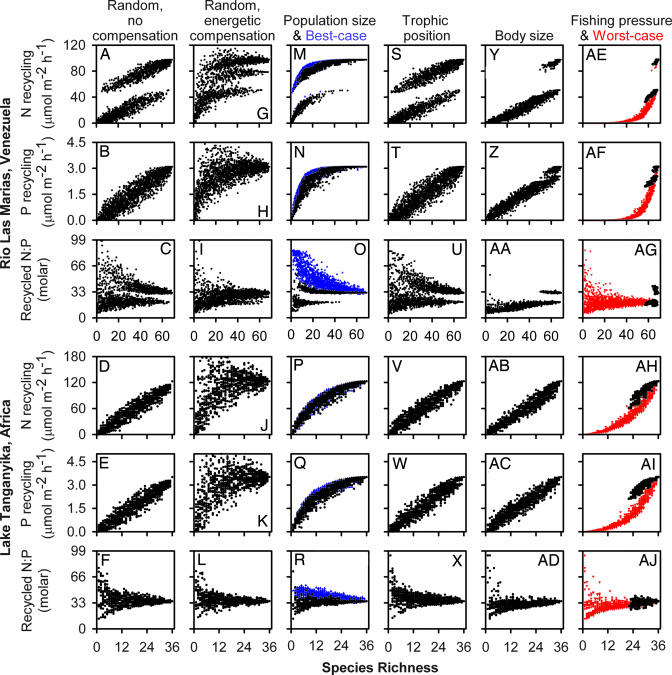
The effects of fish extinctions on nutrient recycling depended strongly on both the capacity of surviving species to compensate and the order in which species were lost [supporting information (SI) Table 1]. In the absence of compensatory responses, recycling of N and P declined linearly with random species extinctions in both ecosystems (Fig. 1A, B, D, and E). Variance in nutrient recycling rates was high but relatively constant, whereas the predictability of the excreted N:P ratio was inversely related to species richness (Fig. 1 C and F). In RLM, a striking bifurcation in predicted N recycling arose from extinction of a single species, Prochilodus mariae (Fig. 1A). Prochilodus alone produced 47% of recycled N, reflecting its combination of large size and high density (10).
Fig. 1.
Effects of extinctions on nutrient recycling by fish in RLM and LT. Shown are 25 simulations of random extinctions without (A–F) or with (G–L) compensation by surviving species, and nonrandom extinctions reflecting increasing risk with small population size (M–R), high trophic position (S–X), large body size (Y–AD), and observed fishing pressure (AE–AJ). For comparison, best- (M-R, blue) and worst-case (AE–AJ, red) scenarios are indicated. A few results exceeded the maximum y axis value in C, H–K, U, and AG. Note bifurcating (e.g., A and C) and trifurcating (G) patterns associated with loss of Prochilodus mariae, which dominates N recycling in RLM.
When populations of surviving species increased to compensate for loss of species from the same trophic guild, mean N and P recycling declined <20% until more than the species were lost from each ecosystem (Fig. 1 G, H, J, and K). In fact, aggregate recycling rates increased by up to 58% in RLM and 208% in LT when species with the highest recycling rates replaced counterparts with lower rates. As species richness dwindled, major decreases in recycling rates were driven by loss of trophic guilds rather than particular species. The importance of guilds was most evident for N recycling in RLM (Fig. 1G). Only one species (Steindachnerina argentea) was available to replace the dominant detritivore (Prochilodus), yielding a trifurcation representing cases where Prochilodus persisted, Prochilodus was extinct but replaced by Steindachnerina, or both species were extinct.
Nonrandom extinction scenarios produced widely divergent outcomes. Loss of rare species had the weakest effects in each ecosystem, and was similar to the best-case scenario in which the order of extinctions minimized declines in recycling (Fig. 1 M, N, P, and Q). Extinction in order of trophic position had intermediate effects that were equivalent to random extinctions without compensation (Fig. 1 S–X). This pattern arose because top predators had low population densities and included small “parasitic” species that feed upon scales, fins, or mucus of larger fish. Size-dependent risk also yielded results similar to random extinctions (Fig. 1 Y–AD), except when early loss of Prochilodus rapidly reduced N recycling in RLM (Fig. 1Y).
Basing extinction risk on observed patterns of fishing pressure (SI Table 2) produced the sharpest reductions in nutrient recycling in both ecosystems (Fig. 1 AE–AJ). Indeed, loss of major fishery species resulted in recycling rates that sometimes approached the worst-case scenario. Overfishing also reduced the aggregate N:P ratio of recycled nutrients, particularly in RLM (Fig. 1 AG and AJ).
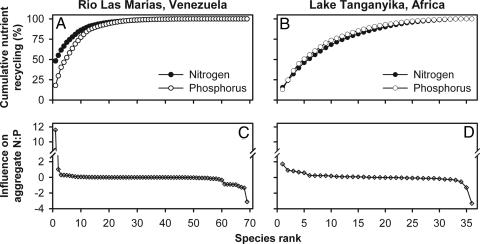
The contrasting patterns arising from alternative extinction scenarios reflect strong skew in the contributions of individual species to aggregate nutrient recycling in both RLM and LT. Estimated contributions to N and P recycling differed among species by more than three orders of magnitude, and the top eight species contributed 61–80% of the total in each system (Fig. 2 A and B). As a result, only 6 of 69 species in RLM and 3 of 36 species in LT affected aggregate recycled N:P ratios by more than ±1.0 (Fig. 2 C and D), despite the broad range of recycling ratios exhibited by individual species (molar N:P ranged from 6 to 176 in RLM and from 13 to 126 in LT; ref. 17). Particularly extreme disparities in contributions among species in RLM, due primarily to Prochilodus, led to discontinuities in N recycling relative to species richness and enhanced the difference between the best- and worst-case scenarios compared with LT (Fig. 1).
Fig. 2.
Roles of individual fish species in aggregate nutrient recycling in RLM and LT. Images illustrate species in rank order of contributions to N and P recycling (A and B), and influence on excreted N:P (C and D). Each curve represents a separate ranking of species; therefore, the order of particular species differs between curves. Note the break in the y axis in C and D to accommodate the dominant influence of P. mariae in RLM.
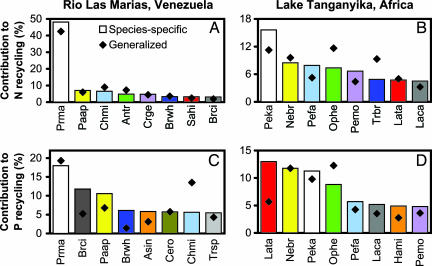
The variation among species in contributions to nutrient recycling arose from differences in both relative biomass within the community and species-specific influences on excretion rates. Across all species, biomass alone explained 99% of variation in contributions to N recycling in RLM (F1,67 = 8986.48, P < 0.001) and 83% in LT (F1,34 = 165.40, P < 0.001). However, biomass explained much less variation in P recycling in RLM (57%, F1,67 = 87.74, P < 0.001) and LT (48%, F1,34 = 31.81, P < 0.001), revealing the importance of species identity. Further evidence of identity effects was provided by comparisons of contributions to aggregate nutrient recycling derived from species-specific versus generalized size-specific excretion rates. Although species-specific and generalized estimates of contributions to N recycling were concordant in RLM (Fig. 3A), they differed in both magnitude and rank order for N recycling in LT (Fig. 3B) and P recycling at both sites (Fig. 3 C and D).
Fig. 3.
Identity of species contributing most to N (A and B) and P (C and D) recycling in RLM and LT. Individual species are indicated by colors and taxonomic abbreviations (see SI Table 3). Note the differences in rank order of species between N and P recycling. Contributions estimated by using species-specific recycling rates (bars) reflect both species identity and biomass dominance, whereas estimates using generalized size-specific recycling rates (diamonds) reflect only biomass dominance. Differences between bars and diamonds represent the influence of species identity.
There were also striking differences between the contributions of individual species to N versus P recycling (Fig. 3). For example, Lamprichthys tanganicae in LT ranked first in P recycling (13.2% of total; Fig. 3D) but only seventh in N recycling (4.8% of total; Fig. 3B). Similar shifts in quantitative contributions and rank order among other species led to a lack of correlation between N and P recycling in LT (n = 10, r = 0.549, P = 0.101), and correlation driven only by Prochilodus in RLM (with Prochilodus, n = 12, r = 0.779, P = 0.003; without Prochilodus, n = 11, r = 0.194, P = 0.567).
Discussion
This study provides a quantitative assessment of how anthropogenic erosion of fish species richness may affect the functioning of ecosystems. Our results indicate that declining fish diversity is likely to alter nutrient recycling. Comparisons among extinction scenarios, sites, and nutrients reveal repeated patterns that may be general, but also underscore the complexity of predicting the ecosystem-level effects of extinctions from species-rich natural communities.
Nonrandom extinction scenarios produced markedly different outcomes than random ones, as observed in previous studies with other taxa (24–27, 30). For ecosystem processes that are positively related to the biomass of organisms, such as nutrient recycling, it is not surprising that loss of rare species would have only minor effects. The similarity between the results of random extinctions and loss of species in order of either body size or trophic position is intriguing, as large species and top predators are frequently considered to be most affected by human activities (3–5). However, our fishery surveys at each site indicate that fishermen target species whose combination of population density and body size give them relatively high biomass (SI Table 2). In this way, fishing can rapidly diminish fish biomass, thereby reducing the role of fish in nutrient recycling and other aspects of ecosystem functioning. In addition to decreasing nutrient recycling rates more quickly than extinctions ordered by population density, trophic position, or body size, overfishing is predicted to decrease the aggregate N:P ratio of recycled nutrients because small fish recycle less N relative to P than larger species (17).
When surviving species were allowed to compensate for extinctions, average reductions in nutrient recycling were relatively small until many species were lost. This pattern could be interpreted as suggesting a decoupling of biodiversity and ecosystem functioning, but in fact the potential for compensatory responses directly reflects the richness of these faunas. We focused upon trophic guilds as the functional grouping most relevant to nutrient recycling, and most guilds included numerous species. Loss of entire functional groups becomes more likely as the proportion of extinct species increases (30, 33); therefore, tropical fish diversity provides “insurance” for ecosystem processes by providing a large pool of functionally similar species that could compensate for extinctions (34). The value of this insurance depends in part on the diversity within each functional group, as evidenced by the drastic decrease in N recycling in RLM when Prochilodus was lost from the species-poor detritivore guild. Moreover, experimental manipulations in both RLM and LT suggest that compensatory responses may not always occur in a predictable fashion. For example, consumptive effects of large algivorous and detritivorous fish cannot be replaced by smaller fish or other taxa (10, 17, 35), and the same probably applies to large predators (8). Thus, certain extinctions may have disproportionately strong effects on ecosystems by eliminating species that play unique roles within functional groups. Unfortunately, the controls on community reorganization after extinctions from complex communities remain poorly understood, therefore our compensatory scenario was necessarily limited to simple rules of energetic replacement.
As reported in previous surveys of how contributions to ecosystem processes are distributed among species in natural ecosystems (25, 30, 32), we found that relatively few species dominated nutrient recycling (Fig. 2). This pattern raises questions about whether results from experimental communities comprising equal densities of all target species are applicable to natural communities (2). Moreover, our species-specific and generalized estimates of contributions to nutrient recycling by individual fish species often differed in magnitude and rank order (Fig. 3). These disparities indicate that the skew in contributions reflected not only the relative size and abundance of each species but also factors such as growth rate, dietary nutrient content, or body stoichiometry (16–18). Hence, our work provides further evidence that the details of species ecology can have ecosystem-level ramifications that are not predicted by relative biomass within the community (1, 31).
The consequences of declining biodiversity also may differ among ecosystem processes (1), and we found that even N and P recycling by fish respond differently to extinctions (Fig. 1). Although both nutrients are derived from dietary sources, interspecific differences in nutritional demands and dietary nutrient content give rise to a broad range of recycling rates and ratios (16–18). Thus, individual fish species differed widely in their relative contributions to N versus P recycling at both sites (Fig. 3). Given the variety of ways in which species influence ecosystems, such inconsistencies between interrelated aspects of nutrient recycling cast doubt on the possibility of summarizing the overall functional importance of particular species to prioritize conservation efforts.
Human alteration of fish communities is widespread (3–6), and we have offered a quantitative approach for assessing the effects of fish extinctions on ecosystem functioning. The parallels in our results from a small Neotropical river and a large African lake indicate that the consequences of declining fish diversity will depend upon the order of extinctions and especially the compensatory responses by surviving species. We have also shown that patterns of community composition and species-specific functional traits can give rise to important differences between ecosystems in the effects of extinctions. Together with earlier demonstrations that N recycling rates increase with the diversity of benthic marine invertebrates (25, 36), our results indicate that eroding aquatic biodiversity is likely to have detrimental effects on ecosystem functioning by altering nutrient recycling.
Sustainable fisheries are critical for human welfare and biodiversity conservation in the tropics. Our work highlights an unseen threat that overfishing poses to ecosystem functioning: the species targeted by fishermen are often major contributors to nutrient recycling. Fish play a significant role in the rapid recycling of nutrients (17–18, 20–21) required to support primary productivity in tropical aquatic ecosystems (22, 23); therefore, fish extinctions have serious implications for ecosystem productivity. If the impoverishment of tropical fish communities through overfishing does reduce nutrient recycling rates and the recycled N:P ratio, as suggested by our results, the high primary productivity that supports tropical freshwater fisheries could be compromised.
Materials and Methods
Study Sites.
Field data were collected at two sites, RLM and LT. RLM is a small piedmont river in the Orinoco basin of Venezuela (9°10′ N, 69°44′ W). LT is the largest African rift lake (650 × 50 km) and is a global hotspot of aquatic biodiversity. Field work on LT was conducted near Kigoma, Tanzania (4° 55′ S, 29° 36′ W). These sites share similar fish species richness, high primary productivity that is limited by nutrient availability, and intense fishing pressure, therefore they represent a class of freshwater ecosystems where fish-nutrient linkages could be important at the ecosystem level. However, they differ markedly in size, physical structure, and fish community composition, thereby enhancing the inferential power offered by concordant results. Further details about each site are available elsewhere (10, 17–18, 35).
Nutrient Recycling Rates.
Aggregate recycling of N and P by fish was calculated by summing population-level estimates for 69 species in RLM and 36 species in LT. Population-level recycling was estimated as the product of per capita recycling rates and population density. We used established methods (ref. 9; approved by the Cornell Institutional Animal Care and Use Committee) to measure excretion of dissolved N (NH4 in RLM; total dissolved N in LT) and P (total dissolved P) by freshly captured fish of 47 species (n = 457) in RLM and 14 species (n = 112) in LT. These species represented 97% and 74% of individuals in the fish communities at each site.
Size-scaling of recycling rates was described for each species by using ordinary linear regression of log10-transformed recycling rates (μmol of N or P individual−1·hour−1) against log10-transformed wet mass (g). Significant equations were applied to size distribution data (n = 8–653 per species) to estimate mean per capita N recycling rates. When N recycling was not predicted by size, we used the mean of measured rates for a species. For species in which N recycling was not measured, expected recycling was estimated by applying a scaling equation from taxonomically related species with similar diet to species-specific size data (n = 1–21 per species). Because of low descriptive power of scaling equations for P recycling (17), P recycling was estimated by multiplying mean per capita N recycling by the ratio of mean P:mean N recycling measured in the species (or related species).
Fish population densities were derived from extensive field censuses. In RLM, we quantified the density of each species throughout a 2.6 km reach using electroshocking and visual counts. In LT, we averaged the densities observed during visual censuses of three large quadrats (each 7 × 8 m) on rocky substrates at each of 12 locations. These methods yielded minimum density estimates (particularly in LT, where many nocturnal species were excluded), but total densities and biomass were high nonetheless in both RLM (11.1 individual m−2; 44.0 g wet mass·m−2) and LT (3.1 individual m−2; 50.6 g·m−2).
Extinction Simulations.
We used probabilistic, numerical simulations (25, 26) to assess the potential consequences of extinctions for nutrient recycling by fish. Four classes of scenarios were investigated: random extinctions without compensatory responses, random extinctions with compensatory responses, nonrandom extinctions without compensatory responses, and extreme best- and worst-cases. In each case, 1,000 simulations were conducted at every level of species richness. Simulations were written and executed in R (version 2.1).
Random extinctions without compensatory responses were implemented by randomly selecting taxa for extinction (i.e., all taxa had an equal probability of extinction), and keeping population densities of surviving species unchanged. Random extinctions with compensatory responses allowed population growth by competitors after extinctions. Estimation of potential compensatory responses drew upon metabolic scaling theory (37), which dictates that a species' energy usage is a function of its population density and body size (energy ∝ mass0.75). The energy of extinct species was reallocated to surviving species from the same trophic guild in proportion to their relative energy usage, then converted into additional individuals that contributed to nutrient recycling. This method preserved both total energy flow to the fish community and energetic partitioning among and within trophic guilds as long as ≥1 species persisted in each guild. Guild designations were based on gut content analyses (17), and included 8 guilds in RLM and 6 in LT.
Nonrandom extinctions without compensation were used to test the implications of differential risk among species. Alternative scenarios set the probability of extinction for each species as directly or inversely proportional to specific traits, and we focused on four real-world patterns: a negative relationship between population density and extinction risk (28), a positive relationship between trophic position in the food web and risk (3–4, 28), a positive relationship between body size and risk (5, 29), and a positive relationship between observed fishing pressure and risk. Trophic position was based on mean stable isotope ratios of nitrogen in dorsal muscle of each species (n = 6 per species in most cases) (38). Fishing pressure was described by using Chesson's α (39), an index that scales the frequency of each species in creel surveys (n = 1,326 individuals of 12 species from RLM; n = 150 individuals of 14 species from LT) against its frequency in the community.
Under best- and worst-case scenarios, risk was considered inversely or directly proportional to the contribution of each species to aggregate N or P recycling, or the N:P ratio. By directly relating risk to functional roles, these scenarios provide minimum and maximum estimates of potential changes in recycling because of extinctions.
Statistics.
Patterns of aggregate nutrient recycling generally displayed power-function relationships with species richness. For each scenario, we fitted the coefficient and exponent of power functions to simulation results using maximum likelihood. Comparisons among scenarios were based on 95% confidence intervals around exponents.
To aid in interpreting simulation results, we analyzed the proportional contribution of each species to aggregate N and P recycling. Species-specific influence on the recycled N:P ratio was calculated as the difference in aggregate N:P between communities including and lacking each species. Three further comparisons were used to assess the relative influence of biomass dominance and species identity on contributions to recycling. First, we regressed contributions to aggregate recycling of N or P against the biomass of each species. The variance not explained by this relationship (i.e., 1 − R2) represents the overall influence of species identity. Second, we quantitatively separated the effect of species identity from that of biomass dominance at the species level by comparing estimates of the contribution of each species to nutrient recycling based on either species-specific rates or generalized size-specific recycling rates. Generalized rates were derived from the size-scaling of N and P recycling across all species (n = 457 in RLM, n = 112 in LT), thereby excluding species-specific influences such as growth rates and nutrient content of body tissues or diet. The difference between species-specific and generalized estimates represents the influence of species identity. Third, we used Pearson product-moment correlations to test the relationship between contributions to aggregate N versus P recycling among species whose contribution to aggregate recycling was disproportionate (i.e., exceeding 1/S, where S is the total number of species). A low correlation was interpreted as evidence that roles in N and P recycling are decoupled in these dominant species.
Supplementary Material
Acknowledgments
We thank E. Michel, G. Kazumbe, H. Mgana, the Nyanza Project, and the Tanzanian Fisheries Research Institute for help in Tanzania. D. Taphorn, J. Hood, D. Lowry, C. Rockwood, R. Hall, B. Taylor, J. Headworth, A. Ulseth, S. Thomas, and J. Allgeier contributed to our work in Venezuela. S. Ellner assisted with extinction models, and M. Brown, A. Galford, and A. Kasson assisted with sample analysis. Comments from N. Hairston, Jr., B. Peckarsky, D. Schindler, J. Moslemi, K. Capps, M. Booth, and anonymous reviewers greatly improved the manuscript. Permission for field work was granted by the Venezuelan government and the Commission for Science and Technology in Tanzania. Funding was provided by the National Science Foundation through a graduate fellowship (to P.B.M.), the Integrative Graduate Education and Research Traineeship in Biogeochemistry and Environmental Biocomplexity at Cornell, Nyanza Project Grants ATM-9619458 and ATM-0223920, and Research Grants DEB-9615349, DEB-9615620, DEB-0321471, and INT-0321443.
Abbreviations
- RLM
Rio Las Marias
- LT
Lake Tanganyika.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608148104/DC1.
References
- 1.Hooper DU, Chapin FS, III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, et al. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 2.Srivastava DS, Vellend M. Annu Rev Ecol Evol Syst. 2005;36:267–294. [Google Scholar]
- 3.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 4.Myers RA, Worm B. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 5.Allan JD, Abell R, Hogan Z, Revenga C, Taylor BW, Welcomme RL, Winemiller K. BioScience. 2005;55:1041–1051. [Google Scholar]
- 6.Ruesink JL. Conserv Biol. 2005;19:1883–1893. [Google Scholar]
- 7.Power ME. Science. 1990;250:811–814. doi: 10.1126/science.250.4982.811. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter SR, Kitchell JF, editors. The Trophic Cascade in Lakes. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 9.Vanni MJ. Annu Rev Ecol Syst. 2002;33:341–370. [Google Scholar]
- 10.Taylor BW, Flecker AS, Hall RO., Jr Science. 2006;313:833–836. doi: 10.1126/science.1128223. [DOI] [PubMed] [Google Scholar]
- 11.Schindler DE, Carpenter SR, Cole JJ, Kitchell JF, Pace ML. Science. 1997;277:248–251. [Google Scholar]
- 12.Froese R, Pauly D, editors. FishBase 2000: Concepts, Design and Data Sources. Los Baños, Laguna, Philippines: International Center for Living Aquatic Resources Management; 2000. [Google Scholar]
- 13.Pough FH, Janis CM, Heiser JB. Vertebrate Life. Upper Saddle River, NJ: Prentice–Hall; 2005. [Google Scholar]
- 14.Food and Agriculture Organization of the United Nations. FISHSTAT Plus: Universal Software for Fishery Statistical Time Series. Rome, Italy: FAO; 2004. [Google Scholar]
- 15.Brashares JS, Arcese P, Sam MK, Coppolillo PB, Sinclair ARE, Balmford A. Science. 2004;306:1180–1183. doi: 10.1126/science.1102425. [DOI] [PubMed] [Google Scholar]
- 16.Schindler DE, Eby LA. Ecology. 1997;78:1816–1831. [Google Scholar]
- 17.McIntyre PB. Ithaca, NY: Cornell Univ; 2006. PhD thesis. [Google Scholar]
- 18.Vanni MJ, Flecker AS, Hood JM, Headworth JL. Ecol Lett. 2002;5:285–293. [Google Scholar]
- 19.Sterner RW, Elser JJ. Ecological Stoichiometry. Princeton, NJ: Princeton Univ Press; 2002. [Google Scholar]
- 20.Meyer JL, Schultz ET, Helfman GS. Science. 1983;220:1047–1049. doi: 10.1126/science.220.4601.1047. [DOI] [PubMed] [Google Scholar]
- 21.Andre ER, Hecky RE, Duthie HC. J Great Lakes Res. 2003;2:190–201. 29S. [Google Scholar]
- 22.Lewis WM., Jr Ann Rev Ecol Syst. 1987;18:159–184. [Google Scholar]
- 23.Kilham P, Kilham SS. Freshwat Biol. 1990;23:379–389. [Google Scholar]
- 24.Ives AR, Cardinale BJ. Nature. 2004;429:174–177. doi: 10.1038/nature02515. [DOI] [PubMed] [Google Scholar]
- 25.Solan M, Cardinale BJ, Downing AL, Engelhardt KAM, Ruesink JL, Srivastava DS. Science. 2004;306:1177–1180. doi: 10.1126/science.1103960. [DOI] [PubMed] [Google Scholar]
- 26.Bunker DE, DeClerck F, Bradford JC, Colwell RC, Perfecto I, Phillips OL, Sankaran M, Naeem S. Science. 2005;310:1029–1031. doi: 10.1126/science.1117682. [DOI] [PubMed] [Google Scholar]
- 27.Gross K, Cardinale BJ. Ecol Lett. 2005;8:409–418. [Google Scholar]
- 28.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Proc R Soc London B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy JE. Ecol Lett. 2003;6:680–687. [Google Scholar]
- 30.Balvanera P, Kremen C, Martinez M. Ecol Appl. 2005;15:360–375. [Google Scholar]
- 31.Power ME, Tilman D, Estes J, Menge BA, Bond WJ, Mills LS, Daily G, Castilla JC, Lubchenco J, Paine RT. BioScience. 1996;46:609–628. [Google Scholar]
- 32.Sala OE, Lauenroth WK, McNaughton SJ, Rusch G, Zhang X. In: Functional Roles of Biodiversity: A Global Perspective. Mooney HA, Cushman JA, Medina E, Sala OE, Schulze E-D, editors. New York: Wiley; 1996. pp. 129–149. [Google Scholar]
- 33.Fonseca CR, Ganade G. J Ecol. 2001;89:118–125. [Google Scholar]
- 34.Naeem S. Conserv Biol. 1998;12:39–45. [Google Scholar]
- 35.Flecker AS. Ecology. 1996;77:1845–1854. [Google Scholar]
- 36.Emmerson MC, Solan M, Emes C, Paterson DM, Raffaelli D. Nature. 2001;411:73–77. doi: 10.1038/35075055. [DOI] [PubMed] [Google Scholar]
- 37.Brown JH, Gillooly JF, Allan AP, Savage VM, West GB. Ecology. 2004;85:1771–1789. [Google Scholar]
- 38.Post DM. Ecology. 2002;83:703–718. [Google Scholar]
- 39.Chesson J. Ecology. 1978;59:211–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.