Abstract
Using Drosophila pseudoobscura, we tested the hypothesis that social constraints on the free expression of mate preferences, by both females and males, decrease offspring viability and reproductive success of mating pairs. Mate preference arenas eliminated intrasexual combat and intersexual coercion. The time female and male choosers spent in arena tests near either of two opposite-sex individuals measured the preferences of choosers. We placed choosers in breeding trials with their preferred or nonpreferred discriminatee when they met the minimum criteria for showing the same preference in two consecutive tests. There was no statistically significant difference in the frequency of female and male choosers meeting minimal preference criteria. There was a significant difference between female and male choosers for offspring viability, with female choice having the greater effect, but there was not a significant difference in the overall reproductive success of male and female choosers. There were significant differences in fitness between matings to preferred and nonpreferred partners. Female and male choosers paired with their nonpreferred discriminatees had offspring of significantly lower viability, as predicted by the constraints hypothesis. Reproductive success, our measure of overall fitness, was greater when males or females mated with the partner they preferred rather than the one they did not prefer.
Keywords: constraints hypothesis
Social constraints on the free expression of mate preferences may affect offspring viability and reproductive success of choosers as suggested by commentators (1) on Partridge's (2) classic study, which was the first to associate offspring viability with mate choice. That mate preferences affect offspring viability also is an important prediction of hypotheses about the cues mediating mate preference (3), particularly those focused on mate compatibility at loci affecting immune function in offspring (4–6). Many studies have demonstrated that females prefer males with more elaborate secondary sexual characteristics (7), and other studies have shown that males with the most exaggerated secondary sexual traits are healthier than other males (8–12). However, relatively few studies (13–15) have shown benefits in offspring viability for females reproducing with males having traits preferred by most females. In contrast, recent studies on the benefits of mate preferences for choosers, without reference to traits of discriminatees that may mediate preferences, showed that mate preferences of females and males affect offspring viability (16–21), productivity of mating pairs (22), and chooser longevity (23). We tested the constraints hypothesis (24–26) that says social or ecological constraints on reproductive decisions, those that prevent individuals from reproducing with their preferred partner(s), result in lower offspring viability and lower overall reproductive success.
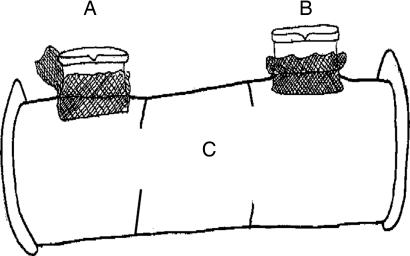
To test predictions of the constraints hypothesis, we used the same methodology with Drosophila pseudoobscura as Drickamer et al. (17, 18), Moore et al. (22, 23), and Bluhm and Gowaty (16, 27) did with other animals, to evaluate individual choosers' mate preferences for two opposite-sex “discriminatees” while experimentally eliminating social constraints such as intrasexual fighting and intersexual coercion (Fig. 1). We picked the discriminatees at random with respect to their phenotypes to control for the possibility that signals also manipulate the decisions of choosers. That is, the experiment evaluated the effects on chooser fitness of enforced, exclusive pairing with discriminatees they did and did not prefer. Thus, this study shows the effects on the fitness of chooser individuals of an experimental constraint on the expression of mate preferences.
Fig. 1.
Experimental mate preference arenas consisted of tygon tubing (0.79-mm internal diameter and 7.6 mm in length), fine plastic mesh, and two Eppendorf tubes. The single chooser was able to move throughout the length of the long corridor. The Eppendorf tubes were the cells (A and B) containing discriminatees of the opposite sex. Opaque tape on one side of each tube blocked visual contact between the discriminatees, one per cell during preference testing. The side of each Eppendorf cell connected to a longer corridor of tubing in which we placed focal individual choosers. Very fine plastic mesh prevented the discriminatees from moving into the corridor and the chooser from entering either of the cells. We divided each corridor into three regions (A and B in front of each Eppendorf cell, and C designating the space between them) designated by dark lines drawn on the surface of the tubing.
Results
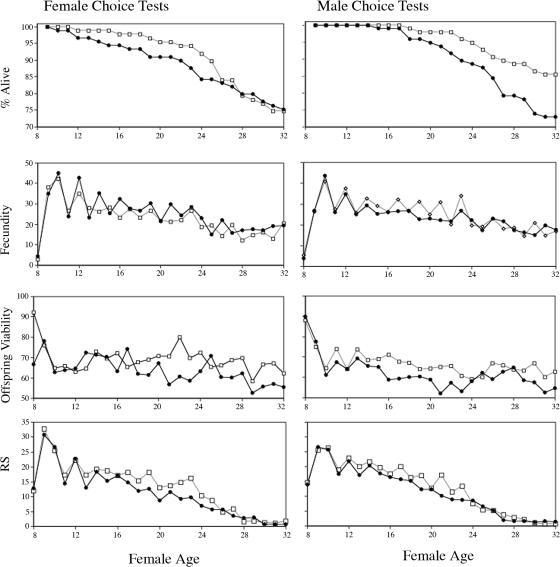
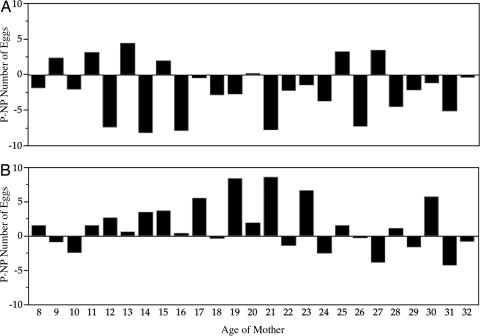
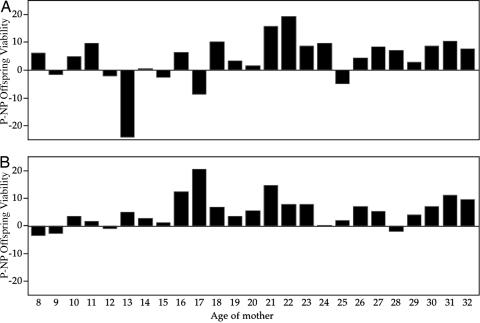
The results of a two-way, fixed-effects ANOVA on fecundity, offspring viability, and chooser reproductive success are given in Table 1. The factors were male or female choice and also mating type, which indicated whether the chooser was paired experimentally with its preferred or nonpreferred discriminatee. Graphs of the time courses of female longevity, fecundity, offspring viability, and reproductive success by chooser sex and mating type are given in Fig. 2. There were no significant differences in female longevity between female or male chooser tests, nor were there significant differences in female longevity between matings to preferred and nonpreferred partners (Fig. 2). There were no significant differences in mean fecundity by chooser sex, mating type, or their interaction (Table 1 and Fig. 2). However, the day-by-day distribution of difference scores (preferred − nonpreferred) showed that in female choice tests, nonpreferred matings had significantly higher fecundity than did preferred matings (Fig. 3). In contrast, for male choice tests, the day-by-day distribution of difference scores (preferred − nonpreferred) for fecundity was higher in preferred matings than in nonpreferred matings (Fig. 3). Mean offspring viability did not differ significantly between male and female choice tests, and likewise, there were no significant differences in reproductive success by chooser sex (Table 1 and Fig. 2). There were statistically significant differences between pairings with preferred and nonpreferred partners for both offspring viability (Table 1 and Figs. 2 and 4) and net reproductive success of mating pairs (Table 1 and Fig. 2), both fitness measures being larger for choosers with their preferred partners. There was no indication of a significant interaction between chooser sex and mating type in either offspring viability or net reproductive success of mating pairs. In female choice tests, the net reproductive success for nonpreferred pairings was 238.08 ± 12.53 SE, and for preferred pairings, it was 286.66 ± 12.53 SE, a highly significant difference between mating types of 48.58 ± 17.72 offspring (P < 0.01). In male choice tests, reproductive success in nonpreferred pairings was 247.79 ± 12.04 SE, and in preferred pairings, it was 289.66 ± 12.33 SE (P < 0.01), a highly significant difference of 41.87 ± 17.23 SE offspring.
Table 1.
ANOVA on fecundity (number of eggs), offspring viability (percentage of egg to adult survival), and reproductive success
| Source | df | Sum of squares | F ratio | Probability < F |
|---|---|---|---|---|
| Fecundity | ||||
| Experiment 1 or 2 | 1 | 23,172.08 | 0.5796 | 0.45 |
| Male or female choice test | 1 | 8,630.68 | 0.216 | 0.64 |
| Mated with P or NP | 1 | 12,324.31 | 0.31 | 0.58 |
| Interaction of chooser × mating type | 1 | 129,653.87 | 3.242 | 0.07 |
| Offspring viability | ||||
| Experiment 1 or 2 | 1 | 71.36 | 0.2520 | 0.60 |
| Male or female choice test | 1 | 1,077.54 | 3.80 | 0.05 |
| Mated with P or NP | 1 | 1,136.23 | 4.01 | 0.046 |
| Interaction of chooser × mating type | 1 | 17.3 | 0.061 | 0.8 |
| Reproductive success | ||||
| Male or female choice test | 1 | 2,491.14 | 0.26 | 0.61 |
| Mated with P or NP | 1 | 126,206.57 | 13.40 | 0.0003 |
| Interaction | 1 | 694.81 | 0.07 | 0.79 |
P, preferred; NP, nonpreferred.
Fig. 2.
Time course for female longevity, offspring viability, fecundity, and reproductive success (RS) of mating pairs in female and male choice tests, for choosers paired with their nonpreferred (filled circles) or preferred partners (open squares). Differences in longevity for females in preferred or nonpreferred matings were not statistically significant by using Kaplan–Meier statistics (for female choice tests: Wilcoxon χ2 = 2.02, df = 1, P > 0.15; for male choice tests: Wilcoxon χ2 = 0.1140, df = 1, P > 0.73).
Fig. 3.
The difference scores for fecundity between preferred and nonpreferred matings in female (A) and male (B) choice studies. The distribution of difference scores was significantly different in both female choice tests (Wilcoxon signed rank t = −81.5, df = 24, P > |t| = 0.013) and male choice tests (Wilcoxon signed rank t = 59.5, df = 24, P = |t| = 0.055). NP, nonpreferred; P, preferred.
Fig. 4.
The difference scores for offspring viability between preferred and nonpreferred matings in female (A) and male (B) choice studies. The distribution of difference scores was significantly different both for female choice tests (Wilcoxon signed rank test = 97.5, P > |t| = 0.006) and for male choice tests (Wilcoxon signed rank test = 134.5, P > |t| < 0.0001). NP, nonpreferred; P, preferred.
Discussion
The highly significant differences in offspring viability and breeder reproductive success between pairings to nonpreferred and preferred partners suggest that our experimental procedure, consisting of the arena (Fig. 1) and the minimal time criteria for preference, which controlled for intersexual and intrasexual interactions that could interfere with mate preferences, allowed choosers to assess meaningful phenotypic differences between prospective mates. Other methods for measuring mate choice in flies, such as the Elens–Wattiaux (E–W) chambers, which allow more than two flies to simultaneously interact, may be better at measuring sexual isolation between geographic strains or between species than they are at measuring less-pronounced differences between prospective mates within a population. In E–W chambers, D. pseudoobscura females mated more often with the first of two males who initiated courtship with them, and males mated often with the first female they courted, who was often the first female they encountered (28). The preference arenas give more opportunity for “sizing up” a prospective mate without actual mating and perhaps even typical courtship, and the arenas control for nonobvious mechanisms of intrasexual interference and intersexual coercion. Because we picked discriminatees at random with respect to obvious phenotypic characteristics and did not note any differences in discriminatee phenotype before, during, or after arena and breeding trials, we could not determine from this experiment just what phenotypic variations might correlate with chooser preferences.
Mate preferences affected the fitness of female choosers, as Darwin (29), Bateman (30), Williams (31), Parker et al. (32), and Trivers (33) predicted. Mate preferences also affected the fitness of male choosers, a more surprising outcome under classical expectations about mate preferences. Under the parental investment hypothesis (32–34), male choice is not predicted to exist in D. pseudoobscura because of the dramatic size asymmetry of gametes, with vastly larger eggs than sperm (35, 36), and also because there is no postzygotic parental care in this species.
The significant differences in offspring viability between matings to nonpreferred and preferred partners are consistent with the predictions of the constraints hypothesis for both female and male choosers. We conclude that constraints on reproductive decisions have important effects on offspring viability in D. pseudoobscura. This result is similar to those in other tests that use the same methodology that we used here. In mice (Mus musculus), offspring viability was significantly lower when female choosers (17), male choosers (19), or both male and female choosers (18) were constrained to reproduce with individuals they did not prefer compared with those they did. In ducks (Anas playtrhynchos), in which only the fitness of female choosers and the fitness of their offspring were tested, female choosers paired with males they did not prefer had offspring of significantly lower viability than female choosers with males they did prefer (16).
Fecundity was significantly greater in nonpreferred than in preferred matings in 18 of 25 (72%) days of egg-laying in our female choice tests, which is a significant difference (Fig. 1; Wilcoxon signed rank t = −81.5, df = 24, P = 0.013). This occurred despite higher offspring viability for females with males they preferred, a tradeoff indicating that female D. pseudoobscura compensated for reduced offspring viability by increasing the number of eggs they laid. Fecundity in the male choice tests was significantly higher in preferred than in nonpreferred matings on 60% of days (Fig. 3). Thus, fecundity in male choice tests is consistent with expectations from classical sexual selection.
Overall fitness, measured as net reproductive success Σ(lxmxvx), was significantly greater for pairings with preferred than nonpreferred partners for both female and male choosers. We conclude that constraints on reproductive decisions have important effects on net reproductive success of mating pairs in D. pseudoobscura. The difference between matings was 18.5% of the average reproductive success of all matings under female choice and 15.6% of all matings under male choice. These differences represent sizable selective disadvantages for choosers mated to their nonpreferred rather than their preferred partners.
Methods
Animals.
W.W.A. collected D. pseudoobscura at Mesa Verde, Colorado in summer 1996 and maintained them as isofemale lines. Eight lines were used to set up a population cage in July 1997. Drosophila were maintained on a food consisting of cornmeal, agar, molasses, and brewer's yeast, with a small amount of propionic acid added to retard the growth of mold. A few drops of a live solution of yeast were added to each bottle or food cup. Twenty cups with this food medium were placed in the population cage, and on Mondays, Wednesdays, and Fridays, an old cup was replaced with a new one. Approximately 12 generations after setting up the population cage, we began separating and storing newly eclosed males and females for behavioral preference tests. We completed two large experiments in fall 1998 and fall 1999, both carried out at 22°C. We chose to do both experiments in the fall, when climatic conditions in the laboratory would be similar.
Preference Tests.
We collected virgin flies from culture bottles every 7 or 8 h. We anesthetized them with CO2, sexed them, and held them until preference testing. Flies were stored in same-sex groups of no more than 20 individuals for 7 days in vials containing food medium.
We tested the preferences of focal individuals using mate preference arenas (Fig. 1) that eliminated direct competitive interactions between discriminatees and potentially coercive interactions between the sexes. We also used criteria for exhibiting a preference that depended on the time a chooser spent near one or the other discriminatee (see below), a methodology similar to those used by other workers for a variety of animals (7).
The design of the mate preference arenas (Fig. 1) allowed visual, olfactory, and auditory contact but prevented tactile contact between the focal individual chooser and the opposite-sex discriminatees. We picked focal individual choosers and the opposite-sex discriminatees at random with respect to any phenotypic variation obvious to us. In the female preference tests, we used 265 focal virgin females and 530 virgin male discriminatees. In the male preference tests, we used 233 focal virgin males and 466 virgin female discriminatees. We aspirated discriminatees into cells a and b, followed by aspiration of the focal chooser into corridor c.
We scored preference for one versus the other discriminatee in terms of (i) the time choosers spent in front of cells a and b and (ii) the repeatability of preference in consecutive tests using the same chooser and discriminatees. Our minimum criteria for declaring a preference in each arena test were (i) the chooser had to spend 60% (12 min) of the first 20-min preference test next to one or the other discriminatee in region a and/or b of the arena, and (ii) the chooser had to spend 60% (7.2 min) of that time with the same discriminatee (in either a or b). We switched the discriminatees between cells in consecutive tests to control for position effects. We performed female and male chooser preference tests on the same days to control for microhabitat and temporal variation that could affect measurements of fitness components. Forty-seven percent (125 of 265) of focal female choosers and 55.3% (129 of 233) of focal male choosers met the minimum criteria for exhibiting a preference.
Breeding Tests.
We discarded all individuals from preference tests in which focal choosers failed to meet the criteria for exhibiting a preference because the hypothesis we were testing was about constraints on reproductive decisions. That is, we excluded from further tests those individuals whose preferences were ambiguous because our experiment was about the effect of constraints. When a focal chooser's associations were ambiguous, indicating no clear choice between discriminates, we could not be sure we were experimentally producing a breeding constraint when we used them in the breeding trials. We randomly assigned the first focal chooser in each series (female choosers and male choosers) to either the preferred or nonpreferred partner. Thereafter, we alternated assignments to breeding vials. We considered choosers placed with their nonpreferred discriminatee to represent greater constraint on reproductive decisions than choosers with their preferred discriminatee. The factors in our overall design thus included two experiments, sex of the chooser (female or male choice tests) and mating type (preferred or nonpreferred).
Measurements of Reproductive Success and Fitness Components.
For each of the two experiments, we measured the fitness of 30–37 replicate choosers of each sex and each mating type. We measured fitness for a total of 247 pairings. We kept all pairs in mating vials for 1 day and then discarded all males. Females laid eggs from the mating that occurred with a single male after the first day of completing a preference trial. For 25 consecutive days, we moved all females to fresh vials containing food medium. We recorded longevity (lx) as the frequency of females surviving to each day of age. We counted all eggs laid on each day, and fecundity at age x, mx, was measured as the number of eggs each female laid during day x. Beginning 14 days after mating, we counted the number of offspring emerging as adults from each vial over a period of 10 days. Egg-to-offspring viability at age x, vx, was measured as the fraction of eggs laid on day x that survived to eclosion. We used reproductive success W or net reproduction (37) as our measure of the overall fitness of mating pairs. In terms of female longevity, fecundity, and offspring viability, reproductive success is Σ(lxmxvx), where the summation is over days of age.
We tested for normality of all variables. We used parametric tests throughout because all variables were normally distributed. We used a fixed-effects ANOVA with the three factors experiment, chooser sex, and mating type to test for differences among offspring viability, chooser fecundity, and chooser reproductive success. An ANOVA of our entire data set showed no significant differences between the two major experiments on any variable, so we combined the data for them. To test for independence among days for difference scores in offspring viability between preferred and nonpreferred matings, we used time series autocorrelation analyses and Fisher's Kappa to test for deviations from the null hypothesis of “white noise.” We failed to reject the null hypothesis, so we concluded that the difference scores were independent between days and tested the distributions for differences using the Wilcoxon signed rank test. We considered results statistically significant if P was <0.05.
Acknowledgments
We thank John Avise, Cindy Bluhm, Lee C. Drickamer, Steve Hubbell, Teri Markow, Allen Moore, and Jerry Downhower for many helpful and insightful conversations and for comments on the manuscript; Randy Nelson for his encouragement; Kyungsun Kim for technical assistance; and William C. Bridges for statistical advice. This work was supported by National Science Foundation Grant IBN-9631801.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kingett PD, Lambert DM, Telford SR. Nature. 1981;293:492. [Google Scholar]
- 2.Partridge L. Nature. 1980;283:290–291. [Google Scholar]
- 3.Hamilton WD, Zuk M. Science. 1982;18:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 4.Brown JL. Behav Ecol. 1997;8:60–65. [Google Scholar]
- 5.Penn DJ, Potts WK. Am Nat. 1999;153:145–164. doi: 10.1086/303166. [DOI] [PubMed] [Google Scholar]
- 6.Wedekind C. In: Evolution in Health and Disease. Stearns SC, editor. Oxford: Oxford Univ Press; 1999. pp. 102–107. [Google Scholar]
- 7.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 8.Kacelnik A, Norris S. Behav Brain Sci. 1998;21:378–385. [Google Scholar]
- 9.Loehle C. Ecol Mod. 1997;103:231–250. [Google Scholar]
- 10.Loyau A, Saint Jalme M, Cagniant C, Sorci G. Behav Ecol Sociobiol. 2005;58:552–557. [Google Scholar]
- 11.Worden BD, Parker PG. Anim Behav. 2005;70:1047–1053. [Google Scholar]
- 12.Zala SM, Potts WK, Penn DJ. Behav Ecol. 2004;15:338–344. [Google Scholar]
- 13.Petrie M. Nature. 1994;371:598–599. [Google Scholar]
- 14.Reynolds JD, Gross MR. Proc R Soc London Ser B. 1992;250:57–62. [Google Scholar]
- 15.Simmons L. Behav Ecol Sociobiol. 1987;21:313–322. [Google Scholar]
- 16.Bluhm CK, Gowaty PA. Anim Behav. 2004;68:977–983. [Google Scholar]
- 17.Drickamer LC, Gowaty PA, Holmes CM. Anim Behav. 2000;59:371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- 18.Drickamer LC, Gowaty PA, Wagner DM. Anim Behav. 2003;65:105–114. [Google Scholar]
- 19.Gowaty PA, Drickamer LC, Holmes S-S. Anim Behav. 2003;65:95–103. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- 20.Ryan KK, Altmann J. Behav Ecol Sociobiol. 2001;50:436–440. [Google Scholar]
- 21.Sandvik M, Rosenqvist G, Berglund A. Proc R Soc London Ser B. 2000;267:2151–2155. doi: 10.1098/rspb.2000.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore AJ, Gowaty PA, Wallin WJ, Moore PJ. Proc R Soc London Ser B. 2001:517–523. doi: 10.1098/rspb.2000.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore AJ, Gowaty PA, Moore PJ. J Evol Biol. 2003;16:523–530. doi: 10.1046/j.1420-9101.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 24.Gowaty PA. In: Black Partnerships in Birds. Black JL, editor. Oxford: Oxford Univ Press; 1996. pp. 21–52. [Google Scholar]
- 25.Gowaty PA. In: Women, Evolution, and Rape. Travis C, editor. Cambridge, MA: MIT Press; 2003. pp. 61–86. [Google Scholar]
- 26.Gowaty PA, Buschhaus N. Am Zool. 1998;38:207–225. [Google Scholar]
- 27.Bluhm CK, Gowaty PA. Anim Behav. 2004;68:985–992. [Google Scholar]
- 28.Kim Y-K, Gowaty PA, Anderson WW. In: Noldus L, Grieco JJF, Loijens LWS, Zimmerman PH, editors. Proceedings of the Fifth International Conference on Methods and Techniques in Behavioral Research; The Netherlands: Wageningen; 2005. pp. 533–535. [Google Scholar]
- 29.Darwin C. The Descent of Man and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 30.Bateman AJ. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 31.Williams GC. Adaptation and Natural Selection. Princeton: Princeton Univ Press; 1966. [Google Scholar]
- 32.Parker GA, Baker RR, Smith VGF. J Theor Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- 33.Trivers RL. In: Sexual Selection and the Descent of Man. Campbell B, editor. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- 34.Parker GA, Simmons LW. Proc R Soc London Ser B. 1996:315–321. [Google Scholar]
- 35.Snook RR, Markow TA. J Insect Phys. 2001;47:957–964. doi: 10.1016/s0022-1910(01)00070-1. [DOI] [PubMed] [Google Scholar]
- 36.Snook RR, Markow TA, Karr TL. Proc Natl Acad Sci USA. 1994;91:11222–11226. doi: 10.1073/pnas.91.23.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Futuyma DW. Evolution. Sunderland, MA: Sinauer; 2005. [Google Scholar]