Abstract
Although studies with primary lymphocytes are almost always conducted in CO2 incubators maintained at atmospheric oxygen levels (atmosO2; 20%), the physiological oxygen levels (physO2; 5%) that cells encounter in vivo are 2–4 times lower. We show here that culturing primary T cells at atmosO2 significantly alters the intracellular redox state (decreases intracellular glutathione, increases oxidized intracellular glutathione), whereas culturing at physO2 maintains the intracellular redox environment (intracellular glutathione/oxidized intracellular glutathione) close to its in vivo status. Furthermore, we show that CD3/CD28-induced T cell proliferation (based on proliferation index and cell yield) is higher at atmosO2 than at physO2. This apparently paradoxical finding, we suggest, may be explained by two additional findings with CD3/CD28-stimulated T cells: (i) the intracellular NO (iNO) levels are higher at physO2 than at atmosO2; and (ii) the peak expression of CD69 is significantly delayed and more sustained at physO2 that at atmosO2. Because high levels of intracellular NO and sustained CD69 tend to down-regulate T cell responses in vivo, the lower proliferative T cell responses at physO2 likely reflect the in vitro operation of the natural in vivo regulatory mechanisms. Thus, we suggest caution in culturing primary lymphocytes at atmosO2 because the requisite adaptation to nonphysiological oxygen levels may seriously skew T cell responses, particularly after several days in culture.
Keywords: glutathione, low oxygen, nitric oxide, proliferation
Virtually all animal cells, whether freshly isolated or established cell lines, are currently cultured in incubators maintained at atmospheric oxygen levels (20% O2; 5% CO2). However, very few cells encounter O2 levels in vivo that are >12% (the level in arterial blood), and most cells are located in tissues that have 3–5% O2 (1–3). This difference between the oxygen levels used in typical cell culture incubators and the oxygen levels that cells encounter in vivo has largely been overlooked. However, as we show here, it is critical to define culture systems that more closely model in vivo conditions and thus make findings from ex vivo studies of cell function more relevant to understanding in vivo processes.
Several studies indicate that incubator oxygen levels can modulate the metabolism (2, 4), gene expression (5, 6), and function of primary cells (3, 7–9). However, the significance of these findings is obscured because the current notation for describing incubator oxygen levels is very confusing: atmospheric oxygen levels are typically referred to as “normoxic,” even though these levels are substantially above the levels animal cells normally encounter in vivo. Furthermore, physiologically relevant oxygen levels (2–5% O2) are commonly referred to as “hypoxic,” a term that is also used to refer to pathophysiological oxygen levels (<2%) that primarily occur in tumors and ischemia. To avoid this confusion, we suggest reserving the term “hypoxic” for oxygen levels <2% and by using the terms “atmospheric” (atmosO2) and “physiological” (physO2) oxygen levels to refer, respectively, to the 20–21% oxygen levels in air, or in typical CO2 incubators, and the 2–12% oxygen levels that approximate in vivo oxygen exposure.
In studies presented here, we have systematically compared primary cells cultured at atmosO2 (20%) and physO2 (5%), chosen to match the average oxygen levels found in most tissues and better able (as we show here) to maintain the intracellular redox environment close to that measured for freshly isolated T cells. In a previous study, we showed that culturing primary T cells without stimulation at the two incubator oxygen levels yields comparable results with respect to cell size, granularity, viability, and expression of classical T cell subset markers (CD3, CD4, CD8) (7). However, we found that when primary T cells are stimulated with concanavalin A or CD3/CD28 cross-linking, there is significantly less proliferation in the cells cultured at physO2 (7).
We confirm this finding here and, focusing on primary T cells stimulated with CD3/CD28 at the two oxygen levels, we further show that (i) the production of intracellular reactive oxygen species (iROS) and NO is higher at physO2 than at atmosO2; (ii) the expression of CD69, an early marker of T cell activation, differs between the two oxygen levels, whereas the kinetics of other T cell activation markers (CD25 and CD71) are unaffected; and (iii) adding N-acetylcysteine (NAC) to the cultures to create a more reductive in vitro environment [and to supply cysteine necessary for glutathione (GSH) synthesis] does not abrogate the differences in CD3/CD28-stimulated T cell proliferation at physO2 versus atmosO2.
In discussing these findings, we suggest that the greater T cell proliferation observed at atmosO2 reflects in vivo T cell responses that occur under inflammatory (oxidative) conditions, whereas the controlled proliferative T cell responses observed at physO2 reflect in vivo T cell function in the healthy immune system.
Results
The Cellular Redox Status of Primary T Cells Cultured at Physiological Oxygen Levels Approximates Their in Vivo Redox Status.
We compared the redox status of T cells in freshly isolated human peripheral blood mononuclear cells (PBMCs), with T cells in the same cell preparation after it was cultured for 3 days at physO2 or atmosO2 without exogenous stimulation. For these studies, we measured intracellular glutathione [intercellular GSH (iGSH) and oxidized iGSH (iGSSG)] by tandem MS. In addition, we used FACS to perform a second iGSH measurement (using monochlorobimane) and to measure intracellular NO [iNO; by using DAF-FM, DA (4-amino-5-methyamino-2′,7′-difluorofluorescein diacetate)]. Each cell preparation was aliquoted to provide a sample for an immediate (baseline) measurement. The remaining cells were cultured for 3 days at each of the oxygen levels and analyzed immediately thereafter.
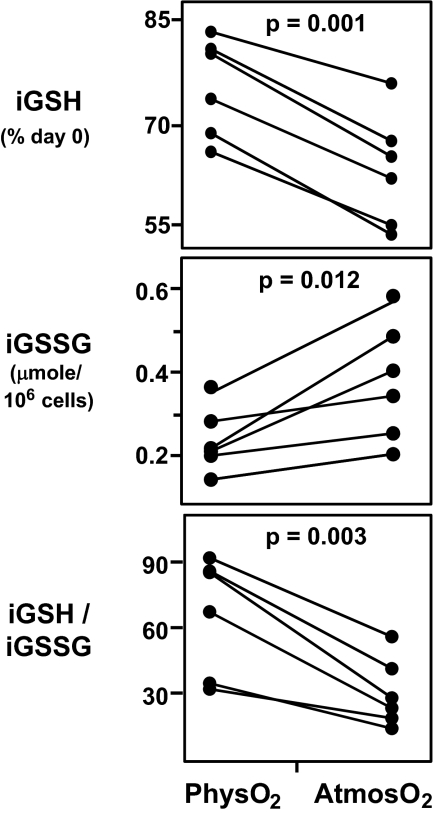
Although tandem MS provides the most accurate measurement of GSH and GSSG available today, there are still relatively few studies in the literature in which this method is used. Our studies use this method to measure iGSH and iGSSG in extracts obtained from “negatively enriched” primary T cells (see Materials and Methods). Results show that T cells cultured at both oxygen levels lose iGSH. However, significantly more iGSH is lost at atmosO2 than at physO2 (41.3 ± 8.5% at atmosO2 and 30.8 ± 9.0% at physO2, P = 0.001; shown as percentage of day 0 iGSH in the figure; Fig. 1).
Fig. 1.
PhysO2 maintain the intracellular redox state closer to in vivo levels than atmosO2. Freshly prepared negatively enriched human peripheral blood T cells (see Materials and Methods) were cultured at physO2 and atmosO2 oxygen for 3 days without exogenous stimulation. iGSH and iGSSG were measured by tandem MS at the beginning and on day 3 of culture. (Top) iGSH levels on day 3 expressed as percentage of iGSH on day 0. (Middle) iGSSG levels on day 3. (Bottom) Intracellular redox state (iGSH/iGSSG) on day 3. Statistics were calculated by using JMP software by least-square fit model with sample and oxygen as independent variables (see Materials and Methods). Each set of connected points represents one subject (n = 6).
Consistent with this finding, iGSSG (the oxidized form of GSH in cells) is significantly higher (1.5- to 2-fold) in T cells cultured at atmosO2 than physO2 (P = 0.012; Fig. 1). In addition, the iGSH/iGSSG ratio, a common measure of intracellular redox state of T cells, is significantly higher at physO2 than at atmosO2 (P = 0.003; Fig. 1), suggesting that the redox status of cells cultured at physO2 is more like the redox status expected for healthy T cells in vivo (10).
Results are similar for iGSH in CD4 T cells in PBMCs cultured for 3 days at each of the oxygen levels, i.e., a 30 ± 10.2% iGSH loss after culture in air and 20.1 ± 8.5% loss at physO2 levels (P < 0.001; shown as percentage of day 0 in Fig. 2)], although the decrease in CD4 T cell iGSH at the two oxygen levels measured is not as high as the decrease measured by tandem MS for the total enriched T cell preparation (Fig. 2).
Fig. 2.
iGSH and iNO levels at physO2 are maintained closer to the in vivo levels compared with atmosO2. Freshly prepared human PBMCs were cultured for 3 days at physO2 and atmosO2 without exogenous stimulation. iGSH and iNO were measured at the beginning and the end of the 3-day culture by FACS (see Materials and Methods). (Upper) CD4 T cell iGSH on day 3 expressed as fraction of iGSH on day 0 (percentage of day 0 iGSH). (Lower) CD4 T cell iNO on day 3 expressed as fraction of iNO on day 0 (percentage of day 0 iNO). Statistics were calculated by using JMP software by least-square fit model with sample and oxygen as independent variables (see Materials and Methods). Each set of connected points represents one subject. n = 16 for iGSH and n = 10 for iNO.
iNO levels in CD4+ T cells, also measured by FACS, show the same trend: significantly more iNO is lost at atmosO2 than at physO2 (25.2 ± 6.5% at atmosO2 and 12.0 ± 6.2% at physO2, P < 0.0001) (Fig. 2).
Taken together, these findings demonstrate that the intracellular redox environment in T cells changes during culture but is maintained closer to the in vivo levels when cells are cultured at physO2.
CD3/CD28-Stimulated T Cell Proliferation Is Lower in Cells Cultured at PhysO2 than at AtmosO2.
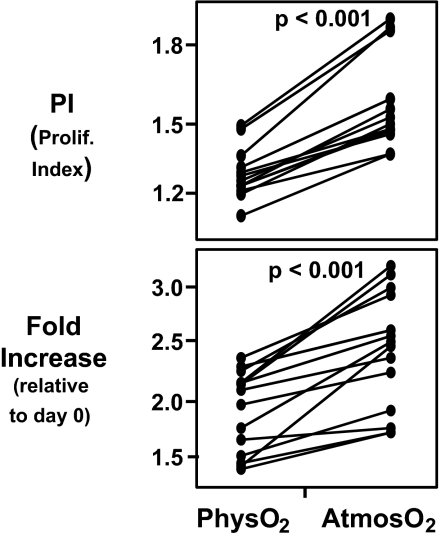
As indicated above, culturing T cells at physO2 results in substantially less deviation from the in vivo redox status. Nevertheless, we have found that stimulation at atmosO2 drives the T cell proliferative responses more intensively than stimulation at physO2 (7). Studies here confirm and extend these findings by showing that T cell proliferation is higher in atmosO2 cultures, whether measured as the proliferative index or the total number of T cells in the culture (Fig. 3).
Fig. 3.
CD3/CD28-stimulated T cell proliferation is higher at atmosO2 than physO2. CFSE-stained human PBMCs were stimulated for 3 days with plate-bound CD3 (1 μg/ml) and CD28 (2 μg/ml). Cell counts were performed by using BD Trucount tubes. Proliferative index (PI) and fold change in the live CD4 T cell number was calculated as described in Materials and Methods. (Upper) Higher proliferation index (1.63 ± 0.17 at atmosO2 versus 1.32 ± 0.09 at physO2). (Lower) Significantly higher fold increase (2.34 ± 0.69 versus 1.47 ± 0.52) in CD4 T cells stimulated at atmosO2 vs. physO2. Statistics were calculated by using JMP software by least-square fit model with sample and oxygen as independent variables. Each set of connected points represents one subject (n = 16).
Consistent with this, the fraction of dividing CD4 T cells is significantly higher at atmosO2 than at physO2 (58.4 ± 9.9 at atmosO2 versus 38.4 ± 7.9 at physO2, P < 0.0001). Furthermore, cell death in stimulated cultures tended to be higher at physO2 than at atmosO2 (P = 0.07). Cell numbers in unstimulated cultures decreased roughly 0.35-fold (0.30- to 0.43-fold) relative to the cell number at the beginning of the culture independent of the oxygen level at which the cells were cultured.
These findings appear paradoxical because, as we have shown above, culturing at physO2 preserves the in vivo redox status better than culturing at atmosO2. However, the differences in the response to stimulation at the two oxygen levels is explained by findings from the studies that follow, which indicate that NO and other redox-dependent immunoregulatory mechanisms known to operate in vivo are incapacitated at atmosO2.
Proliferation Differences at PhysO2 and AtmosO2 Are Not Due to Limiting Cysteine Levels in the Culture Media.
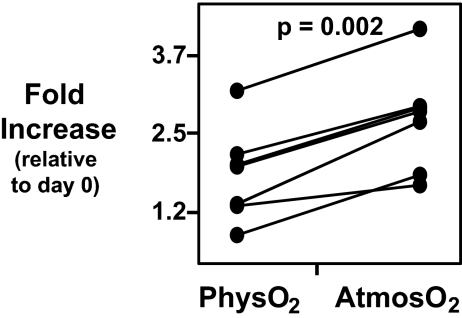
Supplementing the culture medium with NAC, a common physiologically active source of cysteine in vivo or in vitro, does not abrogate the influence of oxygen levels on CD3/CD28-induced T cell proliferation (Fig. 4). In addition, although NAC increases iGSH levels in the cultured T cells (11), this increase in iGSH is equivalent at both oxygen levels (data not shown).
Fig. 4.
NAC does not abrogate the difference in CD3/CD28-stimulated T cell proliferation at atmosO2 and physO2. CFSE-stained human PBMCs were stimulated with plate-bound CD3 (1 μg/ml) and CD28 (2 μg/ml) for 3 days in cultures supplemented with 1 mM NAC. Cell counts were performed by using BD Trucount tubes. Fold increase in CD4 T cells was calculated as described in Materials and Methods. Statistics were calculated by using JMP software by least-square fit model with sample and oxygen as independent variables. Each set of connected points represents one subject (n = 6).
Because NAC acts as an “antioxidant” in the culture media (12), these findings indicate that oxygen levels do not influence proliferation simply by increasing the exogenous oxidant level in the culture. Furthermore, because NAC does not selectively increase iGSH in cells cultured at atmosO2 than cells cultured at physO2, these findings indicate that iGSH per se is not solely responsible for mediating the effect of oxygen on T cell proliferation.
iROS and iNO Levels Are Higher in CD3/CD28-Stimulated T Cells Cultured at PhysO2 Versus AtmosO2.
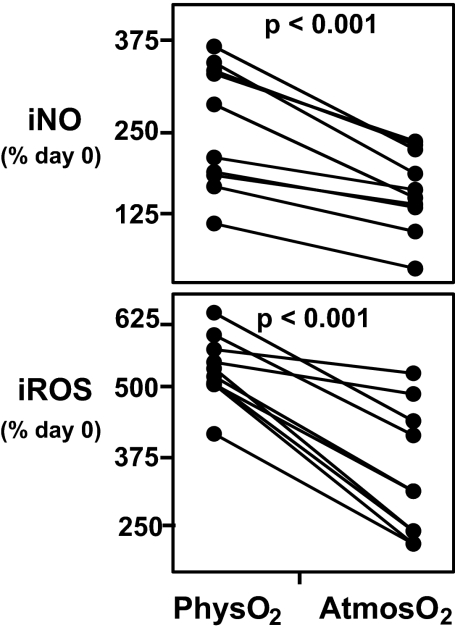
Human PBMCs have been shown to produce iROS (13) and iNO (14) on antigenic stimulation. Consistent with this, we found higher levels of CD4 T cell iNO and iROS levels in CD3/CD28-stimulated cultures (PBMCs) (Fig. 5). However, this increase is substantially greater (1.5- to 2.0-fold) in the T cells stimulated at physO2 (P < 0.001).
Fig. 5.
CD3/CD28 stimulation-induced increase in iNO and iROS is higher at physO2. Human PBMCs were stimulated with CD3/CD28 for 3 days. iNO and iROS were measured at the beginning and the end of culture by FACS as described in Materials and Methods. (Upper) Percentage increase in iNO (percentage increase relative to day 0). (Lower) Increase in iROS (percentage increase relative to day 0) in CD3/CD28-stimulated CD4 T cells. Statistics were calculated by using JMP software by least-square fit model with sample and oxygen as independent variables. Each set of connected points represents one subject (n = 11).
The increased levels of naturally produced iNO in the physO2 cultures very likely explains the decreased proliferation in these cultures because high levels of NO is well known to decrease cell division in cultures maintained at atmosO2 (15, 16).
Incubator Oxygen Levels Differentially Influence Kinetics of CD69 Expression; Subset Markers Are Largely Unaffected.
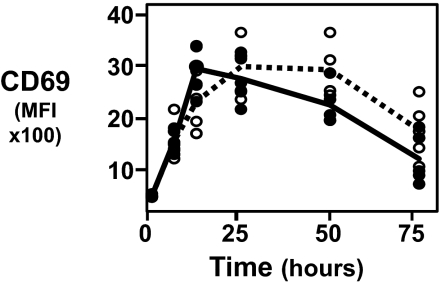
Although T cell phenotypes are relatively similar in PBMCs cultured for 3 days at physO2 and atmosO2 levels, there are striking differences in kinetics of expression of CD69, an early cell surface marker of T cell activation. Consistent with data reported in many studies, CD69 expression on CD3/CD28-stimulated CD4 T cells peaks at 12 h in atmosO2 cultures and gradually decreases by 72 h (17). However, in physO2 cultures, CD69 expression is delayed and does not reach peak levels until 24 h poststimulation. Furthermore, the CD69 expression at physO2 declines more slowly than at atmosO2 (Fig. 6).
Fig. 6.
Peak expression of CD69 is delayed and more sustained at physO2 than atmosO2. Human PBMCs were stimulated with plate-bound CD3 (1 μg/ml) and CD28 (2 μg/ml) for 3 days. Aliquots of cells were stained for CD69 at 6, 12, 24, 48, and 72 h of stimulation. Median fluorescence intensity (MFI) of CD69 for each sample is plotted against time of stimulation in culture at atmosO2 or physO2. Closed circles (fitted by solid line) represent the kinetics of CD69 expression at atmosO2. Open circles (fitted by broken line) represent the kinetics of CD69 expression at physO2 (n = 6).
In contrast, the kinetics of expression of the other T cell activation markers (CD25 and CD71) is not significantly different at the two oxygen levels. Similarly, expression of CD3, CD4, CD8, CD45RA, CD11a, and FACS measures of cell size and granularity of unstimulated T cells are similar at both oxygen levels. However, there is substantially less spontaneous shedding of CD62L in unstimulated T cells at physO2 as compared with T cells at atmosO2 (differences detected in PBMCs from three of five healthy subjects tested; data not shown).
Discussion
In early studies, mammalian cells were cultured in sealed vessels that were initially gassed with CO2 to control pH. Some years later, CO2 incubators were introduced to enable continuous equilibration of the culture medium with a controlled level of CO2 (usually 5% CO2 mixed with air). In these incubators, which rapidly became standard for mammalian cell culture, the oxygen level is determined by atmospheric oxygen levels (≈21%). Thus, nearly all modern functional studies with lymphocytes have been, and continue to be, performed with cultures equilibrated with a gas mixture containing 5% CO2 and 20% O2 (referred to here as atmosO2).
Importantly, atmosO2 is 2–4 times higher than the oxygen levels that, with few exceptions, mammalian cells encounter in vivo. This was recognized by Mishell and Dutton, who showed that antibody responses by B lymphocytes could be readily detected in spleen cell cultures that were maintained in chambers gassed with what we term here as physO2 (18). These studies, which motivated work reported here, were followed over the years by sporadic reports of improved function (antibody production, cell growth, and differentiation) in lymphocytes or other cells (3, 8, 19, 20).
Picking up this thread, we have shown here that the oxygen levels in incubators maintained at atmosO2 are too high to maintain the iGSH levels and other aspects of the intracellular redox environment in a condition comparable to that observed in freshly isolated cells. In contrast, culturing primary lymphocytes at 5% O2, which is closer to the physO2 that cells encounter in vivo, is much less damaging. Thus, after 3 days in culture, the most common measure of intracellular redox state (iGSH/iGSSG ratio) (10) reports the development of a more highly oxidative intracellular environment in T cells at atmosO2 than at physO2.
The maintenance of a higher iGSH/iGSSG ratio in the cells cultured at physO2 indicates that the redox regulatory mechanisms in the cells are operating better to maintain a relatively normal intracellular environment. We have shown here that there is a loss of iGSH at both atmosO2 and at physO2. However, the loss is significantly greater at atmosO2 than at physO2 (41% and 30%, respectively; P = 0.001). Most importantly, as indicated above, despite the iGSH loss in both cases, the iGSH/iGSSG ratio is higher at only physO2, suggesting that cells cultured at physO2 are less stressed than cells cultured at atmosO2.
Curiously, although culturing primary lymphocytes at physO2 better maintains the intracellular redox environment and hence should better maintain the health of the cells, we find that CD3/CD28-stimulated T cell proliferation is greater in cells cultured at atmosO2 than at physO2. In the current culture practice, in which more is usually considered to be better, this finding would appear to indicate that the cultures are not as healthy at physO2 and that functional studies would be better performed at atmosO2, despite the oxidative stress occurring in the culture. However, if the purpose of ex vivo studies were to understand in vivo mechanisms, then physO2 cultures would offer the better means for understanding T cell responses mounted under conditions in which (more of) the in vivo immunomodulatory mechanisms are intact.
This argument is supported by the greater increase in iNO levels that we have demonstrated in CD3/CD28-stimulated T cells. The higher iNO levels in physO2 cultures may indicate increased iNO production at physO2, decreased stability of iNO at atmosO2 (21, 22), or both. In any event, the result is greater bioavailability of iNO at physO2. Artificially raising iNO levels by addition of iNO donors to cell cultures maintained at atmosO2 has been shown to inhibit cell proliferation (16, 23) and promote cell death (24). Thus, the decreased proliferation in the CD3/CD28-stimulated cultures at physO2 can be expected on the basis of the increased iNO and likely reflects the appropriate functioning of iNO an immunomodulatory molecule with a function that is largely lost in cultures maintained at atmosO2.
The prolonged expression of CD69 that we observe at physO2 may also contribute to the lower proliferation observed in the physO2 cultures (25). Furthermore, lower oxygen levels have been shown to stabilize and increase hypoxia-inducible factor 1α expression, which is known to regulate lymphocyte function and development in vivo (26, 27) and has recently been shown to inhibit Ca2+ signaling by accelerating cytoplasmic Ca2+ clearance in CD3/CD28-stimulated cells (28). Thus, the lower proliferation at physO2 may be partly explained by a reduced capacity to flux calcium. Most likely, all of these mechanisms (plus others that are still unknown) contribute to modulating T cell responses in vivo and are responsible for decreasing primary T cell responses to antigenic stimulation at physO2.
Interestingly, although stimulated primary T cells proliferate better at atmosO2 (7, 29, 30), consistent with the findings of Mishell and Dutton (18), B cells have been shown to proliferate and produce antibodies better at physO2 (31, 32). Furthermore, many primary cell types (e.g., fibroblasts, embryonic stem cells) have been shown to grow better at physO2 (19, 33). Therefore, the higher T cell responses at atmosO2 stand out and perhaps represent a physiologically important distinction.
The following question then arises: what is the functional relevance of studies with primary T cells at atmosO2? Studies by Haddad et al. demonstrate that the expression of genes coding for proteins involved in detoxification, inflammation, cell death, and cell repair is higher at atmosO2 than at physO2 (5). This gene response pattern indicates that primary T cells maintained at atmosO2 respond to and function under oxidative stress. Thus, we suggest that responses measured at atmosO2 may be closer to in vivo T cell responses that occur during uncontrolled inflammation associated with oxidative stress than to T cell responses that occur under healthy conditions.
Materials and Methods
Materials.
All monoclonal antibodies (either purified or preconjugated to fluorochromes), BD Trucount tubes, and BD CompBeads were procured from BD Biosciences (San Jose, CA). Phycoerythrin and allophycocyanin were obtained from Prozyme (San Leandro, CA). CFDA-SE [5-(and -6)-carboxyfluorescein diacetate, succinimidyl ester]; monochlorobimane; DAF-FM (4-amino-5-methyamino-2′,7′-difluorofluorescein diacetate); and dihydrorhodamine 123 were obtained from Molecular Probes (Eugene, OR). Probenecid and other high-grade chemicals were obtained from Sigma–Aldrich (St. Louis, MO). RPMI medium 1640 was procured from GIBCO BRL/Invitrogen (Carlsbad, CA). FCS was obtained from Gemini Bio-Products (Calabasas, CA).
Human Subjects.
After informed consent, 20–30 ml of blood was drawn from healthy volunteers in evacuated tubes with heparin (Vacutainer; BD Biosciences). All blood draws were performed between 9:00 and 11:00 a.m. to minimize the effects of circadian variation on end points assayed.
Tri-Gas Incubators.
Cells were incubated at two levels of incubator oxygen. Five percent incubator oxygen tensions were generated in Sanyo MCO-175M O2/CO2 incubators (Sanyo Scientific, Bensenville, IL). Gas phase O2 tensions were controlled by continuous injection of appropriate amount of medical grade N2 to reach the target oxygen level. Cells cultured at atmospheric oxygen levels (20% O2) were incubated in a standard incubator without additional supply of nitrogen. CO2 levels were maintained at 5% in all cases.
Media.
Cells were cultured in RPMI medium 1640 supplemented with 10% FCS (heat inactivated), 100 units/ml penicillin, 100 μg/ml streptomycin, and nonessential amino acids. All media used for isolation and culture of PBMCs were equilibrated to the target oxygen levels at least 12 h before use.
PBMC Isolation and T cell Enrichment.
PBMCs were isolated by Ficoll–Hypaque gradient separation. For measurement of iGSH and iGSSG by tandem MS, T lymphocytes were “negatively enriched” by using RosetteSep for total T lymphocytes according to the procedure provided by the manufacturer (Stemcell Technologies, Vancouver, BC, Canada). The extent of enrichment was determined by staining the cell population with a mixture of CD3, CD4, CD8, CD19, CD14, and CD16. The purity of the enriched T lymphocytes was ≈95%.
CFDA-SE Staining.
T cell proliferation was measured by staining PBMCs with CFDA-SE according to Mannering et al. (34), with some modifications. Proliferation of CD4 T cells was determined by calculation the proliferation index and percent divided cells by using a cell proliferation utility in FlowJo (TreeStar, Ashland, OR).
Cell Culture and in Vitro Stimulation.
T cells or PBMCs (106) (CFDA-SE labeled or unstained) were cultured in 1 ml of RPMI medium 1640 (see Media) in 24-well plates for different periods of time (6, 12, 24, 48, or 72 h). PBMCs were stimulated with plates coated with anti-CD3 (1 μg/ml; UCTH1 clone; BD Biosciences) and anti-CD28 (2.0 μg/ml; CD28.2 clone; BD Biosciences). For experiments with NAC, culture media were supplemented with 1 mM NAC and pH adjusted to 7.4.
Cell Counts and Identification of Viable Cells.
Cell numbers at the beginning and end of the 3 day culture were determined by FACS by using BD Trucount beads according to the instructions provided by the manufacturer. Cell viability was determined by the propidium iodide exclusion method. Fold change in CD4 T cells is calculated as the ratio of live CD4 T cells at the end of the culture (typically 3 days) to the live CD4 T cells at the beginning of the culture (7).
FACS Assays for Intracellular Redox Status.
Intracellular redox state of CD4 T cells was determined by FACS assays for iGSH, iNO, and iROS. Briefly, separate aliquots of cells were stained with 40 μM monochlorobimane (for iGSH) (35), 1 μM DAF-FM, DA (for iNO) (36), or 1 μM dihydrorhodamine 123 (for iROS) (37) for 20 min in staining media (RPMI medium 1640, 4% FCS and 2.5 mM probenecid) at room temperature. The reaction was quenched with excess chilled staining media. Subsequently the cells were centrifuged and resuspended in staining media for further processing for high-dimensional FACS.
High-Dimensional FACS Analysis.
Cells stained with CFDA-SE [5- (and -6)-carboxyfluorescein diacetate, succinimidyl ester], cells that were stained for intracellular redox markers, and unstained freshly prepared or cultured PBMCs were stained with different preparations of fluorochrome-conjugated antibodies (CD3, CD4, CD8, CD45RA, CD11a, CD62L, CD69, CD25, CD71) that were prepared in our laboratory or obtained from BD Biosciences. Surface staining was performed as described (35, 38). “Fluorescence-minus-one” controls (38) were included to determine the level of nonspecific staining and autofluorescence associated with subsets of cells in each fluorescence channel. BD CompBeads (anti-mouse Ig, κ beads) were used for single stain controls for fluorescence compensation. High-dimensional FACS data were collected on BD FACSAria (BD Biosciences). FlowJo (TreeStar) software was used for fluorescence compensation and analysis.
Tandem MS Analysis.
Tandem mass spectrometric analysis of GSH and GSSG was performed according to K.R.A., A.-K.N., and T.C. (unpublished data). Briefly, 2 × 106 lymphocytes were extracted in 80% precipitating solution (20 mM N-ethylmaleimide, 2% sulfosalicylic acid, 2 mM EDTA in 15% methanol). The mixtures were thoroughly vortexed, incubated at room temperature for 20 min, and centrifuged at 13,000 × g for 5 min. Mixtures were then analyzed separately by liquid chromatography tandem MS with internals standards (15N-NEM and GSH-13C for GSH and 15N-NEM and GSSG-13C for GSSG) by using a Shimadzu (Kyoto, Japan) solvent delivery system (model LC-10ADvp pumps and SCL-10Avp controller), a LEAP Technologies (Carrboro, NC) autosampler (model HTS PAL), and an API 3000 tandem mass spectrometer (Applied Biosystems, Foster City, CA) with Turbulon Ion Spray source (PE–Sciex, Concorde, ON, Canada).
Samples were analyzed without chromatographic separation. Ions were detected in the multiple reaction-monitoring mode by using the following transitions: m/z 433.3 to m/z 304.0 (GSH–NEM), m/z 435.3 to m/z 306.1 (GSH-13C, 15N-NEM), m/z 613.2 to m/z 355.2 (GSSG), and m/z 617.2 to m/z 359.2 (GSSG-13C, 15N). Data were acquired with Analyst software (Version 1.2; Agilent Technologies, Palo Alto, CA) and analyzed by using ChemoView (Version 1.2b6; Applied Biosciences).
Statistical Analysis.
Analyses of FACS data, including calculation of proliferation indices, percentage of divided cells, iGSH, iROS, and iNO levels were performed by using FlowJo software (TreeStar). Statistical analyses were performed with the JMP statistical software package (SAS Institute, Cary, NC). Sample (subject) and gas phase oxygen level were entered as model effects in all analyses by using the JMP least-square fit model platform. Visual representations of these analyses are shown in figures generated with Matched Columns utility of the JMP Fit Y by X platform, which connects the data points shown for each subject in the graph. The sample (subject) had significant effect on the analysis (P < 0.001). For all least-square fit models, P < 0.001.
Acknowledgments
We thank the following members of the Herzenberg laboratory (Genetics Department, Stanford University School of Medicine): Glenn Smith and Bahram Aram for excellent and devoted technical support and John J. Mantovani for administrative help, including the preparation of the manuscript. This work was supported National Institutes of Health Grant AI 566223.
Abbreviations
- atmosO2
atmospheric oxygen level
- CFDA-SE
5-(and 6)-carboxyfluorescein diacetate, succinimidyl ester
- DAF-FM
4-amino-5-methyamino-2′,7′-difluorofluorescein diacetate
- iGSH
intracellular glutathione
- iGSSG
oxidized intracellular glutathione
- iNO
intracellular NO
- iROS
intracellular reactive oxygen species
- NAC
N-acetylcysteine
- PBMC
peripheral blood mononuclear cell
- physO2
physiological oxygen levels.
Footnotes
The authors declare no conflict of interest.
References
- 1.Campbell JA. J Physiol. 1925;60:20–29. doi: 10.1113/jphysiol.1925.sp002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laser H. Biochem J. 1937;31:1671–1676. doi: 10.1042/bj0311671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 4.Laser H. Proc R Soc London Ser B. 1952;140:230–243. doi: 10.1098/rspb.1952.0060. [DOI] [PubMed] [Google Scholar]
- 5.Haddad H, Windgassen D, Ramsborg CG, Paredes CJ, Papoutsakis ET. Biotechnol Bioeng. 2004;87:437–450. doi: 10.1002/bit.20166. [DOI] [PubMed] [Google Scholar]
- 6.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Borresen-Dale AL, et al. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkuri KR, Herzenberg LA, Herzenberg LA. Proc Natl Acad Sci USA. 2005;102:3756–3759. doi: 10.1073/pnas.0409910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carswell KS, Weiss JW, Papoutsakis ET. Cytotherapy. 2000;2:25–37. doi: 10.1080/146532400539026. [DOI] [PubMed] [Google Scholar]
- 9.Ebbesen P, Hager H, Aboagye-Mathiesen G, Petersen PM, Lutzhoft J, Villadsen JA, Zdravkovic M, Dalsgaard AM, Zachar V. Exp Gerontol. 1993;28:573–578. doi: 10.1016/0531-5565(93)90046-g. [DOI] [PubMed] [Google Scholar]
- 10.Schafer FQ, Buettner GR. Free Radical Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 11.De Rosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, Mitra D, Watanabe N, Nakamura H, Tjioe I, et al. Eur J Clin Invest. 2000;30:915–929. doi: 10.1046/j.1365-2362.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 12.Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, et al. Proc Natl Acad Sci USA. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MS, Kwon J. Free Radical Biol Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Bogdan C. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin LM, Demple B. Cancer Res. 2005;65:6097–6104. doi: 10.1158/0008-5472.CAN-04-4254. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Schmid T, Brune B. Curr Mol Med. 2004;4:741–751. doi: 10.2174/1566524043359926. [DOI] [PubMed] [Google Scholar]
- 17.Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. J Immunol Methods. 2004;293:127–142. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Mishell RI, Dutton RW. J Exp Med. 1967;126:423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsyth NR, Musio A, Vezzoni P, Simpson AH, Noble BS, McWhir J. Cloning Stem Cells. 2006;8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 20.Lennon DP, Edmison JM, Caplan AI. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 21.Inoue M, Nishikawa M, Sato EF, Ah-Mee P, Kashiba M, Takehara Y, Utsumi K. Free Radical Res. 1999;31:251–260. doi: 10.1080/10715769900300831. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa M, Sato EF, Utsumi K, Inoue M. Cancer Res. 1996;56:4535–4540. [PubMed] [Google Scholar]
- 23.Fiorucci S, Mencarelli A, Distrutti E, Baldoni M, del Soldato P, Morelli A. J Immunol. 2004;173:874–882. doi: 10.4049/jimmunol.173.2.874. [DOI] [PubMed] [Google Scholar]
- 24.Moulian N, Truffault F, Gaudry-Talarmain YM, Serraf A, Berrih-Aknin S. Blood. 2001;97:3521–3530. doi: 10.1182/blood.v97.11.3521. [DOI] [PubMed] [Google Scholar]
- 25.Sancho D, Gomez M, Sanchez-Madrid F. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Kojima H, Sitkovsky MV, Cascalho M. Curr Pharm Des. 2003;9:1827–1832. doi: 10.2174/1381612033454388. [DOI] [PubMed] [Google Scholar]
- 27.Sitkovsky M, Lukashev D. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 28.Neumann AK, Yang J, Biju MP, Joseph SK, Johnson RS, Haase VH, Freedman BD, Turka LA. Proc Natl Acad Sci USA. 2005;102:17071–17076. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen V, Hellung-Larsen P, Sorensen SF. J Cell Physiol. 1968;72:149–152. doi: 10.1002/jcp.1040720302. [DOI] [PubMed] [Google Scholar]
- 30.Loeffler DA, Juneau PL, Masserant S. Br J Cancer. 1992;66:619–622. doi: 10.1038/bjc.1992.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuveny S, Velez D, Riske F, MacMillan JD, Miller L. Dev Biol Stand. 1985;60:185–197. [PubMed] [Google Scholar]
- 32.Ozturk SS, Palsson BO. Biotechnol Prog. 1991;7:481–494. doi: 10.1021/bp00012a002. [DOI] [PubMed] [Google Scholar]
- 33.Ezashi T, Das P, Roberts RM. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannering SI, Morris JS, Jensen KP, Purcell AW, Honeyman MC, van Endert PM, Harrison LC. J Immunol Methods. 2003;283:173–183. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Sahaf B, Heydari K, Herzenberg LA. Proc Natl Acad Sci USA. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacza Z, Snipes JA, Zhang J, Horvath EM, Figueroa JP, Szabo C, Busija DW. Free Radical Biol Med. 2003;35:1217–1228. doi: 10.1016/s0891-5849(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 37.Richardson MP, Ayliffe MJ, Helbert M, Davies EG. J Immunol Methods. 1998;219:187–193. doi: 10.1016/s0022-1759(98)00136-7. [DOI] [PubMed] [Google Scholar]
- 38.De Rosa SC, Herzenberg LA, Roederer M. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]