Abstract
Hec-6st is a highly specific high endothelial venule (HEV) gene that is crucial for regulating lymphocyte homing to lymph nodes (LN). The enzyme is also expressed in HEV-like vessels in tertiary lymphoid organs that form in chronic inflammation in autoimmunity, graft rejection, and microbial infection. Understanding the molecular nature of Hec-6st regulation is crucial for elucidating its function in development and disease. However, studies of HEV are limited because of the difficulties in isolating and maintaining the unique characteristics of these vessels in vitro. The novel pClasper yeast homologous recombination technique was used to isolate from a BAC clone a 60-kb DNA fragment that included the Hec-6st (Chst4) gene with flanking sequences. Transgenic mice were generated with the β-galactosidase (LacZ) reporter gene inserted in-frame in the exon II of Hec-6st within the isolated BAC DNA fragment. LacZ was expressed specifically on HEV in LN, as indicated by its colocalization with peripheral node vascular addressin. LacZ was increased in nasal-associated lymphoid tissue during development and was reduced in LN and nasal-associated lymphoid tissue by LTβR-Ig (lymphotoxin-β receptor human Ig fusion protein) treatment in a manner identical to the endogenous gene. The transgene was expressed at high levels in lymphoid accumulations with characteristics of tertiary lymphoid organs in the salivary glands of aged mice. Thus, the Hec-6s-LacZ construct faithfully reproduces Hec-6st tissue-specific expression and can be used in further studies to drive expression of reporter or effector genes, which could visualize or inhibit HEV in autoimmunity.
Keywords: lymphoid organ, pClasper, peripheral node vascular addressin, sulfotransferase, lymphotoxin
High endothelial venules (HEV) are the site of entry of naïve lymphocytes into the lymph node (LN) parenchyma from the blood stream. Lymphocyte tethering along HEV in LN is mediated by the interaction of l-selectin expressed on the surface of lymphocytes and l-selectin ligands expressed on HEV. The major class of l-selectin ligands are recognized by monoclonal antibody MECA-79 and termed peripheral node vascular addressin (PNAd) (1). PNAd is composed of several scaffold glycoproteins including GlyCAM-1, CD34, and Sgp200 (2, 3). These proteins are posttranslationally modified to generate the sulfosialyl-Lewisx epitope recognized by the prototypic antibody MECA79 by HEV-restricted sulfotransferase activity.
HEC-6ST (4, 5) is a HEV-specific GlcNAc-6-sulfotransferase [gene name Chst4, also termed LSST (6), HEC-GlcNAc6ST, GST3 (7), or GlcNAc6ST-2 (8)] that specifically sulfates C-6 of N-acetylglucosamine in high endothelial cells (HEC). The Hec-6st gene is expressed predominantly on HEV in LN, with only limited expression in other tissues (6, 7). HEC-6ST is required for luminal PNAd expression on HEV (9) because luminal PNAd expression is lacking and lymphocyte homing to the LN is reduced by 50% in Hec-6st deficient mice, indicating its importance in HEV function (9, 10).
Ectopic accumulations of lymphoid cells that have characteristics of organized lymphoid tissue are found in many types of chronic inflammatory stimulation, including autoimmune diseases, such as type I diabetes and rheumatoid arthritis, graft rejection, and microbial infection (8, 11–13). These accumulations, termed tertiary lymphoid organs or tissues (TLO), arise through a process called lymphoid neogenesis (14). HEV found in such TLO frequently express PNAd and HEC-6ST (11, 12). Although it is not clear whether the expression of PNAd in these pathological situations is a prerequisite or the consequence of the chronic inflammatory process, the presence of HEV allows entrance of naïve cells into the site and provides the possibility of further damage through the process of epitope spreading (15). Thus, understanding the regulation of HEV is crucial for gaining insight into their regulation in ontogeny and pathology.
The HEC is highly specialized and loses its characteristic appearance and gene expression soon after isolation from the original microenvironment (16). In fact, even in the ex vivo LN culture, where presumably the microenvironment is preserved to some extent, HEV become flat-walled, with a 45–50% reduction in the capacity to bind lymphocytes within 24 h of culture (17). Thus, it is particularly difficult to study HEV regulation and function in vitro. Therefore, here we aimed to isolate the regulatory elements of a HEV-restricted gene such as Hec-6st and to express a reporter gene under its control in vivo in transgenic mice. This approach allows the generation of transgene constructs that can use Hec-6st regulatory sequences to drive expression of fluorescent marker proteins for visualization of HEV or of inhibitors to prevent the development of TLO in chronic inflammation in vivo.
The yeast-bacteria shuttle vector pClasper was developed to facilitate the functional analysis of genes (18). DNA of up to several hundred kilobases can be carried in pClasper, modified by using yeast genetic techniques, and expanded in bacteria. The inclusion of large genomic regions in the transgene has been shown to produce a more faithful pattern of gene expression at high frequency because distant regulatory elements are included. In this study pClasper was used to capture the mouse Hec-6st gene and modify it by in-frame insertion of a reporter gene in the Hec-6st coding region. The resultant reporter gene construct was used to produce transgenic mice expressing LacZ under control of the Hec-6st gene regulatory elements. The transgene was expressed on HEV in LN, nasal-associated lymphoid tissue (NALT), and TLO. Its expression pattern faithfully recapitulated that of the endogenous Hec-6st gene.
Results
Regulatory Sequences of Hec-6st Are Included in a 60-kb BAC DNA Fragment.
As a highly HEV-specific gene, Hec-6st is of particular interest in our study. It serves as a marker of both the developmental stage and function of HEV. Initially, transgenic mice were generated with constructs with either 4-kb or 9-kb Hec-6st upstream flanking sequence fragments driving the reporter gene. Although several mice contained those transgenes, none exhibited reporter gene mRNA transcripts or protein expression in HEV, demonstrating that even a 9-kb 5′-flanking sequence did not contain the elements required for tissue-specific expression (data not shown). Thus, we concluded that a larger portion of the gene was required to generate HEV-specific expression.
To capture the regulatory elements of Hec-6st, we used the novel pClasper technique to isolate from BAC clone 20473A a 60-kb DNA fragment that included the entire Hec-6st gene and upstream and downstream noncoding sequences. The reporter gene LacZ, along with the URA3 yeast selection marker, was inserted in-frame into exon II of the isolated Hec-6st gene by homologous recombination in yeast, and transgenic mice were prepared (Fig. 1A). The construct produced by this technique did not affect the efficiency of transgenic mouse preparation. Eleven of 43 progeny (25.6%) contained the transgene, as detected by Southern blot analysis of tail DNA (Fig. 1B). No obvious gross phenotypic differences were apparent when the transgene-positive and -negative littermates were compared. Significantly, their lymphoid organs appeared normal. Several transgenic founder mice were bred with C57BL/6 mice.
Fig. 1.
Hec-6st transgenic mice. The Hec-6st gene includes two exons, an intron, and flanking sequences. The entire coding sequence is contained in the second exon. The Hec-6st-LacZ transgene construct was generated by isolating from BAC clone 20473A a 60-kb DNA fragment, which included the entire Hec-6st gene with 40-kb upstream and 18-kb downstream noncoding sequence, and inserting in-frame a LacZ cassette composed of the promoterless LacZ reporter gene and URA3 selection marker in yeast into Hec-6st gene exon II by yeast homologous recombination by using pClasper technology. (A) Schematic diagram of the transgene construct. Fg1 and Fg2 were the matching DNA sequences used to insert the LacZ cassette into Hec-6st gene exon II by homologous recombination. FgA and FgB3 were the DNA sequences used to capture the DNA fragments from the BAC clone using the database sequences. URA3A F and Hec-6st R are the PCR primers used for screening the transgenic mice. 5′–3′ represents the database DNA sequence direction. Arrows indicate the direction of gene transcription. (B) Southern blot analysis of tail DNA of Hec-6st-LacZ founder lines. Positive mice are indicated by the presence of a 3.6-kb BamHI fragment hybridizing with a LacZ probe.
Transgenic LacZ Expression Retraces Endogenous Hec-6st Expression on HEV.
We investigated LacZ expression in secondary lymphoid organs of mice from several independent lines of Hec-6st-LacZ transgenic mice by RT-PCR and LacZ staining. The transgene was transcribed in peripheral LN (PLN), NALT, and mesenteric LN of the transgene positive mice, but not in those organs of the transgene negative littermates (Fig. 2A). The transgene LacZ expression in LN reflected the typical HEV branching pattern including large and small vessels (Fig. 2 Ba, Bc, and Be) (19). The postcapillary venules in LN progress from small highly branched vessels to larger collecting vessels (19). Only the higher orders (orders III–V) express PNAd and are the sites of lymphocyte egress from HEV (20, 21). The LacZ expression clearly follows this specific branching pattern (Fig. 2Be). Previous studies had shown that the endogenous Hec-6st expression is colocalized with PNAd (8). We therefore costained PLN tissue sections for PNAd with the MECA 79 antibody and for LacZ. LacZ expression was exclusively colocalized with that of PNAd, indicating that LacZ was expressed specifically on HEV in LN, faithfully recapitulating Hec-6st expression (Fig. 2C). Interestingly, some HEVs stained positive with the MECA 79 antibody but were negative for LacZ. Several possibilities may contribute to this difference. These could include differences in the sensitivity of the detection methods or a lower level of LacZ protein compared with PNAd. It is also possible that these vessels only express another HEV sulfotransferase, GlcNAc6ST-1 (22). It is possible that these vessels no longer express HEC-6ST once the scaffold glycoprotein has been modified. Peyer's patches were also analyzed. Although abluminal PNAd was detected, LacZ was not colocalized with those vessels, suggesting that abluminal PNAd expression in Peyer's patches is regulated by another sulfotransferase, such as GlcNAc6ST-1. The endogenous Hec-6st gene is known to be expressed in sites other than HEV (6, 7), and there was an unexpected LacZ expression in intestinal villi, which did not correlate with PNAd (data not shown). The villi are sites of lymphocyte recruitment and differentiation into isolated lymphoid follicles (23, 24). Further studies are needed to investigate whether this expression is authentic but had not previously been noted as a trafficking mechanism.
Fig. 2.
LacZ reproduces HEC-6ST expression on HEV in LN and NALT of the transgenic mice. (A) Transcription of Hec-6st and LacZ mRNA in secondary lymphoid organs (PLN, mesenteric LN, and NALT) as detected by RT-PCR using LacZ and Hec-6st primer pairs reported in Materials and Methods. (B) Expression of LacZ in PLN and NALT analyzed by X-Gal staining for 4 h. (Original magnification: ×2.) (Ba, Bb, and Be) PLN. (Bc and Bd) NALT. (Ba, Bc, and Be) Transgene positive mice. (Bb and Bd) Transgene negative littermates. (Be) LN incubated with X-Gal overnight displays the typical HEV branched pattern. (Original magnification: ×5.) (C) Sections of PLN stained for LacZ (Ca) were costained for PNAd (Cb, red) by immunofluorescence. (Original magnification: ×20.)
In the five different lines that were investigated (lines 399, 403, 406, 416, and 427), LacZ was expressed in a branching pattern in the LNs. Line 403 of the transgenic mice had the highest copy of the transgene (Fig. 1B) and the highest levels of LacZ expression. Thus, most of the results shown in this communication were generated by using line 403.
Transgenic LacZ Expression on HEV Mimics That of HEC-6ST in NALT Development.
As a mature HEV marker, HEC-6ST expression is regulated during development (25, 26). It is induced soon after birth and rapidly increases to the adult level in PLN HEV (data not shown), consistent with the switch from MAdCAM-1 to PNAd expression (26). HEV development in NALT is considerably slower than that in LN, requiring ≈5–6 weeks to attain mature HEV status as we have previously reported (25). We thus analyzed LacZ expression in NALT during postnatal development. In 3-week-old mice the NALT was smaller than in the adult although it could easily be distinguished from the surrounding tissue by the accumulation of lymphocytes. PNAd expression was mostly abluminal, and HEC-6ST was barely detectable (Fig. 3Ab). In 6-week-old mice the NALT was fully developed. It was considerably more prominent than in 3-week-old mice, and some HEV expressed HEC-6ST and luminal PNAd (Fig. 3Ad). We then evaluated LacZ expression in NALT of different lines of transgenic mice. Although LacZ levels differed to some extent between the various lines, it was always significantly higher in NALT isolated from 7- to 10-week-old mice (Fig. 3 Bb and Bd) when compared with 3-week-old siblings (Fig. 3 Ba and Bc). No LacZ staining was detected in transgene negative littermates (data not shown). Thus, the Hec-6st-LacZ transgene is regulated in a similar pattern as the endogenous Hec-6st gene during postnatal NALT development.
Fig. 3.
LacZ expression in NALT of transgenic mice mimics wild-type HEC-6ST expression during development. (A) NALT isolated from 3-week-old or 6-week-old C57BL/6 mice. The dotted circles indicate the area of NALT. (Aa and Ac) HE staining. (Original magnification: ×5.) (Ab and Ad) Immunofluorescence double staining for PNAd (red) and HEC-6ST (green). (Original magnification: ×40.) (B) NALT isolated from different lines of Hec-6st-LacZ transgenic mice at 3 weeks or 7–10 weeks of age. (Ba) Line 403, 3 weeks old. (Bb) Line 403, 10 weeks old. (Bc) Line 399, 3 weeks old. (Bd) Line 399, 7 weeks old. LacZ expression was detected by X-Gal staining overnight. (Original magnification: ×2.) Shown are representative results of three experiments of three different Hec-6st-LacZ transgenic mouse lines.
HEC-6ST and Transgenic LacZ Expression on HEV Are Diminished by Treatment with LTβR-Ig (Lymphotoxin-β Receptor Human Ig Fusion Protein).
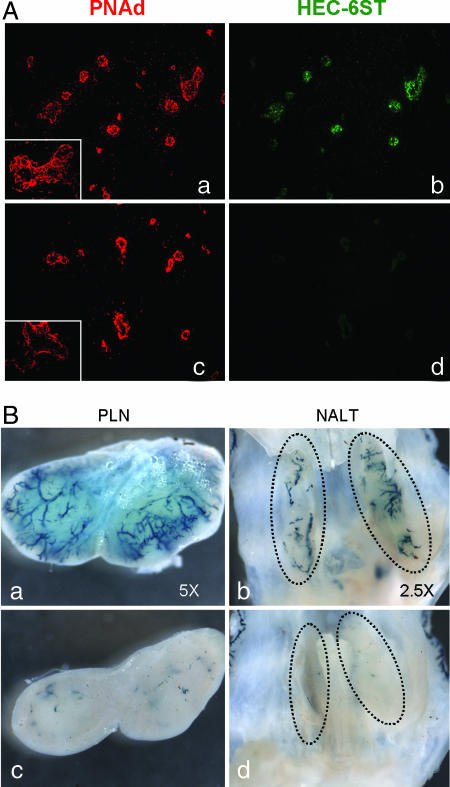
HEC-6ST expression is reduced by the treatment with LTβR-Ig in vivo (27, 28). Seven days after a single injection of 100 μg of LTβR-Ig per mouse, HEC-6ST and luminal PNAd expression are almost completely inhibited in LN HEV. HEV were somewhat flattened, and some displayed only abluminal PNAd expression. No such inhibition was apparent in the control Ig-injected mice (Fig. 4A). We then asked whether the LacZ transgene could be similarly inhibited by LTβR-Ig treatment. Seven days after LTβR-Ig or control Ig injection, PLN, mesenteric LN, and NALT were isolated from the transgenic mice. LacZ activity was drastically reduced in the LN and NALT of transgenic mice treated with LTβR-Ig, but not in transgenic mice treated with control Ig, in a pattern identical to that of the endogenous Hec-6st gene (Fig. 4B).
Fig. 4.
Diminished expression of HEC-6ST and LacZ in HEV after LTβR-Ig treatment. (A) PLN isolated from C57BL/6 mice 7 days after treatment with LTβR-Ig or control Ig. The expression of HEC-6ST (green) and PNAd (red) in PLN was detected by immunofluorescence double staining. (Aa and Ab) Control Ig-treated mouse. (Ac and Ad) LTβR-Ig-treated mouse. Shown is a representative experiment of n > 3 mice per group. (Original magnification: ×20.) (Aa Inset) Typical HEV with luminal and abluminal PNAd staining. (Ac Inset) Typical HEV with abluminal PNAd staining after LTβR-Ig treatment. (Original magnification of Insets: ×100.) (B) PLN and NALT isolated at day 7 after treatment with LTβR-Ig or control Ig. LacZ expression was detected by X-Gal staining overnight. (Ba and Bb) Control Ig-treated mouse. (Bc and Bd) LTβR-Ig-treated mouse. Shown are representative experiments of n = 3 mice per group.
Transgenic LacZ and Hec-6st Are Expressed in HEV in TLO During Chronic Inflammation.
HEV are a frequent characteristic of TLO in chronic inflammation (8, 11–13). Thus, the regulation of HEV is crucial for understanding the role of TLO in pathology. In several strains of laboratory mice deficient in major histocompatibility complex class II gene I–E expression, such as C57BL/6 and SJL (29), TLO develop spontaneously in the salivary glands of aged mice. Accordingly, in a 15-month-old F1 transgenic mouse, but not in a 9-month-old sibling, there was a spontaneous lymphoid infiltration in the salivary gland (Fig. 5 A and E). The cell infiltrate exhibited clear T and B cell compartmentalization (Fig. 5F), lymphatic vessels (Fig. 5G), and HEV that expressed PNAd and low levels of HEC-6ST (Fig. 5G and data not shown), indicating the formation of TLO. LacZ was expressed in the salivary glands of the 15-month-old transgenic mouse, and no such expression was apparent in the salivary gland of the 9-month-old transgene positive sibling, which was negative for cellular infiltrate and HEV (Fig. 5 A–D and H). The X-Gal-stained salivary glands were then sectioned (7 μm) and stained for hematoxylin (HE) or PNAd. The LacZ transgene was expressed only in the TLO area (Fig. 5I) and exclusively colocalized with PNAd, although some MECA 79 vessels are negative for LacZ as noted above (Fig. 5J).
Fig. 5.
HEC-6ST and LacZ expression on HEV in spontaneous TLO in salivary glands. (A–D) Absence of TLO in salivary glands isolated from a 9-month-old transgene positive mouse. (A) H&E-stained section. (B) CD3 (red) and B220 (green) double immunofluorescence stained frozen section. (C) PNAd (red) and lymphatic vessel endothelial hyaluronan receptor 1 (green) double immunofluorescence and HE-counterstained frozen section. (D) X-Gal staining of the entire salivary gland. (Original magnification: ×20 in A–C and ×2 in D.) (E–J) TLO in salivary glands isolated from a 15-month-old transgene-positive mouse (a sibling of the above mouse). (E) H&E-stained section. (F) CD3 (red) and B220 (green) double immunofluorescence stained frozen section. (G) PNAd (red) and lymphatic vessel endothelial hyaluronan receptor 1 (green) double immunofluorescence and HE-counterstained frozen section. Note that the lymphatic sinus is packed with lymphocytes. (Original magnification: ×20 in E–G.) (H) X-Gal staining of the entire salivary gland. (Original magnification: ×2.) (I) HE-counterstaining of a 7-μm section of the X-Gal-stained salivary gland reveals that LacZ is expressed exclusively within the TLO. (J) The X-Gal-stained salivary glands were sectioned and stained for PNAd. LacZ (blue) and PNAd (red) are colocalized in one TLO of the salivary gland. (Original magnification: ×40 in I and J.)
Discussion
The pClasper homologous recombination technology has been used successfully in previous transgenic animal studies to investigate the evolution, regulation, and function of the mouse Hoxc8 gene (30–32), the zebrafish Hoxa-11b gene (33), and the mouse Dlx3 gene, among others (34, 35). It proved essential for the construction of a functional Hec-6st-LacZ transgene capable of directing the tissue-specific expression of the LacZ reporter in register with endogenous Hec-6st gene expression. It allowed the handling of sufficiently large DNA fragments to generate a transgene, which directed the tissue-specific expression of the LacZ reporter in close parallel to endogenous Hec-6st. The failure to achieve this goal with transgenes using a 4- or 9-kb upstream sequence indicates that essential control elements are located outside of the 9-kb Hec-6st flanking sequence. The coexpression of LacZ and PNAd in LN, NALT, and TLO in the transgenic mice, as well as the regulation of the LacZ transgene during development and by LTβR-Ig treatment, indicate that the DNA fragment isolated from the BAC clone faithfully recapitulated the expression of the endogenous Hec-6st gene in HEV. It is of particular interest that LacZ expression clearly delineates the relation between PNAd expression and the branching pattern of the postcapillary venules in LN (17) (Fig. 2Be), which further confirms that the 60-kb Hec-6st-LacZ construct contains the key elements directing specific Hec-6st expression.
LTβR-Ig treatment can inhibit the immune response to foreign antigen and prevent antigen-induced autoimmune diseases such as experimental autoimmune encephalitis and collagen-induced arthritis (27, 36, 37). Treatment with LTβR-Ig can also reverse established insulitis and prevent diabetes (38). Because LTβR is expressed on several cell types in the LN, including HEV and lymphatic vessel endothelial cells, dendritic cells, and stromal cells (28, 39–41), LTβR-Ig treatment can have extensive effects on LN. Thus, investigating the role of LTβR signaling in regulating HEV phenotype and function without affecting other cell types is challenging and requires appropriate tools.
Visualizing HEV in vivo is a very important approach for studying HEV function. At present, in vivo methods to analyze HEV function involve labeling vessels with injected fluorescein-conjugated antibody or injecting fluorescent particles. Hiraoka et al. (10) have described a mouse that is a knockin for egfp in the Hec-6st gene that potentially could highlight vessels in vivo. However, this results in disruption of the endogenous gene. All of these methods potentially disrupt HEV function or nonspecifically highlight all blood vessels. Thus, the use of the regulatory region of Hec-6st to direct HEV-specific reporter gene expression without affecting other cell types significantly improves in vivo HEV studies, which can be extended to effector or inhibitor transgenes.
Exclusive LacZ expression in the spontaneous TLOs in the aged SJL×C57BL/6 mouse was also observed in salivary glands of a 6-month-old SJL×C57BL/6 F1 transgenic mice bred to nonobese diabetic mice (at generation F4), which had several small infiltrates in the salivary gland (data not shown). Thus, the expression of LacZ in the transgenic mice can be used to determine the developmental stage of TLO during chronic inflammation. Given the function of HEV in the progression of TLO in autoimmune disease and chronic rejection in transplantation (8, 11–13), targeting HEV in TLO using the Hec-6st regulatory elements can serve as a novel strategy to control autoimmune diseases.
Materials and Methods
Hec-6st-LacZ Transgenic Mice.
BAC clone 20473A was a gift from Steven Rosen and Stefan Hemmerich (University of California, San Francisco). The nucleotide sequence was based on National Center for Biotechnology Information database sequence contig 1 (CAAA01056405) and contig 2 (CAAA01219588). By an improved pClasper vector (18, 31), a 60-kb DNA fragment, including the entire Hec-6st gene with upstream and downstream noncoding sequence, was captured from the BAC clone. The homologous recombination DNA fragments used to capture the targeted DNA were FgB3 (sequence 42091–42573 of contig 1) and FgA (sequence 43516–44076 of contig 2) (Fig. 1A). The primers used were as follows: FgA forward (NruI), tcgcga GAA ATC TGC CTG CCT CTG CCT CCC; FgA reverse (NotI), gcggccgc TGG ATA GAC CCA CCC GAG AAA AG; FgB3 forward (HindIII), aagctt AGT GTG GTG TTC GCT ATG GGT TT; FgB3 reverse (NruI), tcgcga CAG GCA GGA ATG GGC AGA CT. A DNA fragment including the promoterless LacZ reporter and yeast selection gene URA3 was inserted into Hec-6st gene exon II by homologous recombination in yeast. The homologous recombination Hec-6st sequences used at this step were Fg1 and Fg2 located on 57156–57534 and 56702–57130 of contig 1A, respectively (Fig. 1A). The primers were as follows: Fg1 forward (HindIII), aagctt AAG GCC ACA CTC TGT CAG GGG TGT; Fg1 reverse (SmaI), cccggg GTC CTA CTA CGA CAA CTT CTT TCC C; Fg2 forward (NotI), gcggccgc AGG TCA TCG TTG TAG CTC TCT TCA TC; Fg2 reverse (NotI-BglII), gcggccgc agatct GCT GCT GAC CGC AGA GCA GCT TG. The sequence of the entire transgene construct was ≈69 kb and was verified by restriction enzyme digestion, PCR, and Southern blot analysis (data not shown). Hec-6st-LacZ transgenic mice were generated with the verified construct by pronuclear injection of (C57BL/6J × SJL/J) F2 embryos (Transgenic Mouse Service, Yale University). Several individual lines were prepared by breeding founders to C57BL/6 mice. All of the results shown in this article were generated in F1 or F2 progeny. The screening primers were as follows: URA3 forward, 5′-GCT CAT GAG ACA ATA ACC CTG; Hec-6st reverse, 5′-ATC CCG GGA GCA CAC CAC CAT AGA.
Southern Blot.
The transgene was determined by Southern blot analysis using a total of 10 μg of tail DNA per mouse and digested with BamHI, resulting in a 3.6-kb fragment for the transgene. The probe for LacZ was the PCR product from the LacZ primers and the transgene construct as template. The LacZ primers were 5′-GAC GTC TCG TTG CTG CAT AA (forward) and CAG CAG CAG ACC ATT TTC AA (reverse). No cross-reaction with the transgenic negative tail DNA was observed under the protocol.
RT-PCR.
Tissues were collected from individual mice and immediately placed into lysis buffer for RNA extraction (RNeasy kit; Qiagen, Valencia, CA). The extracted RNA was treated with RNase-free DNase I kit (Qiagen), and first-strand cDNA was reverse-transcribed by using a SuperScript II kit (Invitrogen, Carlsbad, CA). The Hec-6st primers were GGA CCA ACG CCA CGC CTG AGA G (forward) and ATC CCG GGA GCA CAC CAC CAT AGA (reverse). For LacZ primers see Southern Blot.
Immunofluorescence Staining of PNAd and HEC-6ST.
LN were embedded and frozen in O.C.T. (Sakura, Torrance, CA), and 7-μm cryocut sections were fixed with cold acetone. Blocking buffer was 3% BSA (Sigma, St. Louis, MO) and 5% mouse serum (Zymed, San Francisco, CA) in PBS. Detecting antibodies were rat anti-mouse PNAd (MECA-79; BD Pharmingen, San Diego, CA) and rabbit anti-mouse HEC-6ST developed in our laboratory (8, 12). Biotin-conjugated goat anti-rabbit IgG was purchased from Jackson ImmunoResearch (West Grove, PA). Fluorescence-detecting reagents were Cy3-goat anti-rat IgM and Cy2-streptavidin (Jackson ImmunoResearch).
Injection of LTβR-Ig and Control Ig.
Mice were injected i.p. with 100 μg of either LTβR-Ig or control Ig (a gift from Jeff Browning, Biogen/Idec). Lymphoid organs were analyzed 7 days later. These experiments were performed in triplicate with comparable results.
Detection of β-Galactosidase (LacZ) Activity.
Tissue Fixative buffer and solutions A, B, and C were purchased from Specialty Media (Millipore, Billerica, MA). Tissues collected from the transgenic mice were fixed with Tissue Fixative buffer for 1.5 h on ice, rinsed with solution A once, and maintained in solution A for 30 min at room temperature, then rinsed with solution B once and washed for 5 min in solution B. The washed tissues were incubated in prewarmed (37°C) 1% X-Gal (American Bioanalytical, Natick, MA) in solution C for 4 h or overnight, and the blue stain was monitored at regular intervals. The tissue was then rinsed twice with PBS and stored in solution A.
Acknowledgments
We thank Jeff Browning (Biogen/Idec) for LTβR-Ig and Steven Rosen and Stefan Hemmerich for BAC clone 20473A. This work was supported by the Anna Fuller Fund (S.L.) and National Institutes of Health Grants CA16885 (to N.H.R.), DK57731 (to N.H.R.), and GM09966 (to F.H.R.).
Abbreviations
- HEV
high endothelial venule
- LN
lymph node
- PLN
peripheral LN
- NALT
nasal-associated lymphoid tissue
- PNAd
peripheral node vascular addressin
- LTβR
lymphotoxin-β receptor
- TLO
tertiary lymphoid organ
- HEC
high endothelial cell
- HE
hematoxylin.
Footnotes
The authors declare no conflict of interest.
References
- 1.Berg EL, Robinson MK, Warnock RA, Butcher EC. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 3.Hemmerich S, Rosen SD. Glycobiology. 2000;10:849–856. doi: 10.1093/glycob/10.9.849. [DOI] [PubMed] [Google Scholar]
- 4.Shailubhai K, Streeter PR, Smith CE, Jacob GS. Glycobiology. 1997;7:305–314. doi: 10.1093/glycob/7.2.305. [DOI] [PubMed] [Google Scholar]
- 5.Hemmerich S, Butcher EC, Rosen SD. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraoka N, Bronislawa P, Nakayama J, Tsuboi S, Suzuki M, Yeh JC, Izawa D, Tanaka T, Miyasaka M, Lowe JB, Fukuda M. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- 7.Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo FR, Huang CC, Kannagi R, Rosen SD, Hemmerich S. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmerich S, Bistrup A, Singer MS, van Zante A, Lee JK, Tsay D, Peters M, Carminati JL, Brennan TJ, Carver-Moore K, et al. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 10.Hiraoka N, Kawashima H, Petryniak B, Nakayama J, Mitoma J, Marth JD, Lowe JB, Fukuda M. J Biol Chem. 2004;279:3058–3067. doi: 10.1074/jbc.M311150200. [DOI] [PubMed] [Google Scholar]
- 11.Pablos JL, Santiago B, Tsay D, Singer MS, Palao G, Galindo M, Rosen SD. BMC Immunol. 2005;6:6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bistrup A, Tsay D, Shenoy P, Singer MS, Bangia N, Luther SA, Cyster JG, Ruddle NH, Rosen SD. Am J Pathol. 2004;164:1635–1644. doi: 10.1016/S0002-9440(10)63722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 14.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sercarz EE. J Autoimmun. 2000;14:275–277. doi: 10.1006/jaut.2000.0380. [DOI] [PubMed] [Google Scholar]
- 16.Lacorre DA, Baekkevold ES, Garrido I, Brandtzaeg P, Haraldsen G, Amalric F, Girard JP. Blood. 2004;103:4164–4172. doi: 10.1182/blood-2003-10-3537. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Bauer J, Twisk AJ, Breve J, Kraal G. Immunobiology. 1991;182:277–291. doi: 10.1016/s0171-2985(11)80663-7. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw MS, Bollekens JA, Ruddle FH. Nucleic Acids Res. 1995;23:4850–4856. doi: 10.1093/nar/23.23.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson AO, Anderson ND. Am J Pathol. 1975;80:387–418. [PMC free article] [PubMed] [Google Scholar]
- 20.von Andrian UH, M'Rini C. Cell Adhes Commun. 1998;6:85–96. doi: 10.3109/15419069809004463. [DOI] [PubMed] [Google Scholar]
- 21.M'Rini C, Cheng G, Schweitzer C, Cavanagh LL, Palframan RT, Mempel TR, Warnock RA, Lowe JB, Quackenbush EJ, von Andrian UH. J Exp Med. 2003;198:1301–1312. doi: 10.1084/jem.20030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, Muramatsu T, von Andrian UH, Rosen SD. Nat Immunol. 2005;6:1105–1113. doi: 10.1038/ni1258. [DOI] [PubMed] [Google Scholar]
- 23.Newberry RD, Lorenz RG. Immunol Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz RG, Newberry RD. Ann NY Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- 25.Ying X, Chan K, Shenoy P, Hill M, Ruddle NH. Am J Pathol. 2005;166:135–146. doi: 10.1016/S0002-9440(10)62239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. Proc Natl Acad Sci USA. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Liao S, Ruddle NH. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Golden J, Faustman DL. Diabetes. 1993;42:1166–1172. doi: 10.2337/diab.42.8.1166. [DOI] [PubMed] [Google Scholar]
- 30.Bradshaw MS, Shashikant CS, Belting HG, Bollekens JA, Ruddle FH. Proc Natl Acad Sci USA. 1996;93:2426–2430. doi: 10.1073/pnas.93.6.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shashikant CS, Carr JL, Bhargava J, Bentley KL, Ruddle FH. Gene. 1998;223:9–20. doi: 10.1016/s0378-1119(98)00369-2. [DOI] [PubMed] [Google Scholar]
- 32.Carr JL, Shashikant CS, Bailey WJ, Ruddle FH. J Exp Zool. 1998;280:73–85. doi: 10.1002/(sici)1097-010x(19980101)280:1<73::aid-jez9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Chiu CH, Amemiya CT, Carr JL, Bhargava J, Hwang JK, Shashikant CS, Ruddle FH, Wagner GP. Dev Genes Evol. 2000;210:105–109. doi: 10.1007/s004270050016. [DOI] [PubMed] [Google Scholar]
- 34.Sumiyama K, Ruddle FH. Proc Natl Acad Sci USA. 2003;100:4030–4034. doi: 10.1073/pnas.0530119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumiyama K, Irvine SQ, Stock DW, Weiss KM, Kawasaki K, Shimizu N, Shashikant CS, Miller W, Ruddle FH. Proc Natl Acad Sci USA. 2002;99:780–785. doi: 10.1073/pnas.012584999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fava RA, Notidis E, Hunt J, Szanya V, Ratcliffe N, Ngam-Ek A, De Fougerolles AR, Sprague A, Browning JL. J Immunol. 2003;171:115–126. doi: 10.4049/jimmunol.171.1.115. [DOI] [PubMed] [Google Scholar]
- 37.Gommerman JL, Giza K, Perper S, Sizing I, Ngam-Ek A, Nickerson-Nutter C, Browning JL. J Clin Invest. 2003;112:755–767. doi: 10.1172/JCI18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Q, Salomon B, Chen M, Wang Y, Hoffman LM, Bluestone JA, Fu YX. J Exp Med. 2001;193:1327–1332. doi: 10.1084/jem.193.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drayton DL, Bonizzi G, Ying X, Liao S, Karin M, Ruddle NH. J Immunol. 2004;173:6161–6168. doi: 10.4049/jimmunol.173.10.6161. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M, Walter BN, Pike-Nobile L, Fanger NA, Guyre PM, Browning JL, Ware CF, Epstein LB. Cell Death Differ. 1998;5:497–505. doi: 10.1038/sj.cdd.4400374. [DOI] [PubMed] [Google Scholar]
- 41.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]